Band Theory
advertisement

Semiconductors
Bonding in Solids: Metals, Insulators, and Semiconductors: Metals are malleable, meaning
they can be formed into useful shapes or foils. They are ductile, meaning they can be pulled into wires.
Metals are good conductors of electricity and heat.
Models of Metallic Bonding: Looking at metals in general and transition metals in particular,
there is little difference in electronegativity from element to element. So metals and alloys are not likely
to undergo ionic bonding. They are also a long way from having filled subshells, so they would require a
tremendous number of covalent bonds to achieve complete octets. The experience we’ve gained so far
suggests that individual metal atoms are not likely to form such large numbers of covalent bonds. If
neither ionic nor covalent bonding can be expected for a metal, which models can account for the fact
that metal atoms do bind to one another?
The first and simplest explanation of metallic bonding is often referred to as the sea of electrons
model. The essential feature of this model is that the valence electrons of metal atoms are delocalized and
move freely throughout the solid rather than being tied to any specific atom. This model accounts for
several observed properties of metals.
To understand malleability, consider what would happen at the atomic level if a metal were struck
with a hammer. At least some of the atoms would respond to this force by moving relative to others.
If the electrons are moving freely, however, as postulated in the sea of electrons model, they can adjust to
the new position of the atoms and bonding is relatively unaffected. Similarly, pulling a metal into a wire
changes the positions of the atoms, but delocalized electrons can adjust and maintain metallic bonding.
Finally, these delocalized electrons can move in response to any applied electrical field, so the
conductivity of metals can also be understood from the model.
The sea of electrons model provides qualitative understanding, but quantitative models for
metallic bonding also exist. The most important such model is band theory.
1
Use lithium for the simplicity of the s orbital overlap, not because this is the most practical choice.
Because the valence electron configuration of lithium is 1s2 2s1, any bonding interactions between the
metal atoms will have to be based on the 2s orbitals. Those orbitals, of course, are waves, and so we can
use the concept of wave interference to think about how they will interact with one another. We’ll start
with the simplest case: two lithium atoms placed close enough together for their 2s orbitals to overlap one
another and interact. Now recall that waves can interfere either constructively or destructively.
If the pair of 2s orbitals interacts in phase with one another, the interference will be constructive.
The resulting wave, which will have increased amplitude (or electron density) between the nuclei, is
referred to as a bonding molecular orbital. On the other hand, if the pair of 2s orbitals interacts out of
phase with one another, the resulting wave will have a node between the nuclei. This is known as an
antibonding molecular orbital. The term “antibonding” is used because electrons in this orbital will
actually weaken the bond between the atoms
Rather than being associated with either atom individually, these new molecular orbitals can be
thought of as belonging to the pair of lithium atoms together. Figure shows how the energies of these
molecular orbitals are related to that of the original 2s orbitals. Looking at Figure, we can think through
what would happen as more and more lithium atoms are loaded into them. With two atoms, there would
be one bonding and one antibonding orbital. With four lithium atoms, there would be two bonding and
two antibonding orbitals, as shown in the center panel of Figure. (With an odd number of atoms, one
nonbonding orbital would be formed, keeping the number of bonding and antibonding orbitals equal to
each other.)
As we proceed further to very large numbers of atoms, the number of molecular orbitals formed
becomes so large that there is virtually no energy difference from one orbital to the next. At this point,
the orbitals have merged into a band of allowed energy levels, and this is the origin of the term band
theory. Although our presentation here is entirely qualitative, it is important to note that a more thorough
development of these ideas leads to a quantitative model.
A three-dimensional bulk metal is slightly more complicated, and the way that the orbitals will
combine has some dependence on the structure of the solid. For metals with valence electrons beyond the
s subshell, the orbitals that ultimately form bands will include p or d orbitals. These will form additional
bands, and the energy of the resulting s bands, p bands, or d bands may overlap one another.
To understand properties such as conductivity, the band structure of the material provides a very
powerful model.
