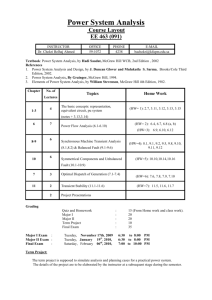
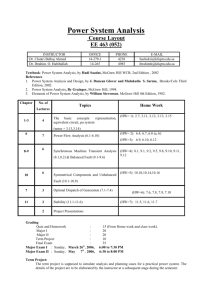
Chemical Engineering
advertisement

B. TECH. DEGREE (Chemical Engineering) REVISED SYLLABUS FOR 2011–2012 onwards Department of Chemical Engineering MAULANA AZAD NATIONAL INSTITUTE OF TECHNOLOGY (MANIT), BHOPAL BHOPAL - 462 051. 1 REFRAMED SYLLABUS FOR B-TECH COURSE I – SEMESTER Course No. PHY101 CHM101 MTH101 C101 HUM101 M101 M141 PHY141 CHM141 Course No. PHY151 CHM151 MTH151 CS151 E151 C151 CS191 E191 C191 M191 E199 Subject Physics I Engg. Chemistry I Mathematics I Environment Engg. Communication skill Engg. Graphics Workshop Physics Lab Engg Chemistry Lab Total Scheme of No. & Studies Periods Duration Per week of Theory Papers L T P No. Hrs 3 1 1 3 3 1 1 3 3 1 1 3 3 1 1 3 3 1 1 3 4 1 3 3 3 3 15 9 9 6 - II – SEMESTER Subject Scheme of No. & Studies Duration Periods Per of Theory week Papers L T P No. Hrs Physics II 3 1 1 3 Engg. Chemistry II 3 1 1 3 Mathematics II 3 1 1 3 Computer Programming 3 1 1 3 Basic Elect/Electronics 3 1 1 3 Engg. Basic Civil & 3 1 1 3 Mechanical Engg. Computer Prog. Lab - 2 Elect. /Electronics Lab 2 Civil Engg. Lab 2 Mech. Engg. Lab - 2 General Proficiency - Total 18 6 8 6 - 2 Credits L 3 3 3 3 3 15 T 1 1 1 1 1 4 9 Total P 3 3 3 9 Credits 4 4 4 4 4 4 3 3 3 33 Total L 3 3 3 3 3 T 1 1 1 1 1 P - 4 4 4 4 4 3 1 - 4 - - 18 6 2 2 2 2 4 12 2 2 2 2 4 36 III - SEMESTER Course No. HUM 201 CH 201 CH 202 CH 203 CH 204 CH 205 CH 241 CH 242 Subject Industrial Economics Introduction to Chemical Engineering Instrumentation & Measurement Chemical Process Calculations Fluid Mechanics Computer ProgrammingII Technical Analysis Lab Fluid Mechanics Lab. Total Scheme Studies Periods week L T 3 1 3 1 of Credits P - No. & Duration of Theory Papers No. Hrs 1 3 1 3 L 3 3 T 1 1 P - 4 4 Per Total 3 1 - 1 3 3 1 - 4 3 1 - 1 3 3 1 - 4 3 3 1 1 - 1 1 3 3 3 3 1 1 - 4 4 18 6 4 4 8 6 - 18 6 4 4 8 4 4 32 IV – SEMESTER Course No. MTH 251 CH 251 CH 252 CH 253 CH 254 CH 255 CH 291 CH 292 CH 299 Subject Numerical Techniques Chemical Engg. Thermodynamics Mechanical Operations Material Science & Technology Chemical Process Technology- I Environmental Protection & Pollution Control Environmental Engg. Lab. Chemical Technology Lab. General Proficiency Total Scheme of studies periods per week L 3 3 T 1 1 P - No. & duration of Theory Papers No. Hrs 1 3 1 3 3 3 1 1 - 1 1 3 3 3 3 1 1 - 4 4 3 1 - 1 3 3 1 - 4 3 1 - 1 3 3 1 - 4 - - 4 - - - - 4 4 - - 4 - - - - 4 4 18 6 8 6 - 18 6 4 12 4 36 3 Credits L 3 3 T 1 1 P - 4 4 Total V – SEMESTER Course No. HUM 301 CH 301 CH 302 CH 303 CH 304 CH 305 CH 341 CH 342 Subject Industrial Project Management Chemical Reaction Engg- I Mass Transfer - I Heat Transfer - I Process dynamics and Control Bio-Chemical Engineering Heat Transfer Lab Mechanical Operation Total Scheme of studies periods per week L 3 T 1 P - No. & duration of Theory Papers No. Hrs 1 3 Credits L 3 T 1 P - 4 3 1 - 1 3 3 1 - 4 3 3 3 1 1 1 - 1 1 1 3 3 3 3 3 3 1 1 1 - 4 4 4 3 1 - 1 3 3 1 - 4 18 6 4 4 8 6 - 18 6 4 4 8 4 4 32 Total VI – SEMESTER Course No. CH 351 CH 352 CH 353 CH 354 CH 355 CH 391 CH 392 CH 393 CH 399 Subject Computational Methods in Chemical Engg. Mass Transfer - II Process Equipments Design & Drawing -I Chemical Process Tech II Heat Transfer -II Reaction Engg. Lab. Mass Transfer Lab. Minor Project & Seminar General Proficiency Total Scheme of studies periods per week L 3 T 1 P - No. & duration of Theory Papers No. Hrs 1 3 3 3 1 1 - 1 1 3 3 3 3 1 1 - 4 4 3 1 - 1 3 3 1 - 4 3 15 1 2 7 4 4 4 12 1 5 3 - 3 - - 15 1 2 7 4 4 4 4 16 4 4 4 6 4 38 4 Credits L 3 T 1 P - 4 Total VII – SEMESTER Course No. Subject CH 401 CH 402 CH 403 CH 411-415 CH 421-425 CH 441 CH 442 CH 443 Scheme of studies periods per week Transport Phenomena Process Equipment Design -II Chemical React. Engg II Elective – I Elective – II Instrumentation & Process Control Lab. Major Project & Seminar Educational training Total Tour CH 452 CH 461CH465 CH 471CH475 CH 492 CH 499 T 1 1 P - 3 1 - 1 3 3 1 - 4 3 3 1 1 4 1 1 3 3 3 3 - - - 1 1 - 4 4 4 4 2 6 - 6 8 - - 2 - - - 2 2 7 10 5 - 15 7 12 34 Scheme Studies Periods week L T 3 1 of Credits P - No. & Duration of Theory Papers No. Hrs 1 3 L 3 T 1 P - 4 - & - VIII – SEMESTER Course Subject No. CH 451 L 3 3 No. & duration of Theory Papers No. Hrs 1 3 1 3 Process Modeling & Simulation Process Engg. & Costing. Elective – III 15 Per Credits L 3 3 T 1 1 P - 4 4 Total Total 3 3 1 1 - 1 1 3 3 3 3 1 1 - 4 4 Elective –IV 3 1 - 1 3 3 1 - 4 Major Project & Seminar General Proficiency Total 12 2 6 12 12 4 - 12 2 6 12 4 16 14 4 34 Elective I CH 411 CH 412 CH 413 Bio- Technology Surface coating Technology Oil Technology 5 CH 414 CH 415 Energy Engg Environmental Impact Assessment & Environmental Audit CH- 421 CH- 422 CH- 423 CH- 424 CH- 425 CH 461 CH 462 CH 463 CH 464 CH 465 Elective II Petroleum & Petrochemical Engg. Fertilizer Technology Industrial Pollution Control Pharmaceutical Technology Corrosion Engg. Elective III Computer Aided Process Control & Design Environmental Pollution Control, Design and Modeling Polymer Technology Computational Fluid Dynamics Risk Analysis & Hazard CH 471 CH 472 CH 473 CH 474 CH 475 CH 472 CH 474 CH 475 Elective IV Process Piping Design Multi Phase Flow Textile Technology Novel Separation Techniques Industrial Catalysis Elective V/VI/VII/VIII Cryogenic Engg. Multi Component Distillation Fluidization Engg 6 CH 361 CH 362 CH 363 CH 364 CH 365 CH 411 CH 412 CH 413 CH 414 CH 415 CH 421 CH 422 CH 423 CH 424 CH 425 CH 461 CH 462 CH 463 CH 464 CH 465 Bio- Technology Chemical Process Industries Petroleum & Petrochemical Engg Advances in Heat Transfer Energy Engg Industrial Catalysis Fertilizer Technology Food Technology Petroleum Processing Technology Pharmaceutical Technology Environmental Pollution : Control Design and Modeling Computer Aided Process Control & Design Polymer Technology Ceramic Technology Risk Analysis & Hazard Novel Separation Techniques Multi Phase Flow Textile Technology Process Piping Design Non Conventional Energy Technology 7 Semester – III INDUSTRIAL ECONOMICS (HUM201) Unit –I Introduction to Economics: Introduction to economics, its Importance, Principals, Approaches, and use of study, Engineering and economics, Economic problems, economic good and wealth, Demand and supply, Competition, Monopoly, theory of firm, Money and its function, theory of money and choice, the bank and its functions, employment and income, Gross National Product, Net National Product Consumption, Savings and investment. Unit –II Features of Indian Economy-I: Broad features of Indian Economy, Natural resources and economic development, Infrastructure in the Indian Economy, Agriculture development, Green revolution, Population, Population theories, Unemployment, Poverty, and Balance Regional Development. Economic Growth and Economic Development, Indian Industries, Industrial Policy, Industrialization in India, role, plan and pattern of Industrialization, Public vs Private Sectors, Economic reforms in India, India’s Five Year Plans. Unit– III Features of Indian Economy-II: The indigenous and modern banking system in India, Reserve Bank of India, Monetary and Fiscal Policies, Financial Institutions and SEBI, Free trade and protection, India’s Foreign Trade and WTO, Balance of Payments, Indian Currency System and Foreign exchange, Foreign Capital Investment, Foreign Aid, and FEMA. Unit –IV Introduction to Business Organization-I: Concept, nature and scope of business, business and its environment, Profit maximization vs social responsibility of business, business ethics, business enterprise, entrepreneurship, Promoters, types and functions, stages in company formation, concept of business growth, rationale and types of growth strategies, joint venture-definition, scope, role and problems of small business, concepts and features of public enterprise, multinationals. Unit– V Introduction to Business Organization-II: Time value for money, Simple and compound interest, annuity, Depreciation, definitions, characteristics, Life and Salvage value, Method of providing for depreciation, relationship between depreciation, repairs, renewals, Depletion cost, Replacement, Amortization, and Present worth. Suggested Text books and references: 1. Indian Economy --Dutt & Sundaram 8 2. Engineering Economics –Tarachand 3. A Text Book of Economic Theory --Stonier & Hague 4. Business Organization --Shukla Introductions to Chemical Engineering (CH-201) Unit I Historical overview of Chemical Engineering: Concepts of unit operations and unit processes, and recent developments. Unit II Fuels –Solid, liquid & Gaseous fuels. Unit III Chemical Kinetics Constant Rate constant order and molecularity of a reaction, zero, 1st, 2nd, and 3rd order reactions. Kinetics of opposing reactions, methods of determination of order of reactions. Reaction rate theories, Arrhenius Parameters, Catalysis (including enzyme catalysis), effect of catalysis on reaction rate. Unit IV Introduction to Heat Transfer, Conduction, Convection, Radiation, Flow Arrangement in Heat Exchangers, Variation of Fluid Temperature in Heat Exchangers, Heat Transfer Equipment, Evaporation, Problems. Unit V Introduction to Mass Transfer, crystallization, distillation, evaporation absorption. Texts/References 1. R. M. Felder and R.W. Rousseau, Elementary Principles of Chemical Processes, 3rd ed., John Wiley, New York, 2. Enderson & Belzil Introduction to Chemical Engineering 3. Bezer & Banchoro, Introduction to Chemical Engineering 4. Salil. K Ghosal, Shyamal K Sanyal, Siddhratha Datta “Introduction to Chemical Engineering, Tata McGraw Hill, New Delhi. INSTRUMENTATION & MEASUREMENT (CH-202) Unit-I Measurement and error. Accuracy and precision, sensitivity, resolution, Types and sources of errors. PMMC, Analog meters, Electronic Voltmeters and Ammeters. (C and DC) Electronic millimeters, AC probes, CROs, VIVM. Unit-II Measurement of inductance and Q of the coil. Maxwell’s Bridge, Wien’sbridge, Schering bridge, wagner’s earth, vector impedance meter transducers (LVDT). Photoelectric transducers. Unit-III Signal generator, function generator. Function generator, sweep frequency generator. Pulse and square wave generator, Heterodyne, Frequency meter frequency counter. 9 Measurement errors. Unit-IV Digital instruments: Advantage of digital instruments, over analog instruments, D-A, A-D conversion. Digital voltmeter, Ramp type DVM, Integrating DVM, Successive approximation DVM Displays (LED. LCD and seven segment etc.) Unit-V Temperature & Pressure measurements, Thermocouples. Photosensitive device, Pressure measuring. Wave Analyzers, Spectrum Analyzer, Instruments used in computer controlled instrumentation, RS232C and IIEE488, GPIB electrical interface, Interfacing transducers to electronic control pressure gauges. Reference Books: 1. Albert D. Cooper – Modem Electric Instrumentation – PHI 2. A.K. Maini-Microwave & RADARS – Khanna Publisher. CHEMICAL PROCESS CALCULATIONS (CH-203) Unit I STOICHIOMETRY: Introduction- Units and Dimensions - stoichiometric principlescomposition relations, density, specific gravity and basis of calculation. Unit II IDEAL GASES AND VAPOR PRESSURE: Behaviors of Ideal gases -kinetic theory of gases application of ideal gas law- gaseous mixtures - volume changes with change in composition. Vapor pressure- effect of Temperature on vapor pressure. Unit III HUMIDITY AND SOLUBILITY: Humidity - saturation - vaporization - condensation - wet and dry bulb temperature, dew point, adiabatic saturation temperature, Solubility and Crystallization-Dissolution -solubility of gases. Unit IV MATERIAL BALANCE: Material Balance - Processes involving with chemical reaction and without chemical reaction - Combustion of coal, fuel gases and sulphur - Recycling operations bypassing streams - Degree of conversion -excess reactant - limiting reactant Unit V ENERGY BALANCE: Thermo chemistry - Hess's law of summation - heat of formation, reaction, combustion and mixing - mean specific heat -Theoretical flame Temperature. TEXTBOOKS: 1. O.A.Hougen, K. M. Watson and R. A. Ragatz, "Chemical Process Principles", Vol-I,CBS Publishers and Distributors, New Delhi, 1995. 