1 , ,..., (0,0,...,0) 2 U U U U x x x x xx x x x
advertisement
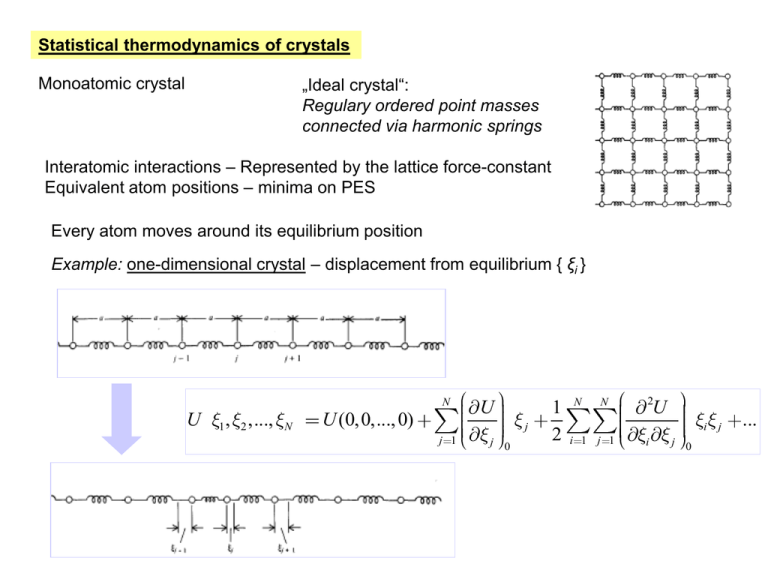
Statistical thermodynamics of crystals
„Ideal crystal“:
Regulary ordered point masses
connected via harmonic springs
Monoatomic crystal
Interatomic interactions – Represented by the lattice force-constant
Equivalent atom positions – minima on PES
Every atom moves around its equilibrium position
Example: one-dimensional crystal – displacement from equilibrium { ξi }
U x1 , x2 ,..., xN
N
U (0, 0,..., 0)
j 1
U
xj
xj
0
1
2
N
N
i 1 j 1
2
U
xi x j
xi x j
0
...
U x1 , x2 ,..., xN
N
U (0, 0,..., 0)
j 1
U x1 , x2 ,..., xN
U
xj
xj
0
1
2
U (0, 0,..., 0)
N
N
i 1 j 1
1
2
N
2
U
xi x j
N
xi x j
...
0
kij xi x j
i 1 j 1
Harmonic approximation – U(ξi) is a quadratic function – „reasonable“ appraoximation
Force constants – kij
U(0,0,...,0) – depends on the lattice parameter → function of ρ = V/N :
≠0
U (0, r)
U x1 , x2 ,..., xN
Depends on ρ
kij
„Coupled harmonic osc.“
3N-6 independent vibrational modes
~ 3N
nj
1 kj
2p m j
1/ 2
kj and μj stands for effective force constant and effective reduced mass
Solving the variational problem of atom cyrstal: transformation into 3N independent harmonic
oscilators.
Frequency of individual oscilators – depends on masses, force constants and type of the
crystal (complicated equation)
nj
kj
kij
V
N
Frequency of normal modes
depends on density !
Partition function of monoatomic crystal:
V
Q
,T
N
e
U ( 0;r ) / kT
3N 6
qvib, j
j 1
(no rotational and translational degrees of freedom)
(atoms are distinguishable !)
Vibrational partition function – harmonic oscilator
en
1
2
hn n
wn
n
1
1 k
2p m
1/2
Vibrational level degeneracy
Zero energy defined as –De
qvib (T )
e
ben
e
n
e
e b hn /2
1 e b hn
b hn n
n 0
Ev
Qv
b hn /2
kT 2
hv / k
ln Qv
T
NkT 2
N ,V
d ln qv
dT
NkQv
1
2
1
eQv /T
1
Vibrational temperature – typically 103 K – just first term needs to be considered
e
Population of vibrational levels: f n T
b hn ( n 1/ 2)
qvib
Fraction of molecule in vibrationally excited states:
fn
0
T
e
fn T
n 1
n 1
b hn ( n 1/ 2)
qvib
1
f0
e
b hn / 2
e
Qv / 2T
Solving the variational problem of atom cyrstal: transformation into 3N independent harmonic
oscilators.
Frequency of individual oscilators – depends on masses, force constants and type of the
crystal (complicated equation)
nj
kj
kij
V
N
Frequency of normal modes
depends on density !