2
3
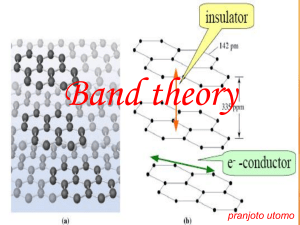
Band Theory and Conductivity
Conductivity: The band structure of a material plays much the same role as
atomic orbital energy levels
evels did for atoms
atoms. Just as the aufbau principle dictates that electrons occupy the
lowest energy orbitals, electrons in bulk materials fi
fill
ll the bands starting at the lowest energy. The highest
energy with electron occupation and the energy gap between this energy level and the next available band
b
provide a means for understanding the electrical conductivity of a material. Electrons carry a current by
moving through a material, and this motion can be thought of in terms of electrons moving from one
orbital to another. But electrons within a fi
filled
ed band cannot move readily to conduct electricity. To have
mobile electrons,
s, we will need to have an unfilled
unfi
band or portion of a band that is close in energy to a
filled band or portion of a band.
Let’s consider these ideas for the band diagram for lithium shown on thee right hand side of Fig
Fig.
The band populated by valence electrons is referred to as the valence band,, and the unoccupied band
above it is known as the conduction band.
band. The conduction band lies directly above the valence band in
energy,
rgy, and the highest occupied energy level sits at the boundary
boundary between these two bands. So an
electron sitting at the top of the valence band needs to gain only a very small amount of energy to move
into the conduction band. This means that lithium should be a good electrical conductor, which it is.
The energy below which an energy level is likely to be filled
lled is called the Fermi level. For a
metal, the Fermi level is at the top of the valence band.
Figure shows some general features of the bands for three different classes of materials: metals,
semiconductors, and insulators. For metals, we see the situation we just described in which addition of
even an infinitesimal amount of energy can promote an
an electron into the valence band. The nonmetal, on
the other hand, has a large energy gap between the filled valence band and the empty conduction band.
So it takes a significant amount of energy to reach that empty band, where electron movement would be
allowed. Under ordinary circumstances, electrons will not have enough energy to overcome this large
band gap, so nonmetals do not conduct and are referred to as insulators. The diamond form of carbon is
an excellent example of a nonconductive material.
The intermediate case is a semiconductor, shown in the center panel of the figure. The highest
filled energy level is at the top of the valence band, but the next band is only modestly higher in energy.
In semiconductors, some electrons have enough thermal energy to reach the upper band even at room
temperature, and therefore they have at least limited electrical conductivity. If the temperature is
increased, more electrons should occupy the upper band, and the conductivity of semiconductors
increases at higher temperatures. Silicon, from the same group as carbon, is the prototypical example of a
pure element that behaves as a semiconductor.
5
6
7
Semiconductors: Pure silicon provides an interesting textbook example of a semiconductor,
but it is not useful in most applications. To generate significant conductivity in pure silicon, somehow we
must promote electrons from the valence band into the conduction band.
This means that we must add energy, and the two most likely ways to do that are by exposing the
silicon to significant heat or by allowing it to absorb light with sufficient photon energy to excite the
electrons across the band gap. Neither of those would be easy to achieve or sustain in most electronic
devices. Looking elsewhere in the periodic table doesn’t really offer better options, though.
There are relatively few elements that can be classified as semiconductors, and none of them is suitable
for widespread use in its pure form. Instead, the semiconductor industry relies on the idea that by cleverly
combining elements, we can produce materials with the specific semiconducting properties desirable for
a particular application. One way to do this is through a process called doping in which carefully
controlled trace amounts of another element are incorporated into a semiconductor like silicon.
Doping a material to derive useful properties is not limited to semiconductors. Many alloys, including
specialty steels, are created by intentional doping.
To understand how doping works, let’s start with the band diagram of silicon shown on the lefthand side of Figure given below. Suppose that we wanted to increase the conductivity of pure silicon
slightly. From what we have learned about the conductivity of metals, we might conclude that to do this
we might put some electrons into the conduction band. So if we had some way to “inject” an extra
electron, it might go into the upper band. Then it could move around in that band. If we could add enough
electrons, then we might get a good conductor. How might we go about adding electrons to silicon? Each
silicon atom has four valence electrons. To increase the number of valence electrons, we might try adding
a small amount of an element with more than four valence electrons.
Suppose we took a sample of pure solid silicon and somehow replaced one silicon atom with
a phosphorus atom. Because we changed only one atom, we could assume that the overall structure of the
crystal and its band diagram shouldn’t be disrupted. But now we do have one extra electron. Because the
valence band is full, that additional electron must go into a higher energy orbital. Because this orbital is
associated with the phosphorus atom, rather than with silicon, it will have slightly lower energy than that
of the silicon conduction band.