2. D. Himmelblau, '"Basic Principles and Calculations in Chemical Engineering", 5th Edn.,Prentice Hall of India Ltd.,N.Delhi,1994. REFERENCES: 1. B.I.Bhatt and S.M.Vora, "Stoichiometry", Tata McGraw Hill Publishers Ltd., New Delhi, 1996. 2. V.Venkataramani and N.Anantharaman, "Process Calculations", Prentice Hall of India Ltd.,N.Delhi,2003. 10 FLUID MECHANICS (CH-204) Unit-I Introduction : Properties of fluid, forces on fluid, stresses, the concept of constitution relations, fluid statics, Normal forces in fluid, pressure Measurement, forces on submerged, forces on submerged bodies, buoyancy, stability. Unit-II Classification of Fluids : Newtonian and Non-Newtonian fluid, Viscosity measurement, Equations of Continuity & Equation of Motion. Navier stokes equation, concept of Reynolds number and friction factor : friction factor for rough and smooth pipes, loss of head due to friction in pipes and fittings. Unit-III Boundary layer theory, Bernoulii‟s equation, fluid machinery pump fans blowers, compressor & vacuum pumps. Power and head requirement for pumps. Unit-IV Flow of incompressible fluid in conduits and thin layers, flow past immersed bodies. Dimensional analysis, Buckingham - theorem, dimensionless numbers and their significances, similitude criteria. Unit-V Measurement of flow: Fluid flow Measurement pitot tube, orifice meter, venture meter, Rota meter weirs and notches. Reference Books: 1. W.L. McCabe & I.C. Smith – UNIT OPERATIONS IN CHEMICAL ENGG. – McGraw Hill & Kogakusha 1976. 2. J.M. Coulson & J.F. Richardson – CHEMICAL ENGINEERING – Vol I & II 3. B.S. Maney, zel (SI) Van Nostand & Reinhold – MECHANICS OF FLUID – ELBS, 1970 4. I. Grannet – FLUID MECHANICS FOR ENGG. AND TECHNOLOGY – Prentice Hall, 1971 5. Maurice G. Larian – FUNDAMENTALS OF CHEMICAL ENGG. OPERATION – Constable and Company Ltd. Landon. 6. S.K. Gupta – MOMENTUM TRANSFER-Newage Publication. COMPUTER PROGRAMMING – II (CH-205) Unit I Objects And Classes: Concepts in object-oriented programming, classes and objects, C++ programming basics, object-oriented analysis, object-oriented design methods. Working With Classes: Operators and Friends: Operator overloading, Friend functions and operators. Arrays, Pointers and References. Unit II Class Inheritance: Derived classes, the protected access specifier, derived class constructors, overriding member functions, Class hierarchies, Public and Private 11 inheritance, multiple inheritances. Unit III Polymorphism: Virtual functions, Abstract base classes and pure virtual functions. Unit IV Files And Streams: Introduction to object-oriented database - case studies. TEXT BOOK: 1. Robert Lafore, "Object Oriented Programming Turbo C++", Gaogotia Pub. 1992. REFERENCES : 1. Neill Graham, "Leaning C++", McGraw Hill Inc. Intl. Edn.,1991. rd 2. Roger S. Pressman, "Software Eng.," A Practitioner‟s Approach, McGraw Hill 3 Edn ., 1992. TECHNICAL ANALYSIS LAB (CH-241) List of Experiments: 1. Application of pH meter to find acidity and alkalinity of a solution. 2. Proximate analysis of coal sample 3. To determine the moisture content of coal sample. 4. To determine the ash content of coal sample. 5. To determine the turbidity in wastewater sample by Nephlometer, chloride in wastewater sample. 6. Determination of pH, temperature and conductivity of given sample by electrode method. 7. Determination of the strength of unknown strong acid by titrating it against weak base by conductometric method. 8. Determination of pH of mixture of CH3COOH and CH3COONa and the dissociation constant of the acid. 9. Chemical analysis of cement. 10. Analysis of fertilizer. FLUID MECHANICS LAB (CH-242) List of Experiment: 1. To determine the local velocity pressure with the help of pilot tube. 2. To find out the terminal velocity of a spherical body in water. 3. To determine the viscosity of a given viscous liquid by capillary tube flow method. 4. To find the pressure drop in a packed bed. 5. To study the flow behaviour of a non-Newtonian fluid and to determine to flow constants. 6. To determine to power-number-Reynolds number curve for an agitated vessel. 7. To differentiate between laminar and turbulent flow using Reynolds experiment. 8. To study the characteristics of an air compressor. 9. To study the characteristics of a centrifugal pump. 10. To study the flow of a fluid in a pipeline and to prepare the friction factor – Re plot. 11. To determine velocity through orifice meter, venture meter. 12. To prepare the calibration curve for an orifice meter and Rota meter. 12 13. To Calculate to prepare the calibration curve for venturimeter. Semester – IV NUMERICALTECHNIQUES (MTH-251) Unit I NON-LINEAR EQUATIONS: Solving non linear equations-Regula Falsi methodNewton's method-order of convergence-Graeffe's method and Bairstow's methodincluding the case of pairs of complex roots-Newton's method for f(x,y)=0,g(x,y)=0. Unit II LINEAR SYSTEMS: Solution of linear equations-Gaussian elimination,Gauss Jordan and Crout's methods-Finding the inverse of a matrix using elementary row transformationsGauss Seidel and Jacobi iterative methods-Power method to find dominant eigen value and eigen vector. Unit III INTERPOLATION AND CURVE FITTING: Newton's forward and backward interpolation-Lagrange's interpolation-Newton's divided difference method-cubic spline interpolation-natural splines-choosing appropriate curve and fitting to data-curve fittingMethod of least Squares-regression equations. Unit IV NUMERICAL SOLUTION OF ODE (Ordinary Differential Equation):Euler's methodEuler's modified method-Taylor's method - Runge-Kutta method of second and fourth order-Simultaneous equations and higher order equations by Taylor's method and RungeKutta method-Multistep method-Milne's and Adams' predictor-corrector methods. Unit V NUMERICAL SOLUTION OF PDE (Partial Differential Equation):Boundary value problems - finite difference methods - second order linear PDEs - solution of Laplace and Poisson equation by Liebmann's method - solution of one Dimensional heat flow and wave equations. TEXT BOOKS: 1. M. K. Venkataraman, "Numerical Methods in Science and Engineering" NPC 2 nd Edn.,(For Unit I)1986. 2. M.K.Jain, SRK lyengar and R.K.Jain, "Numerical Methods for Scientific and Engineering Computation", Wiley Eastern, 1992. REFERENCE: 1. C.F. Gerald, "Applied Numerical Analysis", 2nd Edn., Addison Wesley Publishing Company (For Unit I) 1978, 4th Edn., 1989. CHEMICAL ENGINEERING THERMODYNAMICS (CH-251) Unit I FUNDAMENTALS OF THERMODYNAMICS: Laws of thermodynamics as applied to open and closed system - reversible and irreversible processes - state and point function Absolute entropy - Thermodynamic property changes for ideal gas. Unit II PVT RELATIONS: PVT relationships for gases and liquids - equations of state - Z charts - gas mixtures. Compression - expansion. Refrigeration: Principles and application. 13 Unit III THERMODYNAMIC RELATIONS: Thermodynamic relations - Maxwell's relations Jacobian algebra - estimation of thermodynamic properties. Unit IV PHASE EQUILIBRIA: Phase equilibria - pure component and mixtures - Latent heat correlation - van Laar, Margules equations - Gibbs' - Duhem equation - consistency tests partially miscible and immiscible systems - Azeotropes - retrograde condensation thermodynamic diagrams. Unit V CHEMICAL EQUILIBRIA: Chemical equilibria - heat effects, industrial reactions - Free energy calculations - Homogeneous and heterogeneous reactions - Industrial reactions like NH3 synthesis, SO3 production etc. TEXTBOOKS: 1. J. M. Smith and Van Ness, "Introduction to Engineering Thermodynamics", McGraw Hill, New York, 1994. 2. V.C. Rao – Chemical Engg Themodynamics. REFERENCE: 1. B.F. Dodge, "Chemical Engineering Thermodynamics", McGraw Hill, New York, 1971. MECHANICAL OPERATIONS (CH-252) Unit I CHARACTERISTICS OF PARTICULATE SOLIDS AND SIZE REDUCTION & SCREENING: Properties and characterization of particulate solids, analysis and technical methods for size and surface area distribution of powder; Introduction to size reduction equipment, energy and power requirement in milling operations, computer simulation techniques for mill performance. Mechanical classifiers: Screening equipment, capacity and effectiveness. Unit II FILTRATION: Filtration equipment, filtration media and filter aids, principles of filtration and clarification, estimation of filtration parameters for compressible and incompressible cakes and calculations, centrifugal filtration equipment and principles of operation. Unit III SETTLING AND SEDIMENTATION: Separation based on the motion of particles through fluids, gravity settling processes, Sedimentation, Kynch theory of sedimentation, equipment for sedimentation thickness, rate of sedimentation and sedimentation zones in continuous thickeners, design of thickeners and clarifiers, principles of centrifugal sedimentation and characteristics and sedimenting centrifuges. Unit IV AGITATION AND MIXING OF LIQUIDS: Introduction to agitation and mixing of liquids, agitation equipment, Axial and radial flow impellers and flow patterns in agitated vessels ,prevention of swirling, power consumption in agitated vessels, Blending and mixing, dispersion operations, mixing of solids and pastes and types of mixers. Unit V 14 STORAGE AND CONVEYING OF SOLIDS:Introduction to storage and conveying of solids, bins, hoppers and silos, flow out of bins, design consideration of bins, loading and unloading of solids. Bucket elevators, apron conveyors. Belt conveyors: types of belt conveyors, selection considerations. TEXTBOOKS: 1. McCabe and J.C.Smith,"Unit Operation of Chemical Engineering", 5th Edn., McGraw Hill, New York, 1993. 2. J.M.Coulson and J.F .Richardson, "Chemical Engineering", Vol.II, 4th Edn., Butterworth - Heinemann, 1991. 3. L.N. Foust 4. Brown REFERENCE : 1. Raymond A. Kulweic, "Materials Handling Handbook",2nd Edn, WileyInterscience Publications, 1985. 2. Badger and Banchero, "Introduction to Chemical Engineering", 1 st Edn., McGraw Hill, NewYork, 1954. MATERIAL SCIENCE & TECHNOLOGY (CH-253) Unit-I Mechanical, Thermal &Electrical properties of Materials and their measurement. Unit-II Atomic structure, Inter atomic attraction, Molecular structure, crystallanity, Solid solutions, crystal imperfections, Electronic structure and Electromagnetic properties/ Unit-III Single phase metal deformation, Failure of Metals, Theories of alloying, phase relationship, ironcarbon diagram, Nomenclature of steels, utilization of cast iron, mild steel, stainless steel, lead and graphite in Chemical Engg. System. Unit-IV Theories of Corrosion and corrosion – control, stability of materials in service: Chemical, Thermal and Radiolytic stability. Unit-V Composite materials; Semiconductors, Superconductor, surface Modifications using linings of plastics, rubber, glass, ceramics etc., Nano-materials. Reference Books: VAMBLACK, MATERIAL SCIENCE 2. WOOLEF,: VOL. 1,2,3,4. 3. Robert H. Perry & Don W. Green-PERRYS CHEMICAL Engineering H AND BOOK – VII Ed. – McGraw Hill. 4. O.P. Khanna – MATERIAL SCIENCE & METALLURGY – Dhanpat Rai Publication. 