F HG I KJ chch F HG I KJ ch
advertisement
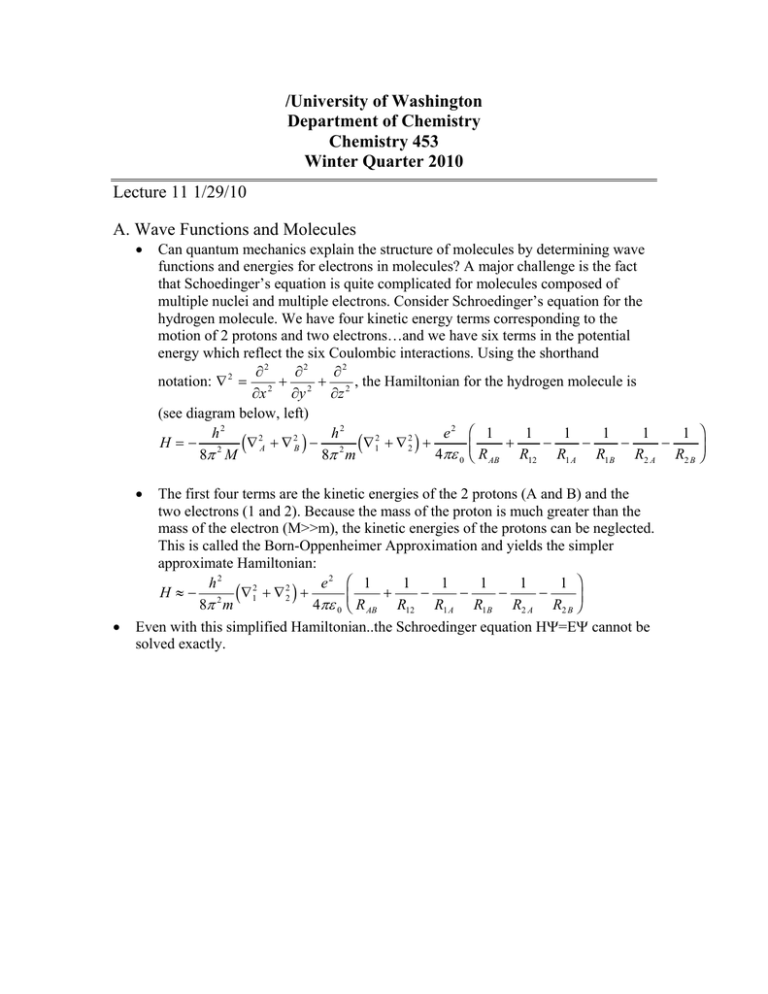
/University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2010 Lecture 11 1/29/10 A. Wave Functions and Molecules • Can quantum mechanics explain the structure of molecules by determining wave functions and energies for electrons in molecules? A major challenge is the fact that Schoedinger’s equation is quite complicated for molecules composed of multiple nuclei and multiple electrons. Consider Schroedinger’s equation for the hydrogen molecule. We have four kinetic energy terms corresponding to the motion of 2 protons and two electrons…and we have six terms in the potential energy which reflect the six Coulombic interactions. Using the shorthand ∂2 ∂2 ∂2 2 notation: ∇ = 2 + 2 + 2 , the Hamiltonian for the hydrogen molecule is ∂x ∂y ∂z (see diagram below, left) h2 h2 e2 1 1 1 1 1 1 2 2 2 2 H=− 2 ∇ A + ∇ B − 2 ∇1 + ∇ 2 + + − − − − 4πε 0 R AB R12 R1 A R1B R2 A R2 B 8π M 8π m c h c h FG H • The first four terms are the kinetic energies of the 2 protons (A and B) and the two electrons (1 and 2). Because the mass of the proton is much greater than the mass of the electron (M>>m), the kinetic energies of the protons can be neglected. This is called the Born-Oppenheimer Approximation and yields the simpler approximate Hamiltonian: 1 1 1 1 1 1 h2 e2 2 2 H ≈ − 2 ∇1 + ∇ 2 + + − − − − 4πε 0 R AB R12 R1 A R1B R2 A R2 B 8π m Even with this simplified Hamiltonian..the Schroedinger equation HΨ=EΨ cannot be solved exactly. c • h FG H IJ K IJ K + Hydrogen Molecule H2 Hydrogen Molecule Ion H2 B. Variational Principle and the LCAO Method • • • If an exact wave function cannot be obtained by directly solving Schroedinger’s equation, approximate wave functions and energies can be obtained using the Variational Principle. The Quantum Mechanical Variational Principle states: “Given any approximation wave function satisfying the boundary conditions of the problem, the expectation value of the energy calculated with this wave function will always be higher than the true energy of the round state of the system”. This principle provides a method for obtaining approximate wave functions and energies in situations where the Schroedinger equation cannot be solved… + Example: The simplest molecule is the hydrogen molecule ion H2 , which has only a single electron. The Hamiltonian is (see diagram above, right) h2 e2 1 1 1 H ≈ − 2 ∇2 + − − , where the Born-Oppenheimer 4πε 0 R AB R1 A R1B 8π m Approximation has again been used. To obtain the expectation value or the average value of the energy, multiply the Schroedinger equation on the left by the complex conjugate of the wave function: Ψ * H Ψ = Ψ * E Ψ = E Ψ * Ψ . Note the wave function Ψ* cannot be shifted to the right of the Hamiltonian because the Hamiltonian operates on anything to its right… Integrate both sides of the Schroedinger equation over all space Ψ * H Ψdτ = Ψ * E Ψdτ = E Ψ * Ψdτ where dτ=dx dy dz. FG H • • z z IJ K z z Ψ * H Ψ dτ z • Solve for E which is the expectation value E = E = • We could solve for the energy if we knew the wave function. But the best we cannot do is guess a probable form for the wave function. We guess that the wave function is Ψ * Ψ dτ • a linear combination of atomic orbitals (LCAO), specifically the 1s orbitals: Ψ ≈ c Aψ 1As + c Bψ 1Bs , where cA and cB are constants…to be determined…and the wave functions are the 1s wave functions for hydrogen atoms A and B. Substituting the trial wave function into the energy