We say that the presence of the phosphorus atom introduces a donor level that lies close to the
energy of the conduction band. Because the gap between this donor level and the conduction band is very
small, the electron from the donor level can be promoted into the conduction band fairly easily. So in this
thought experiment, adding one phosphorus atom into our silicon crystal will boost its conductivity ever
so slightly.
8
Figure❚ Imagine that we add electrons to pure silicon by incorporating a trace of phosphorus. The added electrons will
occupy a donor level just below the conduction band. This leads to an n-type semiconductor. The donor electrons do not
need to cross the band gap to be promoted into unfilled levels of the conduction band, so they boost conductivity. We can
control the number of these donor electrons by adjusting the extent of doping.
Figure❚ In a p-type semiconductor, the addition of electron-deficient dopant atoms provides an acceptor level just above
the top of the valence band. Electrons can be promoted into this level, leaving behind vacancies or “holes” in the valence
band.
It is not possible to carry out such a single atom replacement, of course. Even if it were,
the resulting change in conductivity would be immeasurably small. But the same idea will hold true if we
add more phosphorus atoms. The fifth valence electron from each phosphorus atom will go into a new
band similar to the donor level we introduced above. Then, the donor level electrons can be promoted
into the conduction band, leading to a band diagram like that shown on the right-hand side of Figure 8.12.
The donor level of the phosphorus atoms provides a source of electron density near the conduction band,
increasing conductivity. Moreover, the extent of that conductivity can be adjusted by controlling the
amount of phosphorus present. This makes what is known as an n-type semiconductor. The name comes
from the fact that we have added additional electrons, each of which has a negative charge. (Note that the
overall lattice remains neutral because each phosphorus atom also has an extra proton compared to the
silicon atom it replaces). Those negatively charged electrons allow the semiconductor to carry a current.
9
A typical n-type semiconductor might contain on the order of 0.00001% phosphorus added to a
silicon crystal. (Note that to be able to do this, the silicon itself must be incredibly pure.) We say that the
silicon is doped with phosphorus, and this doping usually takes place as the material is grown. As long as
the dopant atoms are randomly dispersed throughout the solid, the overall effect on the crystal structure
will be minimal. Other elements with more than four valence electrons could also be used.
But phosphorus is a convenient choice because it has an atomic radius close to that of silicon, so it won’t
disrupt the crystal lattice appreciably.
The second common category of doped semiconductors is called the p-type. The idea here is to
provide a way to decrease the number of electrons in the valence band, so that conductivity can be
achieved without needing to promote electrons across the band gap. The strategy is similar to what we
described for the n-type, except that now we want to use a dopant with fewer than four valence electrons.
This introduces an acceptor level that is slightly higher in energy than the top of the valence band.
The most common choice for the dopant in this case is aluminum, and Figure 8.13 shows how the
band diagram will change as the material is doped. Electrons from the valence band can easily be
promoted into the acceptor level, leaving behind vacancies or “holes” in the valence band. These holes
behave as if they had a positive charge, so electrical engineers refer to this as a p-type material.
The extent of doping controls the level of conductivity achieved, just as for the n-type.
For a macroscopic analogy of the importance of size, think of a pile of oranges at a grocery
store. If you were to replace an orange near the bottom and still maintain the pile, which would be
easier to use—a tangerine (about the same size) or a large grapefruit?
Although much less common than silicon devices, germanium-based semiconductors can also be
fabricated. Which kind of material (n- or p-type) would result if pure germanium were doped with
(a) gallium, (b) arsenic, or (c) phosphorus?
Strategy Like silicon, germanium has four valence electrons per atom. So a dopant with fewer
than four valence electrons will produce a p-type material, whereas a dopant with more than four valence
electrons will give an n-type material. We can use the periodic table to fi nd the number of valence
electrons in each element.
Solution (a) Gallium is in Group 13, so it has three valence electrons. Germanium doped with
gallium will be a p-type semiconductor. (b) Arsenic is in Group 15, so it has five valence electrons.
Germanium doped with arsenic will be an n-type semiconductor. (c) Phosphorus is also Group 15, so
germanium doped with phosphorus will be an n-type semiconductor, just as in (b).