5. S.K. Hajra Choudhury – MATERILS SCIENCE & PROCESSES – Indian Book Distributing Co. 1. CHEMICAL PROCESS TECHNOLOGY-I (CH 254) 15 Unit-I. ALKALIES: Chlor-alkali Industries: Manufacture of Soda ash, Manufacture of caustic soda and chlorine - common salt. Unit-II. ACIDS: Sulphur and Sulphuric acid: Mining of sulphur and manufacture of sulphuric acid. Manufacture of hydrochloric acid . Unit-III. CEMENT AND GLASS Cement: Types and Manufacture of Portland cement. Glass: Manufacture of glasses and special glasses. Ceramics: Refractories. Unit-IV. INDUSTRIAL GASES & PAINTS: Industrial Gases: Carbon dioxide, Nitrogen, Hydrogen, Oxygen and Acetylene Manufacture of paints - Pigments. Unit-V. FERTILISERS: Nitrogen Fertilizers: Synthetic ammonia, nitric acid, Urea, Ammonium Chloride, CAN, Ammonium Sulphate - Phosphorous Fertilizers: Phosphate rock, phosphoric acid, Super phosphate and Triple Super phosphate, MAP, DAP. Potassium Fertilizers: Potassium chloride and Potassium sulphate. TEXTBOOKS: 1. N.Shreve, "Chemical Process Industries", 5th Edn., McGraw Hill, New York, 1984. 2. Austine G.T. – Shreeves Chemicals Process Industries – 5th Ed. McGraw Hill 1984. 3. Dryden C.E., M. Gopala Rao – Outlines of Chemical Technology – 3rd Ed. Affiliated EastWest Press, New Delhi. 4. Pandey G.N. – Chemical Technology Volume – I – Lion Press, Kanpur. REFERENCES: 1. R.Gopal Rao and M.Sittig,"Dryden's Outlines of Chemical Technology", 3rd Edn.,Affiliated East-West Publishers,1997. 2. S.D. Shukla and G.N. Pandey, "Text book of Chemical Technology", Vol. I, 1977. Environmental Protection & Pollution Control. (CH-255) Unit I Introduction: Interaction of man and environment, overall picture of environmental pollution, environmental air and water quality criteria, standards and acts, effects of pollution, gaseous liquid and solid wastes and their disposal, noise pollution and its control. Unit II Air Pollution: Types of pollutants - natural and man made, dispersion of pollutant in the atmosphere, Gaussian dispersion model meteorological factors, stability and inversion of atmosphere, plume behavior, control of air pollution from stationary and mobile sources, classification, selection and introduction of equipments like cyclones, electrostatic precipitators, bag filters, wet scrubbers, settling chambers, etc. Methods of measuring and sampling of gaseous and particulate pollutants in ambient air and industrial waste gases, measurement of smoke density and visibility. Unit III Water Pollution: Waste liquid characteristics - physical, chemical composition, biochemical oxygen demand (BOD), pathogenic bacteria and chemical toxicity, types of pollutants in liquid wastes of chemical industries, methods for the treatment of liquid 16 wastes to control pollution, classification viz. physical, chemical and biological methods, selection of equipment like hydrocyclone, settling tanks, filters, ion exchange etc, Methods of sampling, preservation of samples and analysis. Unit IV Solid Waste Disposal: Characterization of solid wastes, problems of collection and handling, various processing techniques used in solid waste management such as compaction incineration, composting landfills and biological processing solid waste as resource material Unit V. NOISE POLLUTION AND ODOUR CONTROL: Sources of noise pollution - noise control criteria, noise exposure index. Control of noise pollution. Odour and its control. TEXTBOOKS: 1. M. N. Rao and H. V .N. Rao, "Air pollution", Tata McGraw Hill, New Delhi. 2. C. S .Rao , "Environment Pollution Control and Environmental Engg.", Tata McGraw Hill, New Delhi. 5. Metcalf & Eddy, “Wastewater Engineering Treatment and Reuse” Tata McGraw Hill Edition. 6. Peavy and Row, “Environmental Engineering” 7. Buckingham, “Hazardous waste” REFERENCE: 1. A.C. Stern," Air Pollution - Engineering Control of Air Pollution", Vol IV, 3rd Edn., 1977. 2. J. O .M. Bockris, "Environmental Chemistry", 1977. INDUSTRIAL POLLUTION ABATEMENT LAB (CH-291) List of Experiments: 1. To determine the dissolved oxygen content of the given sample by Winkler method. 2. To determine the biochemical oxygen demand of the given wastewater sample. 3. To determine the chemical oxygen demand of the given wastewater sample. 4. To study the BOD kinetics of the given wastewater sample and to determine the kinetic constant. 5. To determine the fluoride content in the given water sample. 6. To determine the total suspended solids & dissolved solid in the water sample. 7. To determine bulk density of the soil sample. 8. Determination of sulphate content in wastewater. 9. Estimation of Ammonical Nitrogen of wastewater sample. 10. To Study the Water/Wastewater Analyzing kit. 11. Preparation of laundry soap and to determine its yield. 12. To Study the atomic absorption spectrophotometer. 13. Adsorption study CHEMICAL TECHNOLOGY LAB (CH-292) 1. To determine the viscosity of a viscous liquid by falling sphere method. 2. Determination of saponification value of oil sample 3. Application of pH meter to find acidity and alkalinity of a solution. 17 4. To Study the Colorimeter. 5. To Study the Ion-Analyzer. 6. To study the hydrolysis of cane sugar solution in the presence of an acid by Fehling‟s solution method and find out the reaction constant. 7. To Study the adsorption of benzoic acid on animal charcoal and room temperature and to determine the Freundlich constants k,n. 8. Determination of the strength of unknown hydrochloric acid by titrating it against caustic soda by conducto-metric method. 9. To determine the % composition of a given binary liquid solution by polarimeter. 10. To determine the solubility of a sparingly soluble salt in water by conductance measurement. 11. Determination of pH of mixture of CH3COOH and CH3COONa and the dissociation constant of the acid. 12. Preparation of laundry soap and to determine its yield. 13. To study the Gas Chromatograph. Semester – V INDUSTRIAL PROJECT MANAGEMENT (HUM 301) UNIT–I Fundamentals of Management – I: Management: Evolution, development, characteristics, principles, philosophy, Nature and function, (MBO), (MBE) their importance characteristics and applications. UNIT–II Fundamentals of Management – II: Organizational Behaviour, Human behaviour, group dynamics. Leadership theories, styles and modern philosophies, motivation approaches and theories, communication, barriers and breakdowns, management information system, use of Computer in Management. UNIT–III Introduction to Personnel Management: Employees, Personnel Management practices, methods, recruitment, selection, interviews, group discussions, training, placement and employees development, wages and incentives, labour welfare, conflict, Negotiations, best practices. UNIT–IV Introduction to Marketing and Sales Management: Marketing concept, principles, functions, market survey and research, concepts of sales and distribution, channels of distribution, salesmanship, sales promotions, methods of advertising, copy right, sales management practices. UNIT–V 18 Introduction to Financial Management: Nature and scope of Financial Management, goals of financial management, Sources of finance, Permanent long term, Short term Sources, Interest rates, annuity cost of capital, capital structure, decisions, Break-even Analysis, Financial Planning. Suggested Text books and references: 1. Management Stonier & Freeman 2. Principle of Marketing Philip Kotler 3. Industrial Management K.K. Ahuja 4. Financial Management S.K. Banerjee Chemical Reaction Engineering – I (CH-301) Unit-I Classification of reaction, Defintion of reaction rate, Variables affecting the rate, concept of reaction equilibria, order of reaction and its determination, theoretical study of reaction rates, collision and activated complex theory, Mechanism of reaction series, Parallel and consecutive reaction autocatalytic reactions, chain reaction polymerization reaction. Interpretation of kinetic data, Integral and differential method of analysis, variable volume reactions, total pressure method of kinetic analysis. Unit-II Classification of Reactors: Concept of ideality, Development of design equations for batch, semi batch, tubular and stirred tank reactor, Design of Isothermal and non-isothermal batch, CSTR, PFR, reactors. Combination of reactors, Reactors with recycle, yield and selectivity in multiple reactions. Unit-III Multiple Reactions in Batch, continuous stirred tank and plug flow reactors uniqueness of steady state in continuous stirred tank reactor, optimum temperature progression, thermal characteristics of reactors. Unit-IV Non-ideal reaction, RTD dispersion model, Tank and series model, recycle model, segregated flow in mixed models, evaluation of RTD characteristics. Suggested Readings: nd 1. O. Levenspiel – Chemical Reaction Engineering – 2 Ed. Willey Eastern, Singapore. 2. Houghen Watson & Ragatz – Chemical Process Principles Part – III – (Kinetics & Catalysis) 2nd Ed. Asian Publishing House Bombay. nd 3. Fogler, H.S. – Elements of Chemical Reaction Engineering – 2 Ed. Prentice Hall of India Pvt. Ltd. New Delhi – 1999. rd 4. J.M. Smith – Chemical Engineering Kinetics – 3 Ed. McGraw Hill. nd 5. K.G. Denbigh & K.G. Turner – Chemical Reaction Theory an Introduction – 2 Ed. United Press and ELBS 1972. 6. G. Copper & GVJ Jeffery‟s – Chemical Kinetics and Reactor Engineering – Prentice Hall 1972 19 Mass Transfer – I (CH-302) Unit-I Diffusion Phenomenon: Molecular and eddy diffusion in gases, liquids and solids, interface mass transfer, Mass transfer theories: film theory, Penetration theory and surface renewal theory. Unit-II Concepts of Mass Transfer Coefficient: Individual and film coefficients, overall mass transfer co-efficient and their inter relationships. Continuous contact and differential contact, mass transfer concepts of NTU and HTU, their inter relationship for dilute phase and concentrated phase contact absorption, extraction and distillation. Unit-III Humidification and Drying: Humidification: General Theory, psychometric chart, fundamental concepts in humidification & dehumidification, wet bulb temperature, adiabatic saturation temperature, measurement of humidification calculation of humidification operation, cooling towers and related equipments. Drying : Equilibrium mechanism theory of drying, drying rate curve. Batch and continuous drying for tray driers, Drum dryers, spray and tunnel dryers. Unit-IV Leaching and Extraction: Leaching: solid liquid equilibrium, Equipment, principles of leaching, concurrent and counter current systems and calculation of number of stage required. Unit-V Liquid-Liquid extraction: Liquid equilibrium & Ponchon – Savarit method, packed & spray column, conjugate curve and tie line data, plait point, ternary liquid-liquid extraction, operation and design of extraction towers analytical & graphical solution of single and multistage operation in extraction, Co-current, counter current and parallel current system. Suggested Readings: 1. McCabe, W.L. Smith J.M. – Unit Operation in Chemical Engg. – 5th edition Tat McGraw Hill-Hogakucha, Tokyo, New Delhi. 2. Coulson J.M. Richardson J.F. – Chemical Engg. – Vil-2 Edition-2, Butserworth Heinmann, Oxford, New Delhi. 