equation we c 2 H + 2c A c B H AB + c B2 H BB obtain… E ≈ A AA2 c A + 2c A c B S AB + c B2 • H AA = ψ 1As Hψ 1As dτ • H BB = ψ 1Bs Hψ 1Bs dτ • H AB = ψ 1As Hψ 1Bs dτ = ψ 1Bs Hψ 1As dτ • S AB = ψ ψ dτ = ψ 1Bsψ 1As dτ • The Variational Principle states that the energy calculated using the trial wave function Ψ ≈ c Aψ 1As + c Bψ 1Bs will be higher than the “true” ground state energy. Therefore, the true values of cA and cB can be obtained by minimizing the energy of the system with respect to these constants. This can be done using methods that are standard in the calculus. z z z z A 1s B 1s z z C. Variational Principle: Bonding and Anti-bonding Orbitals… • • Applying the variational principle, the energy of the electron in H2+ can be obtained by minimizing the variational energy with respect to the constants cA and cB… We obtain two secular equations: ∂ E = 0 = c A H AA − E + c B H AB − S AB E ∂c A b ∂ E ∂c B g b b g b = 0 = c A H AB − S AB E + c B H BB − E g g H AA ± H AB 1 ± S AB • Solving these two equations for the energy we get two solutions… E • where we have used the fact that HAA=HBB. Substituting each of these energies back into the secular equations we obtain two solutions c A± = ± cB ± . This yields two orbitals…each with a different energy… Ψ ± = c± (ψ 1As ± ψ 1Bs ) • from which we obtain… c± = • z We require that both orbitals be normalized: Ψ±* Ψ± dτ = c 2A 1 2 ± 2S AB c h zc ± = h 2 ψ 1As ± ψ 1Bs dτ = 1, 1 ψ 1As + ψ 1Bs is called the bonding orbital and is 2 + 2 S AB H + H AB given the shorthand σ1s. The bonding orbital σ as an energy E + = AA 1 + S AB Bonding Orbital: Ψ+ = • The Coulomb integral HAA has the form… ψ 12s 1 e2 e2 H AA = E1s − ψ 12s dτ = E1s − dτ − 4πε 0 4πε 0 R1B R AB FG Hz zc h = • • • z z ψ 2 1s R 1 z ψ FG ψ Hz R 2 1s 1B dτ − IJ K 1 .. R AB 2 1s dτ , are both less than 4πε 0 R1B 4πε 0 R1B 1 zero…They reflect the stabilization of the molecule as a result of the attraction of the electron for the second nucleus, and would be expected to result in a more stable orbital energy than the isolated hydrogen atom. But as the atoms approach 1 the nuclear repulsion term > 0 grows larger and hence the net 4πε 0 R AB contribution of HAA to stabilization is small. The stabilization of the H2+ ion derives from the resonance integral: ψ 1As * ψ 1Bs e2 1 A B H AB = ψ 1s * H 0ψ 1s dτ − dτ − ψ 1As * ψ 1Bs dτ R1B R AB 4πε 0 The first to terms E1s − • 1 IJ K zc h ψ 1As * H 0ψ 1Bs dτ − e2 4πε 0 dτ = − Fc h GH z F cψ h *ψ GH z R A 1s 1B bg B 1s 2 − dτ − 1 R AB zc h I S J K I JK AB Physically this integral is the stabilization deriving from the fact that the electron can shift from the ψ 1sB orbital to the ψ 1sA orbital. It is called the resonance integral because of the resemblance to the coupling or resonance of two classical oscillators. As a result of the negative resonance integral, the + energy of an electron in the bonding orbital of H2 is less than the energy of the electron in the 1s atomic orbital of a hydrogen atom. A plot of E+ , the energy of the σ1s molecular bonding orbital as a function of the internuclear distance RAB is shown below, right. The Anti-bonding Orbital: Ψ − = ψ 1As −ψ 1Bs 2 − 2 S AB is called the anti-bonding orbital and is H AA − H AB . This 1 − S AB energy will be higher than the energy of an isolated hydrogen atom. This means that + if the electron were in the σ∗1s orbital of H2 , the molecule would be less stable than the separated hydrogen atom and ion. given the shorthand σ∗1s. The bonding orbital has an energy E − =