10
Intrinsic [Ge or Si]
Hole formation in semiconductors
11
N-type Semiconductor [Ge or Si]
P-type Semiconductor [Ge or Si]
12
Conductivity in Intrinsic Semiconductors: Conduction takes place in an intrinsic semiconductor
due to the movement of holes and electrons. Electrons and holes being deflected by atoms and lattice
defects, take an irregular path through the conductor. The average rate at which they move is called the
drift velocity. The drift velocity per electric field represents the mobility of the charge carrier.
The conductivity of the semiconductor due to holes is given by . . where p represents the number of holes per unit volume of the semiconductor, µ represents the mobility
of the hole and e is the charge on the electron (1.6 x 10-19 Coulomb).
Similarly, the conductivity of the semiconductor due to electrons is given by . . where n represents the number of free electrons per unit volume of the semiconductor, µ represents the
mobility of the electron and e is the charge on the electron (1.6 x 10-19 Coulomb).
Therefore, the Total Conductivity of the semiconductor, . . . . . . .
The temperature dependence of the intrinsic concentration of electrons and holes in the
semiconductor is given by . where n is constant at a given temperature, E is
the energy gap, k is the Boltzmann constant and T is the temperature in Kelvin. The mobility of charge
carriers (electrons and holes) vary as !" over a temperature range of 100 to 400 K (For Si, m = 2.5 for
electrons and 2.7 for holes and for Ge, m = 1.66 for electrons and 2.33 for holes).
Conductivity in Extrinsic Semiconductors: (i) In n-type semiconductors, hole concentration (p)
is negligible and electron concentration (n) is far greater than the hole concentration. But the
concentration of the electrons (n) will be approximately equal to the concentration of donor atoms (ND).
But from the law of mass action (under thermal equilibrium), p x n remains constant and also
p x n remains independent of the amount of doping by Donor (or Acceptor) impurities.
# where ni represents the intrinsic concentration. Thus in an n-type semiconductor, the
concentration of holes is given by $%
The Total Conductivity of the semiconductor, . . But in n-type semiconductors, && ' or . && '. and $%
Therefore, . . . $% . Thus, the conductivity of n-type semiconductor is given by . $% . (ii) In p-type semiconductors, && , or . && ,. and also $- , the concentration of
the Acceptor atoms. Therefore, the conductivity of p-type semiconductor . / is given by . $- . 13
Effect of Temperature on the Conductivity of n-type Semiconductor: As temperature
increases, the number of electrons jumping from the donor level to the conduction band increases and
thus the conductivity increases, this rise in conductivity with temperature takes place only upto a
particular level till all the electrons from the donor level are transferred to the conduction band.
Thereafter, the conductivity remains constant because all the electrons of the donor level are now in the
conduction band. This is called donor exhaustion zone. Beyond this zone, increase in conductivity with
rise in temperature occurs entirely due to intrinsic conductivity.
Effect of Temperature on the Conductivity of p-type Semiconductor: As temperature
increases, the number of electrons jumping from the valence band to the acceptor level increases and thus
the conductivity increases, this rise in conductivity with temperature takes place only upto a particular
level till all the acceptor atoms receive electrons from the valence band and reach the acceptor saturation
zone. In this temperature zone, the conductivity remains constant since the accepter level is now
completely filled or maximum numbers of holes have already been formed in the valence band.
This is called acceptor exhaustion zone. Thereafter, the intrinsic conductivity becomes predominant on
further rise in temperature.
Preparation of Ultrapure Silicon: silicon of extreme purity (99.9999%) is required for the
preparation
of
semiconducting
materials.
It
is
achieved
through
the
following
steps.
[a] Distillation: Tetrachlorosilane (SiHCl3) is taken in a series of distillation stills and a layer of HCl
is kept over it (to remove arsenic impurity). Chlorine gas is then passed through the charge, which is kept
heated electrically. The emerging vapours are passed through a fractionating column and collected finally
in an ice-cold receiver. Pure SiCl4 so obtained is then treated with extra pure water to get SiO2,
which
is
then
reduced,
in
an
atmosphere
of
hydrogen
to
obtain
elemental
silicon.