3. Treybal R.E. – Mass Transfer Operation- 3rd edition, McGraw Hill ok Co. New York. Heat Transfer -I (CH-303) Unit I Introduction to heat transfer and general concepts of heat transfer. by conduction, convection and radiation. Mechanism of Heat Transfer, Heat Transfer flux and resistance. heat rate equations, 20 Unit II Conduction: Basic law of heat conduction – Fourier‟s law, thermal conductivity, its dependence on temperature, steady state heat conduction through a composite solid and its electric analogue, steady state heat conduction through cylinders, spheres and variable area of solids, different insulating materials and their applications for process equipment and pipelines, Fourier‟s law in three dimensions, lumped capacity method of unsteady state conduction. Unit III Convection: Convection heat transfer and the concept of heat transfer coefficient, individual and overall heat transfer coefficient, heat transfer between fluids separated by plane wall, heat transfer between fluids separated by cylindrical wall (pipes), critical/ optimum insulation thickness, heat transfer through extended surfaces. Forced Convection: Over a flat plate, thermal boundary layer, dimensionless groups and dimensional analysis, Buckingham Pi-theorem, heat transfer correlations- internal and external flows, laminar and turbulent flows. Free convection: Heat transfer correlations for free convection, free convection from flat surfaces, free convection from a cylinder. Unit IV Radiation: Basic principle of radiation from a surface, blackbody radiation, Planck‟s law, Wein‟s displacement law, the Stefan Boltzmann law, Kirchoff‟s law, gray body, radiation exchange between black bodies & gray bodies. Unit V Heat Transfer with phase change: Boiling phenomena and analysis of boiling curve, correlation for nucleate boiling, critical heat flux, condensation phenomena, film condensation on a vertical surface (Nusselt equation, effect of non-condensable gases, drop wise condensation. Evaporation: Types of evaporators, single and multiple effect evaporators, capacity and economy, boiling point elevation. Suggested Readings: 1. Donald Q. Kern – Process Heat Transfer – Tata McGraw Hill. 2. Alan J. Chapman – Heat Transfer – IV Ed. – Collier Mcmillan. 3. Heat Transfer by Y.V.C. Rao. 4. J.P. Hallman, “Heat Transfer”, 9th Ed., McGraw Hill. 2001 5. Vinay K. Dutta – PHI (Heat Transfer). 21 PROCESS DYNAMICS & CONTROL (CH 304) Unit-I First Order Systems: Linear open loop systems - First order and Linearised first order systems - Response to various disturbances. Unit-II Higher Order Systems: First order in series - Higher order systems - Response to various disturbances. Unit-III Block Diagram: Controls - Block Diagram - closed loop transfer function - Transient response - Simple alarm Modes of control and controller characteristics. Unit-IV Stability Analysis: Stability - Routh analysis - Frequency response - Control system design - Controller tuning. Unit-V Special Controls: Cascade - feed forward and ratio control - dead time compensation - Internal Model Control - Control valves - Process identification. TEXT BOOKS: 1. D. P. Coughnowr & Koppel, "Process Systems Analysis and Control", McGraw Hill, New York, 1991. 2. C. A. Smith and A. B. Corripio, "Principles and Practice of Automatic Process Control", Wiley, New York, 1989. 3. Stephnopalis PHI REFERENCES: 1. P. Harriot, "Process Control" , Tata McGraw Hill, New Delhi, 1984. 2. D.P. Eckman, "Industrial Instrumentation", Wiley Eastern Ltd., New York 1990. BIOCHEMICAL ENGINEERING (CH 305) Unit-I Introduction To Bioscience: Types of Microorganisms: Structure and function of microbial cells. Fundamentals of microbial growth , batch and continuous culture. Isolation and purification of Enzymes from cells. Assay of Enzymes. Unit-II Functioning Of Cells And Fundamental Molecular Biology: Metabolism and bio-energetics, Photosynthesis, carbon metabolism, EMP pathway, tricarbocyclic cycle and electron transport chain, aerobic and anaerobic metabolic pathways. Synthesis and regulation of biomolecules, fundamentals of microbial genetics, role of RNA and DNA . Unit-III Enzyme Technology And Kinetics: Applied Enzyme catalysis , Applications of enzymes in industry and medicine. Immobilization of enzymes. Kinetics of enzyme catalytic reactions involving isolated enzymes. Reversible inhibition. Unit-IV Reactions Catalysed By Enzymes, Reactors, Analysis: Reactor Design and Analysis for soluble enzyme systems. Cofactor regeneration . Membrane reactor . Effect of mass transfer in immobilised enzyme particle systems. Reactors for immobilised enzyme systems. Unit-V Bio Reactors , Effect Of Transport Processes: Introduction to Bioreactor design: Continuously Stirred aerated tank bioractors. Mixing power correlation .Determination of volumetric mass transfer rate of oxygen from air 22 bubbles and effect of mechanical mixing and aeration on oxygen transfer rate, heat transfer and power consumption. Multiphase bioreactors and their applications. Downstream processing and product recovery in bioprocesses . TEXT BOOKS: 1. J. E. Bailey and D. F. Ollis. " Biochemical Engineering Fundamentals", 2 nd Edn., McGraw Hill, New York , 1986. 2. Trevan, Boffey, Goulding and Stanbury," Biotechnology", Tata McGraw Hill Publishing Co., New Delhi, 1987. REFERENCE: M. L. Shuler and F. Kargi, “Bio Process Engineering: Basic concepts”, 2nd Edn., Prentice Hall of India, New Delhi, 2002. HEAT TRANSFER LAB (CH-341) List of experiments: 1. To determine the thermal conductivity of metal rod. 2. To determine the equivalent thermal conductivity of composite wall. 3. To determine heat transfer coefficient in force convection. 4. To determine heat transfer coefficient in Natural convection. 5. To determine heat transfer coefficient with the help of Steafan Boltzman Apparatus. 6. To calculate emmissivity of the test plate by emissivity measurement apparatus. 7. To determine heat transfer coefficient in double pipe heat exchanger. 8. To study the heat transfer characteristics of a shell and tube heat exchanger (heating/cooling) of water. 9. To determine heat transfer coefficient in parallel and counter flow heat exchanger. 10. To measure the rate of evaporation using an open pan evaporator. 11. To measure the rate of condensation of pure water vapour and to determine the heat transfer coefficient. 12. Demonstrate the film-wise drop-wise condensation and determination of the heat transfer coefficient. 13. To study the single effect evaporator and find out the transfer corfficient. MECHANICAL OPERATIONS LAB (CH-342) 1. Sphericity factor on friction losses. 2. Agitated vessel 3. Settling studies 4. Drag studies 5. Filtration (constant rate) 6. Filtration (constant pressure) 7. Screening 8. Elutriation Clarification, cyclone separator 9. Jaw crusher 10.Ballmill 11. Particle size distribution 12. Storage of Solids 13. Cyclone studies, Multiple cyclone, mixer – screw type ribbon type 23 Semester – VI Computational Methods in Chemical Engineering (CH-351) Unit-I Treatment of Engineering data – Graphical representation. Empirical equations, Interpolation, Newton‟s formula, Lagrange‟s Interpolation formula, extrapolation, Integration, graphical Integration, Graphical Construction of Integral curves, Numerical Integration. Unit-II Interpretation of Engineering Data – Significant figure, Classification of Measurements, Propagation of Errors, Variation and Distribution of Random Errors, Properties of Variance and Distribution of Random Errors, Properties of Variance, Confidence limits for small samples. Unit-III Ordinary Differential Equations – Formulation, Application of Law of Conservation of Mass – Mixing in flow process. Classification of ordinary Differential Equations and its applications to common Chemical Engineering problems. Unit-IV Numerical Solutions of Ordinary Differential Equations-Linear Secondorder Equations with variable coefficients, Numerical solution by Runge – Kutta Method. Its application to higher – order equations. Unit-V Formulation of partial Differential Equations. Finite difference, linear finite difference equations, nonlinear difference equations. Optimization, types of methods, its application relating to chemical processes. Suggested Readings: 1. Mickley, H.S. Sherwood, T.S. Read – Applied Mathematics in Chemical Engineering – Tata McGraw Hill pub. 2. Jenson & Jeffrey‟s – Mathematical Methods in Chemical Engineering. 3. S.K. Gupta - Mathematical Methods in Chemical Engineering. MASS TRANSFER – II (CH 352) Unit-I Fundamentals of Mass Transfer : Analogies in transfer processes Determination of mass transfer co-efficient; two phase flow in packed beds, co-current and counter current processes loading flooding, column internals: types of trays/plates and packing, point and plate efficiency. Unit-II Distillation: Vapour liquid Equilibria, Me-Cave Thefe Method Boiling point diagram, Relative volatility, flash and differential distillation for two component mixture, steam distillation, azeotropic distillation, extractive distillation. Unit-III Continuous and Differential contact Distillation: Rectification, reflux ratio, calculation of numbers of plates by NTU, optimum reflux ratio, open steam, multiple feed and multiple product calculations, Enthalpy 24 concentration diagram, Panchon-Savarit method for calculation of number of theoretical plates. Approximate equation; Fensky and undeinrood equation for minimum numbers of plate calculation, Polarison Oililand method for actual numbers of plate calculation, Batch distillation. Unit-IV Absorption: Absorption and Extraction in continous contact columns, cocurrent and cross current contacting fluids, calculations of NTU and HTU, concept of HETP. Unit-V Adsorption: Adsorption theories, types of adsorbent; activated carbon, silica and molecular sieves. Batch and column adsorption. Break through curves, Liquid percolation and gas adsorption, BDST models for adsorption calculation. Suggested Readings: rd 1. Treybal R.E. – Mass Transfer operation – 3 Ed. McGraw Hill. rd 2. McCabe W.L. Smith J.M.- Unit operation in Chemical Engineering – 3 Ed. Tat McGraw Hill. nd 3. Coulson J.M. Richardson J.F. – Chemical Engineering – Vol 2 2 Ed. rd 4. Sherwood, T.K. Pigford R.L. and Wllke, C.R. – Mass Transfer- 3 Ed. McGraw Hill. PROCESS EQUIPMENT DESIGN –I (CH-353) Unit-I Mechanics of materials : Stress – Strain relationships of elastic materials subjected to tensile, compressive and shear forces, Elastic and plastic deformation, Bending moment and bending stress, Torsion, creep and fatigue, theories of column; Thermal stress, membrane stresses in stresses of revolutions, stress concentrations, Theories of failures. Unit-II General design considerations; Design codes; Design pressure; Design temperature; Design stress; materials; welded joint efficiencies; corrosion allowances; Design loads, liquid storage tank codes, classification, design of shell, bottom plates, self supported, and column supported roofs, wind girder, nozzles and other accessories. Unit-III Unfired pressure vessel: Pressure vessel codes, classification of pressure vessels, Design of cylindrical and spherical shell under internal and external pressures; Selection and design of flat plate, torisperical, ellipsoidal, and conical clousures, compensations of openings. High pressure Vessels: Stress analysis of thick walled cylindrical shell, Design of monobloc and multiplayer vessels. Unit-IV Tall vertical & horizontal vessels: Pressure dead weight, wind, earthquake and eccentric loads and induced stresses; combined stresses, Shell design of skirt supported vessels. Vessel supports; Design of skirt, lug, and saddle supports. Unit-V Bolted Flanges: Types of Flanges, and selection, Gaskets, Design of non-standard 25 flanges, specifications of standard flanges. Fabrication of equipment major fabrication steps; welding, non destructive tests of welded joints, inspection and testing, vessel lining, materials used in fabrication of some selected chemical industries. Suggested Readings: 1. Brownell, N.E. and Young, H.E. – Process Equipment Design – John Wiley (1959) 2. Bhattacharya, B.C. – Introduction of Chemical Equipment Design – CBS Publishers, New Delhi. 3. I.S. : 2825-1969- Code for Unfired Pressure Vessels. 4. I.S.: 803-1962, Code For Practice For Design, Fabrication and Erection of Vertical And Mild Steel Cylindrical Welded oil storage Tanks. 5. Joshi, M.V. – Process Equipment Design. 6. Steel tables. 7. Data files as supplied by university. 