Partition function of monoatomic crystal:
V
Q
,T
N
e
U ( 0;r ) / kT
3N 6
qvib, j
j 1
qvib
V
Q
,T
N
3N
j 1
e
hn j / 2 kT
1 e
hn j / kT
e
U ( 0;r ) / kT
(no rotational and translational degrees of freedom)
(atoms are distinguishable !)
e
hn / 2 kT
1 e
hn / kT
Large number of vibrational modes (3N) – continuous distribution from 0 to νmax
Define „frequency density“ g(ν)dν – number of normal vibrational models in an
interval (ν,ν+dν)
E
kT
ln Q
2
ln Q
T
V
Q
,T
N
N ,V
U (0; r )
kT
ln 1 e
0
hn / kT
3N
j 1
e
hn j / 2 kT
1 e
hn j / kT
e
U ( 0;r ) / kT
CV
E
T
hn
g (n )d n
2kT
g (n )d n
„Normalization“ condition:
3N
0
We need a suitable approximation for g(ν); TD properties can be obtained
E
U (0; r )
0
hn e
1 e
hn / kT
hn / kT
hn
g (n )d n
2
2
CV
hn / kT e
k
0
Almost exact (harmonic approximation only) –g(ν) is missing
=> Various approaches to find g(ν)
1 e
hn / kT
g (n )d n
hn / kT 2
N ,V
I. Classical thermodynamics
Dulong-Petit law
Each vibrational degree of freedom contributes based on equipartition theorem
CV
3Nk
3R
6 cal / deg.mol
Works for numerous crystals at high temperatures
Fails at low temperatures
Qualitative failure at very low temperatures (CV approaches 0 K as T3 – experimentally)
Silver crystal
II. Einstein model
1907
Quantization of vibrational energy (similar to Planck model of black body)
Each atoms vibrates around its equilibrium position independently of other atoms
3N independent oscillators with the same frequency νE
g (n )
Using g(ν):
3N d n
nE
(delta function)
νE ... Frekvency (Einstein’s) 3N independent oscillators
Specifc for each crystal – depends on the PES details
E
hn / kT
hn e
1 e
U (0; r )
0
hn / kT
E
T
CV
2
CV
hn / kT e
k
0
CV
hn E
3Nk
kT
2
e
1 e
1 e
hn
g (n )d n
2
V
hn / kT
hn / kT 2
g (n )d n
hn E / kT
hn E / kT 2
QE
hn E
k
Einstein temperature:
CV
Q
3Nk E
T
hn E
k
QE
2
QE / T
e
1 e
QE / T 2
Only parameter (Einstein temperature):
Works remarkably except for very low temps.
T
0 : CV
Q
3Nk E
T
2
e
QE / T
A. Einstein, Ann. Physik, 22 (1907) 180.
Heat capacity of diamond
ΘE = 1320o K
Dependence of CV on reduced temperature (ΘE/T) is
universal for all crystals
III. Debye model
Einstein model – fails at low temps
Oscillator energy depends on frequency
T→0 : Low energy modes become
important
Norma mode frequency varies from 0 do 1013 Hz
Below – normal modes in 1-D crystel (high and low energy models depicted below)
A mode having the highest frequency: wavelength ~ 2a – atoms move against each other
A mode with minimal frequency – atoms moves in the same direction
Debye: modes with wavelength » lattice constant – independent of material – crystal
behaves as continuous elastic body
Wave with amplitude A and frequency ω=2πν and moving in the direction k :
u(r, t )
Aei (k r
k je wave vector; 2π/λ
v ... Velocity of the wave
u
wt )
w/k
nl
Superposition of waves moving in opposite direction:
Standing wave
u
2 Aeik r cos wt
To form a standing wave - its imaginary part must be zero on the border (crystal edge):
kx L
nx p
ky L
ny p
kz L
nz p
k
k
2
p
n
L
p
L
Frequency depends on k
u
nx2
ny2
Number of vawes with wavenumber in
interval (k, k+dk)
nz2
F(k )
Distinguishing the direction
Of the wave
g (n )d n
p Lk
6 p
g (n )d n
3
L3k 3
6p 2
dF
dk
dk
w (k )dk
n
1
4pV n 2 d n
3
ul
nl
2
Number of waves having wavevector smaller
than k.
2
ut3
w/k
u
l
uk
2p
4pV n 2
dn
3
u
Vibrational modes in the direction perpendicual (or parallel)
Vk 3
6p 2
Vk 2 dk
2p 2
3
u03
Introducing average velocity:
12pV 2
n dn
u03
g (n )d n
2
ut3
1
ul3
Exact expression for low energy modes
nD
g (n )d n
Debye frequency – Maximal frequency of the crystal – follows from
0
3N
4pV
nD
1/ 3
u0
g (n )d n
9N 2
n dn
3
nD
n
0
2
CV
hn / kT e
k
0
CV
1 e
T
9 Nk
QD
3
nD
nD
hn / kT
hn / kT 2
QD / T
0
n
0
g (n )d n
x 4e x
ex 1
2
dx
QD
hn D
k
Debye temperature
3N
T
D
QD
Debye function:
T
3
QD
3
x 4e x
QD / T
0
e
x
1
2
dx
One-parameter equation, numerical solution
CV
3Nk D
T
QD
For temperature approaching 0 K:
12p
T
Nk
5
QD
4
T
0K :
CV
3
A proper behavior
Even for T goes to 0
Heat capacity as a function of T/ΘD – single universal curve
Aluminium
428 K
Cadmium
Chromium
Copper
Gold
Iron
Lead
Manganese
Nickel
Platinum
209 K
630 K
343.5 K
165 K
470 K
105 K
410 K
450 K
240 K
Silicon
Silver
Tantalum
Tin (white)
Titanium
Tungsten
Zinc
Carbon
Ice
645 K
225 K
240 K
200 K
420 K
400 K
327 K
2230 K
192 K