8. Applied Process deign in Chemical and Petrochemical Plants, E.E. Ludwig – Gulf Publishing Co. 1964, Vol – I. Chemical Process Technology – II (CH 354) Unit I. NATURAL PRODUCTS PROCESSING: Production of pulp, paper and rayon. Manufacture of sugar, starch and starch derivatives. Gasification of coal and chemicals from coal. Unit II. INDUSTRIAL MICROBIAL PROCESSES AND EDIBLE OILS: Fermentation processes for the production of ethyl alcohol, citric acid and antibiotics. Refining of edible oils and fats, fatty acids. Soaps and detergents. Unit III. PETROLEUM REFINING AND PETROCHEMICAL PRECURSORS: Petroleum refining to produce naphtha, fuel hydrocarbons and lubricants. Processes for the production of petrochemicalprecursors: ethylene,propylene, butadiene, acetylene, synthetic gas, benzene, toluene and xylene. ( Cracking, Catalytic reforming and separation of products) Unit IV. POLYMER BASED INDUSTRIES AND THEIR CHARACTERISTICS: Plastics: Production of thermoplastic and thermosetting resins such as polyethylene, polypropylene, phenolic resins and epoxy resins; Polymers and their applications in engineering practice. Unit V. FIBRE FORMING AND ELASTOMERIC POLYMERS: Synthetic fibres: polyamides, polyesters and acrylics from monomers. Processes for the production of natural and synthetic rubbers. TEXTBOOKS: 1. G.T. Austin, " Shreve's Chemical Process Industries", 5th Edn., Mcgraw Hill Book Co., NewYork, 1984. 2. R. Gopal Rao and M. Sittig, " Dryden's Outline of Chemical Technology",3 rd Edn., Affiliated East-West Publishers, 1990. REFERENCE: 1. S.D.ShukIa and G.N. Pandey, "Text book of Chemical Technology", Vol. I, 1977. Heat Transfer – II (CH 355) Unit I 26 Review of convective heat transfer correlations and Heat Exchangers: Importance of heat exchangers in process industries, various types of heat exchange devices and their selection. Double pipe, shell and tube heat exchangers; Design and rating, baffles and their types, FT-correction factor, liquid-liquid, gas-liquid and gas-gas systems. Extended surfaces for heat transfer, concept of effectiveness and NTU of a heat exchanger. Unit II Boiling & Evaporator: Boiling characteristics. Nucleate pool boiling and forced convection boiling, boiling mechanism, boiling curve and heat transfer correlations, heat pipes. Classification and use of evaporators in process industries, effect of boiling point elevation and hydrostatic head on evaporator performance, estimation of surface area in multiple effect evaporator, evaporator calculations in process industries, fouling in evaporators. Unit III Condensation: Mechanism and types of condensation of vapour, Nusselt equation for film wise condensation on vertical surface and its extension to inclined and horizontal surfaces, condensation number, film condensation inside horizontal tubes. Unit IV Crystallization: Mechanism, crystallization from mixed solutes, crystallizer seed and particle size distribution, classification of crystallizers, enthalpy -concentration diagram, crystallizer-material and energy balance. TEXTBOOKS: 1. Holman J.P., “Heat Transfer”, 9th Ed., McGraw Hill. 2001 2. Kreith F. and Bohn M., “Principles of Heat Transfer”, 6th Ed., Brooks Cole. 2000 3. Hewitt G.F., Shires G.L. and Bott T.R., “Process Heat Transfer”, Begell House. 1994. 4. Incropera F.P. and Dewitt D.P., “Fundamentals of Heat and Mass Transfer”, 5th Ed., John Wiley. 2002 REACTION ENGG. LAB. (CH 391) List of Experiments: 1. To determine velocity rate constant of hydrolysis of ethyl acetate by sodium hydroxide. 2. To study the rate constant of hydrolysis of an ester-catalyzed by acid. 3. To study a consecutive reaction system (hydraulic model) 4. To study a parallel reaction system (hydraulic model) 5. To study a homogeneous reaction in a semi-batch reactor under isothermal conditions. 6. Study of non- catalytic homogeneous saponification reaction in CSTR. 7. To study a non-catalytic homogeneous reaction in a plug flow reactor. 8. To study the residence time distribution behavior of a back mix reactor. 9. To study the RTD behavior of a tubular reactor. 10. To study the RTD behavior of a packed bed reactor. Mass Transfer Lab. (CH 392) List of Experiments: 1. To Study the flooding and loading of packed columns using different types of packing. 2. To study different types of plates and packing. 3. To prepare the vapor-liquid equilibrium and Boiling point diagram for a binary liquid 27 mixture. 4. Determination of relative volatility of a given system of acetic acid water. 5. To verify Rayleigh equation for differential distillation of binary system. 6. To carry out the steam distillation. 7. To study batch distillation. 8. To study continuous distillation. 9. Studies on packed tower distillation unit. 10. Studies on the sieve plate distillation uint. 11. Studies on bubble cap distillation column. 12. To study the absorption of a gas in a packed column and calculation of NTU and HTU. 13. To perform batch adsorption and verify Freundlich law and Langmir isotherm. Minor Project & Seminar (CH 393) The Project work will involve experimental work, modeling and simulation. Semester – VII Transport Phenomenon (CH 401) Unit-I Momentum Transport: Viscosity and the mechanism of momentum transport, Newton‟s law of viscosity, pressure and temperature dependence of viscosity, theory of viscosity of gases at low density, theory of viscosity of liquids. Velocity Distributions in Laminar Flow: Shell momentum balances: boundary conditions, flow of a falling film, flow through a circular tube, flow through an annulus, adjacent flow of two immiscible fluids. The Equations of Change for Isothermal Systems: To equation of continuity, the equation of motion, the equation of mechanical energy. Unit-II Thermal Conductivity and the Mechanism of Energy Transport: Fourier‟s Law of heat conduction, temperature and pressure dependence of thermal conductivity in gases and liquids, theory of thermal conductivity of gases at low density, theory of thermal conductivity of liquids, thermal conductivity of solids. Temperature Distributions in solids and in Laminar Flow: Shell energy balances; boundary conditions, heat conduction with an electrical heat source, heat conduction with a chemical heat source, heat conduction through composite walls: Addition of Resistance, Forced Convection, Free Convection. The Equations of change for Nonisothermal systems: The equations of energy, the energy equation in curvilinear coordinates, the equations of motion for forced and free convection in nonisothermal flow, summary of the equations of change, use of equation of change to set up steady – state heat transfer problems. Unit-III Diffusivity and the Mechanism of Mass Transport: Definition of concentrations, velocities and mass fluxes, fick‟s law of diffusion, theory of ordinary diffusion in gases at low density, theory of ordinary diffusion in liquids. Concentration Distributions in Solid and in Laminar Flow: Shell mass balances: boundary 28 conditions, diffusion through a stagnant gas film, diffusion with heterogeneous chemical reaction, diffusion with homogeneous chemical reaction, diffusion into a falling liquid film l forced –convection mass transfer, Analogies between Heat, mass and momentum and transfers Suggested Reading 1. Bird R B, Stewart W E and Light fort R N, “Transport Phenomena”, John Wiley and Sons (2002). 2. Welty J R , Wilson R E and Wicks C E , “Fundamentals of Momentum , Heat and Mass Transfer”,4th ed,John Wiley and Sons (2001 ). 3. John C Slattery, “Momentum, Energy and Mass transfer in continua”, McGraw Hill, Co. (1972). PROCESS EQUIPMENT DESIGN – II (CH 402) Unit-I Scale up criteria and scale up of process equipment. Process design calculations for heat exchange equipment shell and tube heat exchangers general description, heat transfer coefficients and pressure drop by Kerm‟s Bells methods rating on existing unit. Unit-II Design of a new system having one or more units in series single effect evaporation, multiple effect evaporator with boiling point elevation. Unit-III Process design calculations for mass exchange equipment plate and packed column for distillation and adsorption including column diameter and height. Unit-IV Detailed process and mechanical design, flash drum, Kettle reboiler, condenser, cooling tower & rotary drier. Suggested Readings: th 1. Perry, Robert H., Green Don W 7 Ed- Perry‟s Chemical Engineering HandbookMcGraw Hill New Delhi. 2. E.E. Ludwig – Applied Process Design in Chemical Petrochemical Plants – Gulf Publishing Co. 1964 Vol. – 2. 3. B.D. Smith – Design of Equilibrium Stages. th 4. Coulson J.M. Richardson J.F. – Chemical Engineering Vol-6 Ed. Pergaman Process. CHEMICAL REACTION ENGINEERING – II (CH 403) Unit-I Heterogeneous processes: Catalysis and adsorption; Classification of catalysts, Preparation of catalysts. Promoters and Inhibitors, General mechanism of catalytic reactions surface area and pore size distribution Rate equation of fluid solid catalytic reactions, Hougen-Watson & law models, Procurement and analysis of kinetic data, kinetics of catalyst deactivation. Unit-II External transport processes and their effects on heterogeneous reactions yield and selectivity Reaction and diffusion in porous catalysts, isothermal and nonisothermal effectiveness factors, Effect of intraphase transport on yield, selectivity and 29 poisoning, Global reaction rate. Unit-III Design of catalytic reactors, Iso thermal & adiabatic fixed bed reactor staged adiabatic reactors, Non-Iso thermal non-adiabatic fixed bed reactors, Fluidized bed reactors, Slurry reactors, Trickle bed reactors. Unit-IV Models for fluid-solid non-catalytic reactions, controlling mechanisms, Diffusion through gas film controls. Diffusion through ash layer controls, Chemical reaction controls, fluidized bed reactors with and without elutriation. Unit-V Gas-liquid reactions and liquid-liquid reaction, Rate equation based on film theory, Reaction design for instantaneous reactions ad slow reactions, Aerobic Fermentation, Application to Design Tools for Fast Reactions. Suggested Readings: rd 1. J.M. Smith-Chemical Engineering Kinetics – 3 McGraw Hill nd 2. O. Levenspiel – Chemical Reaction Engineering – 2 Ed. Wiley Eastern, Singapore. nd 3. Fogler, H.S. : Elements of Chemical Reaction Engineering 2 Ed. Prentice Hall of India Pvt. Ltd. New Delhi – 1997. nd 4. K.G. Denbig & K.G. Turner – Chemical Theory – An Introduction to Re-Actors – 2 Ed. United Press & ELBS 1972 5. G. Cooper & G.V.J. Jefferys Chemical Kinetics and Reactor Engineering Prentice Hall 1972. 6. Hougen, Watson & Ragatz, Chemical Process Principles Part 3 (Kinetics & Cataysis) 2nd Ed. 7. Bio-Process Technology (CH-411) Unit-I Introduction to Bio-Chemical Engineering: Aspects of microbiology, cell theory structure of microbial cells, classification of microorganism, Essential chemicals of life lipids, Sugars and Polysaccharides, RNA and DNA, Amino acids and proteins. Unit-II Metabolism and Energetic: Assimilatory and dissimilatory process, metabolic mechanism of the cells. Biochemical Kinetics: Simple enzyme reactions in heterogeneous systems. Unit-III Growth cycle, phases for Batch cultivation, mathematical modeling of batch growth, products synthesis Kinetics, overall kinetics and thermal death kinetics of cells and spores. Unit-IV Unit Operations in Biochemical Process: Agitation and aeration, gas liquid mass transfer, Determination of oxygen transfer rates, Determination of Kga and KLa scaling of mass transfer equipment. Heat balance 30 and „heat transfer correlation for biochemical systems, sterilization, filtration and drying. Unit-V Design and Analysis of Bio-Reactors Classification and characterization of different bioreactors. Batch and continuous reactors, tubular, CSTR and tower reactors. Aerobic and anaerobic fermentation – process, design and operation of typical aerobic and anaerobic fermentation processes, Manufacture of microbial products e.g. antibiotics alcohol/wine etc. Use of immobilized enzyme and whole cells for industrial processes. List of Experiments: 1. To carry out isolation and identification of microorganism from a soil sample. 2. To examine and study the effectiveness of various techniques used for the preservation of microorganisms. 3. To study the kinetics of ethanol fermentation. 4. To determine the kinetic constants 1.1 max and km for the growth of microorganisms. 5. To identify bacterial species using Gram staining tests. 6. To determine the reducing sugar in the given fermentation medium. 7. To determine the protein in the given fermentation medium. 8. To determine the total sugar content in the given fermentation medium. 9. To study the kinetics of methane fermentation. 10. To study the kinetics of an enzyme catalyzed reaction. 11. To study the activity of enzymes in free and immobilized States. 12. To study the activity of whole cell enzymes in free and immobilized States. Suggested Readings: 1. Baily, J.E. and Ollis D.F. – Biochemical Engineering Fundamentals – II Ed. McGraw Hill (1986). 2. Coulson and Richardson – Chemical Engineering – Ed. III SURFACE COATING TECHNOLOGY (CH 412) Unit I History and development of paint industry, paint its definition, function and classification. Unit II Raw material for industry, drying oils, bodies oils natural and synthetic resins, pigments and extenders. Unit III Auxiliaries like driers, plasticizers, softeners, dispersing and flatting agents varnishes and lacquers, Unit IV formulation and manufacturing of paints, machinery used in paint manufactures, methods of application, Unit V Applications of industrial and architectural finishes. Common defects in paint and varnishes. 31 OIL TECHNOLOGY (CH 413) Unit I General survey of oils and oil based industries sources of oils and fats, their classification, General properties and utilization, composition of glycosides. Unit II Non-glyceride components of oils & fats, Fatty acids & waxes, Methods of introduction of oil fats, extraction solvents and extraction rendering. Unit III Refining and hydrogenation of oils, vanspati, margarine, Shorteming. Unit IV Soaps raw materials and methods of manufactures, introduction to synthetic detergents Unit V Fat splitting, fractionation of fatty acids and recovery of glycerin, Essential oils and cosmetics. ENERGY ENGG (CH 414) Unit 1 Energy Scenario Commercial & Non commercial energy, primary energy resources, commercial energy production, final energy consumption, energy need of growing economy, long term energy scenario, energy pricing, energy sector reform, energy & environment, energy conservation and its importance, re- structuring of the energy supply sector, energy strategy for future, energy conservation act. Unit II Energy Management & Energy Planing Definition & significance, energy strategy, energy policy & energy planning, two sides of energy management, sectors of supply side energy management, objective of energy management, hierarchical levels of supply side energy management, trade off b/w energy management, energy strategies & energy planning, energy & economy, essential imperatives & steps in supply side energy planning, energy planning flow for supply side, essential data for supply side energy planing, infrastructure planning, transportation of energy, per capita energy consumption, imperatives & steps in user side energy planning, energy management & control system for demand side, seven principal of energy management, energy policy of a supply organization & demand side organization, organization for energy management, training & human resource development, motivation. Unit III Energy Audit & Energy Monitoring, Targeting and Conservation Introduction, need, types & procedure of energy audits, modern techniques and instruments for energy audit. 32 Defining monitoring & targeting, element of monitoring & targeting, data & information analysis, techniques- energy consumption, production & cumulative sum of differences (CUSUM). Energy conservation opportunity, electrical & thermodynamic ECOs, ECOs in chemical process industries, waste management & recycling of discard material and energy. Unit IV Advancement In Technologies & Future Energy Alternatives Recent advancement in energy technology towards 21st century, transport of energy, ethanol as a fuel. Fusion – introduction potential, condition for fusion, magnetic confinement fusion reactor, cold fusion laser induced fusion. Biomass –introduction, municipal waste, biomass conversion, wood combustion Geothermal energy – introduction, origin, nature, resources and exploration, environment impact, low temperature geothermal resources. Unit V Case Studies Energy conservation in alcohol industry. Energy conservation in fertilizer industry and pulps & paper industry. Energy conservation in different units of refinery likes FCCU, HCU & ADU. Text Books 1. Hinrich & Kleinbach “Energy : its use and the environment” III ed. Harcourt. 2. Boyle “Renewable Energy : Power for a sustainable future” Oxford. 3. Rao S. & Parulckar B.B. ”Energy technology” khhanna publisher 4. Capenart & Turner “ Guide to energy management ” 6 ed. Keinnedu fairmant press. Environmental Impact Assessment& Audit (CH 415) Unit – I Study of Ecology and Ecosystem Ecology and Environment, Biosphere as an Ecosystem, Functions of an Ecosystem, Habitats of Biological Species, Ecological Succession, Food-chains and Food-webs, The Bio-geo-chemical Cycles of Elements and Minerals Unit – II Biodiversity and its Conservation Introduction to Biodiversity, Components of Biodiversity, Importance of Biodiversity, Threats to Biodiversity, Factors causing Loss of iodiversity, Endangered and Endemic Fauna and Flora of India, In situ and Ex situ Techniques for Conservation of Biodiversity Unit – III Environment and Human Population 33 Global Population, Population Growth and Population Explosions, Environment and Human Health, Environment and Human Rights, Value based Environmental Education, Environmental Movements Unit – IV Environment Impact Assessment (EIA) Concept of EIA, Origin of EIA, Procedure of EIA, Evaluation Methodology for EIA, Scope Studies, Preparation and Review of Environment Impact Statement (EIS), Introduction of Life Cycle Assessment, Environmental Parameters in LCA, Concept of Environmental Audit, Necessity and Importance of EA, Audit procedures Unit – V Environmental Management System (EMS) Introduction, Terminology and Certification, Environmental Standards, the International Standard Organization (ISO), the ISO 9000 and the ISO 14000 Family of Standards, Guides and Technical Reports, ISO 14001 Certification as a Tool for Sustainable Development. Books Recommended 1. Anjaneyulu Y., “Environment Impact Assessment Methodologies”, B S Publications (2002). 2. Canter L. W., Environment Impact Assessment”, McGraw Hill, Second Edition (2005). 3. Garg S. K., Garg R. and Garg R., “Ecological and Environmental Studies”, Khanna Publishers, First Edition (2006). 4. Santra S. C., “Environmental Science”, New Central Book Agency (P) Ltd., Second Edition (2006). 5. Uberoi N. K., “Environmental Management”, Excel Books, Second Edition (2006). Petroleum & Petrochemical Engg. (CH 421) Unit-I Primary Processing Of Crude Oil: Classification of crude oil, Atmospheric distillation, Vacuum distillation of residueProducts and distillation practice. Unit-II Secondary Processing Of Crude Oil: FCCU, Hydro cracking, Visbreaking, Thermal cracking, Coking, Reforming, Alkylation, Polymerisation and Isomerisation process. Unit-III Treatment Techniques: Treatment techniques for removal of objectionable gases, Odours, to improve performance, Storage stability, Extraction of aromatics, Olefins and recovery operations from petroleum products. Unit-IV Petrochemicals: Chemicals from methane and synthetic gas: Ammonia, Methanol and Hydrogen Cyanide, Chemicals from olefins: Ethylene derivatives, Propylene derivatives and Butylenes derivatives, Aromatics, intermediates for synthetic fibres, Plastics and rubber. Unit-V Environmental And Safety Aspects In Refinery And Petrochemicals: 34 Waste water and effluent gases treatment from alkylation units and petrochemical units, safety aspects in the above industries. TEXT BOOKS: 1. W.L. Nelson, “Petroleum Refinery Engineering”, 4th Edn., McGraw Hill, New York,1985 2. B. K. Bhaskara Rao, "Modern Petroleum Refining Processes", 2nd Edn., Oxford and IBH Publishing Company, New Delhi, 1990. REFERENCES : 1. G. D. Hobson and W. Pohl., " Modern Petroleum Technology", Gulf Publishers, 2 nd Edn., 1990. st 2. R. A. Meyers, "Hand book of Petroleum Refining Processes”, McGraw Hill, 1 Edn., 1980. FERTILISER TECHNOLOGY (CH 422) Unit-I Introduction: Macro- and micro-nutrients, fertilizer grades, development of fertilizer industry, different types of fertilizers, their demand and production in India. Fuel stock availability and energy consumption pattern in fertilizer industry. Unit-II Nitrogenous Fertilizers: Various feed stocks, merits/demerits, synthesis gas production by steam reforming and partial oxidation, purification methods; design considerations and developments in primary reformer, shift converters, CO 2 removal and final gas purification. Unit-III Ammonia Synthesis: Different types of reactors, their design consideration, operation and comparison of various processes. Unit-IV Urea and other Nitrogenous Fertilisers: Physico-chemical considerations, various processes and plant practices for industries: urea, calcium ammonium nitrate, ammonium sulphate. Unit-V Phosphatic Fertilizers: Raw material and limitation in their use, uncertainties in their availability and their impact on the existing plants and future planning, normal and triple super-phosphates, phosphoric acid, processes of manufacture and their limitations, design considerations and developments. Books Recommended 1. Dryden C E, “Outlines of Chemical Technology”, East –West Press Pvt. Ltd., New Delhi, 2nd Edition (1973 ) 2. Austin G T, “Shreve‟s Chemical Process Industries”, McGraw Hill Book Company, New Delhi 5th Edition (1986 ) 3. Chemical Engineering Education Development Centre– “Chemical Technology I, II, III , IV , Manual of Chemical Technology, Indian Institute of Technology , Madras”. 4. Shukla S D and Pandey G N, “A text book of Chemical Technology Vol I”, Vikas Publishing House Pvt. Ltd., New Delhi. 5. Shukla S D and Pandey G N, “A text book of Chemical Technology Vol lI”, Vikas Publishing House Pvt. Ltd., New Delhi Industrial Pollution Control (CH 423) 35 Stream sanitation. Different equations of self-purification, River standards, Effluent standards, Minimal national standards (MINAS). Sources and effects of various pollutants, Disposal of industrial wastes-on land, in creeks and the sea, in inland streams, into impoundments. Importance of planning location of industries and industrial estates, Common effluent treatment plants, their economics and management. Detailed considerations of wastes from industries such as textile (Cotton, wool, rayon, synthetics), sugar, pulp and paper, distilleries, oil refineries, petrochemicals, pharmaceuticals, dairy, food processing, soaps and detergents, mining, iron and steel, pickling, plating, galvanizing, tanning slaughterhouse, fertilizers, pesticides, dyes and dye intermediates, radioactive wastes. Recovery of hyproducts, reuse of wastewaters with or without treatment. PHARMACEUTICAL TECHNOLOGY (CH 424) Unit-I Practice of the following unit operation in pharmaceutical industries: Heat transfer, evaporation, distillation, dry, mixing size reduction, crystallization, filtration, size separation, conveying, humidification, air conditioning and refrigeration. Unit-II Formulation, development of sterile dosage forms. Production facilities, environmental control and personnel in the production of sterile dosage form, compounding, processing, filtration, sealing, sterilization, packing and labeling of sterile dosage forms. Quality control tests like sterility, pyrogen, clarify, safety and leakage testing. Unit-III Types of tablets. Manufacturing of tablets by wet granulation, dry granulation and direct compression. Tablet processing problems and defects, tablet standardization: hardness, friability, weights variation, disintegration, dissolution and content uniformity tests. Unit-IV Capsules: Hard gelatin capsule, capsule size, formulation and preparation of filled hard gelatin capsules, soft gelatin capsule, soft gel – manufacturing procedures. Quality control of capsule. Unit-V Cosmetics and Toiletries: Introduction, factors to be considered in the formulation of facial cosmetics, dentifrice‟s, deodorant, antiperspirants, shampoos, hairdressing and hair removers. Unit-VI Pharmaceutical packing: Packing components, types of packing containers and closures, materials used for and their pharmaceutical specification, method of evaluation, stability aspects of packaging materials. Suggested Readings: 1. Leon Lachman, H.A. Lieberman, J.L.K. – The Theory and Practice of Industrial Pharmacy – Verghese Publishing House, Hind Rajasthan Building Dadar, Mumbai – 36 40001. 2. Ganderton – Unit Process in Pharmacy. 3. D. Hershey, Ed – Chemical Engineering in Medicine And Bodogy – Plenum Press, New York. 4. Chemical Engineering in Medicine – Chern. Engg. Prpgrer Syrnp Series No. c 66, Vol 62. Corrosion Engg. (CH 425) Basic concepts: Definition and importance; Electrochemical nature and forms of corrosion; Corrosion rate and its determination. Electrochemical thermodynamics and kinetics: Electrode potentials; Potential-pH (Pourbiax) diagrams; Reference electrodes and experimental measurements; Faraday‟s laws; Electrochemical polarization; Mixed potential theory; Experimental polarization curves; Instrumentation and experimental procedure. Galvanic and concentration cell corrosion: Basic concepts; Experimental measurements, and determination of rates of galvanic corrosion; Concentration cells. Corrosion measurement through polarization techniques: Tafel extrapolation plots; Polarization resistance method; Instrumental methods and Errors in measurement of polarization resistance; Commercial corrosion probes; Other methods of determining polarization curves. Passivity: Basic concepts of passivity; Properties of passive films; Experimental measurement; Applications of Potentiostatic Anodic Polarization; Anodic protection. Pitting and crevice corrosion: Basic concepts; Mechanisms of pitting and crevice corrosion; Secondary forms of crevice corrosion; Localized pitting. Metallurgical features and corrosion: Inter-granular corrosion; Weldment corrosion; Dealloying and dezincification. Environmental induced cracking: Stress corrosion cracking; Corrosion fatigue cracking; Hydrogen induced cracking; Some case studies; Methods of prevention and testing; Erosion, fretting and Wear. Environmental factors and corrosion: Corrosion in water and Aaqueous Ssolutions; Corrosion in sulphur bearing solutions; Microbiologically induced corrosion; Corrosion in soil; Corrosion of concrete; Corrosion in acidic and alkaline process streams. Atmospheric and elevated temperature corrosion: Atmospheric corrosion and its prevention; Oxidation at elevated temperatures; Alloying; Oxidising environments. Prevention and control of corrosion: Cathodic protection; Coatings and inhibitors; Material selection and design Books: 1. Fontana, M.G., “Corrosion Engineering”, McGraw-Hill. 37 2. Jones, D.A., “Principal and Protection of Corrosion”, Prentice-Hall INSTRUMENTATION AND PROCESS CONTROL LAB.(CH-441) List of Experiments: Each student should design a complete process plant with mechanical design details of at least three major equipments. Major Project and Seminar (CH –442) The student would be allotted a project in the beginning of the VII semester itself. The project will be based on the industry where he/she has undergone inplant training in industry during summer vacations. He/She would be expected to submit a detailed plant design report later in the (VIII) semester for the project course(CH-492). In this semester he/she will be assessed for the work that he/she does during the seventh semester under the supervision of a faculty of the department. Educational Tour and Training (CH-443) The students are required to undergo inplant training in some chemical industry for a six weeks period during their summer vacations following VI semester. He/ she is required to collect information‟s relating to process details and other information‟s related to process material, utilities and their properties to prepare a report to be submitted to the department. The student would be assessed in the VII semester through a Viva-voce to be conducted by the teacher incharge training of Chemical Engineering Department. Semester – VIII Process Modeling & Simulation. (CH 451) Unit-I The Role of Analysis: Chemical Engineering Problems, basic concepts analysis: The analysis process, A simple example of estimating an order. Source of the model equation: conservation equations, constitutive equation, control volumes, Dimensional analysis, System of units, Dimensional consistency in mathematical descriptions, Dimensional analysis and constitute relationships, Final observations. Unit-II Non-reacting Liquid Systems: Introduction, equation of continuity, simple mass balance, application of the model equations, component mass balances. Model behavior : Steady state behavior, Unsteady state behavior, density assumption, Numerical intercal integration methods of ordinary differential equation. Reacting liquid systems: Introduction, basic model equations for a Tank-Type reactor, The reaction rate, The batch reactor, pseudo First-order reactions, Reversible reactions, multiple reactions: consecutive reactions, parallel reactions, complex reactions, 38 constant density assumption, order and stoichiometry. Unit-III Treatment of Experimental Data: Introduction, criteria for Best Slope-I, Best Slope-II, Best straight line, Physical property correlations, Fitting a quadratic. Simulation examples of gravity fluid flow, heat and mass transfer, monte-carto simulation. Unit-IV Dynamic modeling of simple processes, sequential, simultaneous modular and equation oriented approaches, partitioning and tearing. Unit-V Computer programming of various iterative convergence methods such as NewtonRaphson, False position, wegstein, Muller methods. List of Experiments: 1. Process dynamics experiments like flow of incompressible fluids at variable flow rate. 2. Dyynamics of a tank draining through an orifice in the bottom. Differential equation formulation and verification with the experimental data. 3. Mass balance in a tank filling at certain rate and emptying at another rate. Rectangular and wedge-shaped tank and incompressible fluid. nd 4. Modeling a batch reactor-verification of 151 and 2 order rate kinetics. 5. Counter current double pipe heat exchanger modeling-data analysis by iterative methods. 6. Simulation of a distillation column-binary systems, equimolal overflow, constant relative, volatility. 7. Input-Output response study in non-ideal flow reactors. 8. Simulation of perfectly mixed reactor with heat transfer. Derivation of a mathematical model and solving for study state heat transfer. Suggested Readings: 1. Russell T.W.F. – Introduction to Chemical Engineering Analysis – John Wiley & Sons New – York. 2. Luyben W.L. – Process Modelling, Simulation and control for Chemical Engineers – II Ed. McGraw Hill Publishing Co. New York – 1990. Process Engineering & Costing (CH 452) Unit-I System and subsystem in process engineering, System analysis, Economic degree of freedom various algorithms, Synthesis of processes, Flow sheeting, Mathematical representation of steady state flow sheet. Unit-II Equal time value of money, equivalence comparisons, discrete interest and continuous interest, development of its formula, comparison of alternative investment based on capitalized cost. Unit-III Economic Design Criteria Terms involved in profitability analysis, Gross 39 income, depreciation, taxes, net profit, rate of return, venture profit, payout time break even point. Unit-IV Time value of money, net present value and venture worth. Capital cost and manufacturing cost estimation methods, Economic analysis and evaluation. Sensitivity & risk analysis, simplifying scale-up cost estimation. Unit-V Analysis of R&D investment, Technological forecasting for the process industries, Interaction between design and cost equation for optimal design of equipments, Inflation. Energy conservation and environmental control. List of Experiments: 1. Industrial process costs, Fixed and working capital estimation for chemical industries like sulfuric acid, caustic soda, ammonia, urea, ethanol. Indirect and other. 2. Factorial method of cost estimation. Indirect costs, Lang factors, estimation of purchased equipment costs, characteristic size parameters, heat transfer area, vessel height, plate number, accessories. 3. Operating costs, labor – supervision, running testing laboratory, local taxes insurance, license fees and royalty payments. 4. Economic evaluation of projects, cash flow diagrams depreciation inflation and sensitivity analysis. 5. Computer methods for costing and project evaluation. Development of computer programs. Suggested Readings: 1. Peters, M.S. and Timmerhaus, K.D. – Plant Design and Economics for Chemical Engineers – Ed. McGraw – Hill. 2. Schwery H.E. – Process Engineering Economics – McGraw Hill (1955). Computer Aided Process Control. (CH 461) Hardware: Analog and digital interfacing, sensors and transducers. System software: real time programming, Application software: data logging, filtering, digital control: Ztransforms, discrete time dynamic systems, adaptive control, introduction to MIMO control systems. Laboratory exercises Environmental Pollution: Control, Design and Modeling. (Ch 462) Air pollution –Atmospheric pollutants: Photochemical smog in troposphere; 03 depletion in stratosphere; Aced Rain, Chemical equilibria , Aerosols : Atmospheric deposition, nucleation, Troposphere , Troposphere Energy Balance: Pressure and Temperature relationship; Stability criteria: Stack plume rise, Puff and Plume dispersion, Control of Pollutents: Absorption; Adsorption: Break through Analysis, Particles; Mechanism of Particles capture; Water Pollution – Organic/ inorganic/ Biological; Waste Water 40 Treatment; Aerobic and Anerobic digesters, Dissolved 02 model, Activated sludge process reactor design, Bio-tower reactor design. POLYMER TECHNOLOGY (CH 463) Unit-I Introduction: Concepts and classification of polymers Functionality , Glass transition temperature, Addition, condensation , step- growth and chain –growth polymerization Unit-II Molecular weight estimation: Average molecular weight – Number and weight average, Sedimentation and viscosity average molecular weights, Molecular weight and degree of polymerization, Significance of molecular weight. Polymerization Processes: Bulk, solution, emulsion and suspension polymerization, Comparison of polymerization processes. Unit-III Polymerization Kinetics: Chemistry of step reaction polymerization, Mechanism and kinetics of polycondensation reactions and free- radical chain polymerization. Unit-IV Synthetic Fibres: Types of Fibres, Spinning Techniques, Manufacturing Technology and Applications of different types of fibres: cellulosic fibres, polyamides, acrylics, vinyls and vinylidines, fluorocarbons. Unit-V Plastics: Manufacturing Technology and applications of different types of plastics: Polyester, polyethylene, Phenolics, Rubbers, structure, properties and preparation natural rubber synthetic rubbers: SBR, rubber compounding and reclaiming. Books Recommended 1. Gowariker V R , Viswanathan N V and Sreedhar J “Polymer Science” New Age International Publishers (1996) 2. Billmeyer F W “Text Book of Polymer Science” Wiley Tappers (1994) 3. Ghosh P, “Polymer Science and Technology of plastics and rubber” Tata McGraw Hill (2001). 4. Gupta R K and Anil Kumar, “ Fundamentals of Polymer Engineering”, 2nd Ed., Marcel Dekkar (2003) 5. Fried J R “Polymer Science and Technology” PHI Computational Fluid Dynamics (CH 464) Conservation equations for mass, momentum and energy; Comparison of various numerical techniques for CFD; Review of finite difference and finite element methods; Solution to discretised algebric equation; Finite-volume method for diffusion problems; Finite-volume method for convection and diffusion problems – pressure velocity coupling; Construction of geometry and discreation using Gambit-Fluent‟s manuals; Commercial CFD solvers; Turbulance modeling; Implementation of boundary conditions; Introduction to multiphase flow; Customizing commercial CFD solver; Unsteady state simulations. Suggested Readings: 1. Anderson, J.D., “Computational Fluid Dynamics: The Basics with Application” McGraw-Hill Co. Inc. 2. Anderson, D.A., Tannehill, J.C. and Pletcher, R.H., “Computational Fluid Mechanics and Heat Tranasfer”, Hemisphere Publishing Corporation. 41 3. Patankar, S.V., “Numerical Heat Transfer and Fluid Flow”, Hemisphere Publishing Corporation. 4. Ferziger, J.H. and Peric, M., “Computational Methods for Fluid Dynamics”, Springer. 5. Versteeg, H.K. and Malalasekera, W., “ An Introduction to Computational Fluid Dynamics: The Finite Volume Method”, Prentice-Hall Inc. Risk Analysis and Hazard (CH 465) Origin of process hazards, Laws Codes, Standards, Case Histories, Properties of Chemicals, Health hazards of industrial substances. Toxicology: Toxic materials and their properties, effect of dose and exposure time, relationship and predictive models for response, Threshold value and its definitions, material safety data sheets, industrial hygiene evaluation. Fire & explosion: Fire and explosion hazards, causes of fire and preventive methods. Flammability characteristics of chemical, fire and explosion hazard, rating of process plant. Propagation of fire and effect of environmental factors, ventilation, dispersion, purifying and sprinkling, safety and relief valves. Other Energy Hazards: Electrical hazards, noise hazard, radiation hazard in process operations, hazards communication to employees, plant management and maintenance to reduce energy hazards. Risk Analysis: Component and plant reliability, event probability and failure, plant reliability, risk analysis, HAZOP AND HAZAN, event and consequence analysis (vapour cloud modelling ) Designing for safety, measurement and calculation of risk analysis. Hazard Assessment: Failure distribution, failure data analysis, modeling for safety, safety training, emergency planning ad disaster management, case studies. Text/Reference Books 1. Crawl D.A. and Louvar J.A., Chemical process safety fundamentals with applications,” Prentice Hall of India, New Delhi. 2. Wentz, C.A., “Safety health and environmental protection,” McGraw Hill, 2001. 3. Smith, B.D., “Design of equilibrium state process,” McGraw Hill l. 4. Van Winkle, “Distillation,” McGraw Hill. Process Piping Design (CH 471) Unit-I Classification of pipes and tubes, IS & BS codes for pipes used in chemical process industries and utilities. Unit-II Pipes for Newtonian and non-Newtonian fluids, sudden expansion and contraction effects, Pipe surface roughness effects, pipe bends, Shearing characteristics. Unit-III Pressure drop for flow Newtonian and non-Newtonian fluids through pipes. Resistance to flow and pressure drop. Effect of Reynolds and apparent Reynolds number. 42 Unit-IV Pipes of circular and non-circular cross section – velocity distribution, average velocity and volumetric rate of flow. Flow through curved pipes (Variable cross sections). Effect of pipe-fittings on pressure losses. Unit-V Non-Newtonian fluid flow through process pipes, Shear stress, Shear rates behavior, apparent viscosity and its shear dependence, Power law index, Yield Stress in fluids, Time dependant behavior, Thixotropic and rheopetic behavior, mechanical analogues, velocity pressure relationships for fluids, line. Unit-VI Pipe line design and power losses in compressible fluid flow, Multiphase flow, gas-liquid, solid-fluid, flows in vertical and horizontal pipelines, Lockhart Martinelli relations, Flow pattern regimes. Suggested Readings. 1. Coulson JM and Richardson J.F. – Chemical Engineering – Vol I , VI Edition, Butterworth Heinemann, British Library, Publications, Oxford, 1999. 2. Govier, G.W. and Aziz K. – The flow of Complex Mixtures In Pipe – Krieger Publication, Florida, 1982. 3. Green DW and Malony, perrys – Chemical Engineers Handbook – VII Edition McGraw Hill, Bew York, 1997. Multiphase Flow (CH 472) Introduction to the flow of multiphase mixtures: gas or vapor liquid, liquid-liquid, liquidsolid, gas-solid, solid-liquid-gas and gases carrying solids (pneumatic transport) stratification and dispersion, Flow regimes and flow patterns. Gas (Vapor) and Liquid Flows: Horizontal flow, Vertical flow, pressure, momentum and energy relations, methods of evaluating pressure drop, Lockhard - Martinell, Chisholm correlations, critical flow, non-Newtonian flow. Solid-Gas Flow: Effect of pipeline diameter, inclination, bends, valves and length. Liquid and its physico-chemical properties, rheology, corrosive nature, viscosity, Solid particle size, distribution phase, and density i.e. their factors effecting behavior in a fluid, Concentration of particles and the flow rates of both solids and liquid. Solid-Gas Flow: Horizontal flow, Suspension mechanism, determination of voids, energy requirements for conveying, pressure drop and solid velocities in dilute phase flow, dense phase conveying, vertical transport. Bubble and drop formation: Phase holdups, Interfacial areas, mixing and pressure drops, multiphase (gas liquid solid) operations. Text/Reference Books 1. Govier, G.W. and Aziz, K., “THE FLOW OF COMPLEX MIXTURES IN PIPE,” Krieger Publication Florida, 1982. 2. Coulson JM and Richardson J.F., “CHEMICAL ENGINEERING,” Vol I, Butterworth-Heinmann, Oxford, 1999. 43 TEXTILE TECHNOLOGY (CH 473) Unit-I Classification of fibres: Natural fibres of vegetable origin: jute; hemp; sunn; Urena. The leaf fibres : Sisal, Abaca (manila); seed and fruit fibres; cotton. Natural fibres of animal origin: Wool; Mohair; Cashmere; Persion goat hatosilk; vicuna; fur fibres; Man made fibres; Rayon‟s Polyamide fibres; polyester fibres, polyvinyle derivative fibres; polyolefin & Polyurethane fibres. Unit-II Weaving : Various steps in weaving manufacturing for fibres, design and construction, and weaving fundamentals to the various modern methods of weaving slashing process calculations; woven fabric construction and weaving process calculation & problem solving. Unit-III Physical Testing of textiles : Introduction: Reasons for textile standardization of testing sampling, measurement errors; Effect of atmosphere on physical properties; Fibre tests; Fibre fitness; Fibre length; yarn tests; Linear density twist, yarn evenness; Hairness, friction, Strength tests; Definition; Load elongation curve. Unit-IV Recycling Textile Wastes: Recycling and recovery strategies turning environmental concern into real profit Re-claimed fibres, the sources and usage; Industrial wastewater minimization and treatment. The fibre industry and water management; Production of high tenacity tapes from polyprophyene. The role of process stabilizers in recycling polyoefins. Unit-V Modern Textiles: Challenges for Textile research & development in the 21 st century; fibres textiles and materials for future military use; Development in man made fibre technology-airbages, Textiles in filterations; Textiles in medicine, defence, transport and geotextiles. Suggested Readings: 1. J.Gardon Cook- Handbook of Tesxtile Fibres Vol-I Natural Fibres, Vol-II Man-made fibres. 2. S. Adanur – Handbook of waving – Deptt of Textile Engg. Auburn University, U.S.A. 3. Dan J. Mc. Geight, James B. Bradshow, Everett E. Back & Michael-Weavers Handbook of Textile Calculation – S. Hill Institute of Textile technology, USA. 4. B.P. Saville-Physical Testing of Textieles-Uni. Of Huddersfied, UK. 5. A.R. Horrocks-Recycling Texties and Plastic Wste. 6. Prasad Potluri – Tommorrow‟s Textiles 20012-Deptt. Of textiles, UMIST 7. A.R. Horrocks and Anad – Handbook of Technical Textiles- The Boston Institute UK. Novel Separation Techniques (CH 474) Unit-I Limitations of common separation techniques – sedimentation, screening, filtration, evaporation, distillation, absorption, liquid-liquid and soil0liquid extraction. 44 Unit-II Principles of membrane separation process classification, characterization and preparation of membrane, Analysis and modeling of membrane separation, Membrane modules and application. Unit-III Reverse Osmosis and ultra filtration, membrane characteristics and applications, lon selective membranes and their application in electrolysis. Per vaporization and gas separation using membranes, Liquid membrane, Industrial applications. Unit-IV Foam and bubble separation, principle, classification, foam and surfactants, Separation techniques, Column Separations: Unit-V Zone melting and Zone refining, electrophoresis, desalting by freezing, centrifugation. Unit-VI Parametric pumping, thermal parametric pumping, batch, continuous pumping, multi-component separation, pH-parametric pumping, heatless parametric pumping. Suggested Readings: 1. The McCabe WL and Smith JC-Unit Operation of Chemical Engineer-ING-V Edition, Tata McGraw – Hill, New York. 2. King J. – Separation Process – McGraw Hill. 3. Kaup EC – Design Factors In reverse osmosis – Chemical Engineering 80 (1973). 4. Arden TV – Water Purification By ION Exchange – Butterworth, London, 1968. 5. Industrial Catalysis (CH 475) Unit I Review of Heterogeneous Catalysis. Unit II Transport Processes: Analysis of external transport processes in heterogeneous reactions in fixed bed, fluidized bed and slurry reactors. Intrapellet mass transfer, heat transfer, mass transfer with chemical reaction and simultaneous mass and heat transfer with chemical reaction. Unit III Catalyst Selectivity: Effect of intrapellet diffusion on selectivities in complex reactions, effect of external mass transfer on selectivities. Unit IV Catalyst Deactivation: Modes of deactivation – poisoning, fouling and sintering. Determination of deactivation routes, combined effect of deactivation and diffusion on reaction rates, effect of deactivation on selectivity. Unit V Reactor Design: Design calculation for ideal catalytic reactor operating at isothermal, adiabatic and non-adiabatic conditions. Deviations from ideal reactor performance. Design of industria J fixed-bed, fluidized bed and slurry reactors. Thermal stability of packed bed and fluidized bed reactors. Text/Reference Books 1. Smith, J. M., “Chemical Engineering Kinetics,” 3rd ed., McGraw-Hill,1981. 2. Carberry, J. J., ”Catalytic Reaction Engineering,” McGraw-Hill,1977. 3. Lee, H. H., “Heterogeneous Catalytic Reactors,” Butterworth. 45 4. Tarhan, M. O., “Catalytic Reactor Design,” McGraw-Hill, NY, 1983. 5. Anderson, J. R. and Boudart, M., “Catalysis, Science and Technology,” Vol. 7, Springer Verlag,NY. 6. Thomas, J. M. and Thomas, W. J., “Introduction to the Principles of Heterogeneous Catalysis,” Academic Press, 1967. 46