[b] Zone Refining: This method is based on the principle that the impurities are more soluble in the melt
than in the solid state of the metal. A circular mobile heater is fixed at one end of a rod of the impure
metal. The molten zone moves along with the heater which is moved forward. As the heater moves
forward, the pure metal crystallises out of the melt and the impurities pass on into the adjacent molten
zone. The process is repeated several times and the heater is moved in the same direction. At one end,
impurities get concentrated. This end is cut off. This method is very useful for producing semiconductor
and other metals of very high purity, e.g., germanium, silicon, boron, gallium and indium.
14
Semiconductivity in non-elemental materials: Semiconducting materials are two types;
(a) Elemental (e.g., Si and Ge) and (b) Non-elemental. The non-elemental semiconducting materials
include stoichiometric and non-stoichiometric semiconducting compounds.
Stoichiometric Semiconducting Compounds: Many intermetallic compounds can be formed by
the combination of elements of Group III and Group V or Group II and Group IV.
They have crystal structures and band structures similar to those Si and Ge and contain on an average
four valence electrons per atom. For e.g., Ga-As, Ga-P, Ga-Sb, In-As, In-Sb
Major characteristics are: (i) they generally possess wide energy gap. So they can be used as
semiconductors in wider range of temperatures than elemental semiconductors (ii) it is easy to modify the
energy gap in such compounds. Generally the energy gap in stoichiometric semiconducting compounds
can be increased by substituting one of its elements by an element of lower atomic number belonging to
the same group (iii) they can be doped further to get n or p type semiconductors.
Non-stoichiometric
semiconducting
compounds:
These
compounds
act
as
n-type
semiconductors when there is an excess of cations or deficiency of anions. Similarly, they act as p-type
semiconductor when there is an excess of anions or deficiency of cations. For instance, compounds like
Fe0.61-0.94O and Zn1.1O which have vacancies in their structure and have non-stoichiometric compositions
can behave like n-type or p-type semiconductors.
For example, the non-stoichiometric compound Zn1.1O is formed on exposing ZnO to a reducing
atmosphere. In this process, some Zn2+ ions get reduced to Zn+ ions. These Zn+ ions being richer by one
electron than Zn2+ ions, it donates an electron which can be promoted to the conduction band. Thus Zn+
ions create a donor level in the energy gap between the valence band and conduction band. Consequently,
ZnO acts as a n-type semiconductor.
In the same way, FeO when heated under oxidising conditions, some Fe2+ ions get oxidised
to Fe3+ ions and a non-stoichiometric compound Fe0.9O is formed. Since Fe3+ ion has one electron less
compared to Fe2+ ion, it may be regarded as a positive hole. Under the influence of an electric field,
electrons can hop from Fe2+ to Fe3+ producing conductivity. This type of semiconductor is called hoping
or p-type semiconductor since the Fe3+ ions (acceptor ions) create an acceptor level in the energy between
the valence band and conduction band, thus helping the conductivity.
One of the disadvantages of this semiconductor is the difficulty in controlling their conductivity.
This is because their conductivity depends on the temperature and oxygen pressure used during their
preparation, as the valency of the metal ion cannot be controlled. This problem can be overcome using
controlled valency semiconductors.
15
34
Controlled Valency Semiconductors: Ni.2
Ni35
.4 O is a hopping semiconductor which shows
hopping Semiconductivity by the hoping of electrons from Ni2+ to Ni3+ ions. The concentration of
Ni3+ ions and hence the conductivity can be controlled by the addition of calculated amount of Li+ ions.
34
35
So the formula of the compound Li3
.8 Ni.9 Ni.8 O. It can be used over a wide range of temperature up to
200ºC, so it finds use in thermally sensitive resistors.
Syllabus
Semi conductors – Band theory – intrinsic and extrinsic semiconductors – conductivity in
semiconductors – Effect of temperature on conductivity - semi conductivity in non-elemental materials –
stoichiometric and nonstoichiometric semiconducting compounds – ultrapure silicon production –
zone refining
Previous University Questions
[1] Write a note on the band theory of semiconductors {10 marks}
[2] Differentiate between Intrinsic and Extrinsic semiconductors {2 marks}
[3] Explain the conduction process in Intrinsic and Extrinsic semiconductors {10 marks}
[4] Explain the process of ultrapure silicon production {5 marks}
16
Highlights
Band Theory of Semiconductors: When atoms come together to form a compound, their atom
orbital energies mix to form molecular orbital energies. As more atoms begin to mix and more molecular
orbitals are formed, it is expected that many of these energy levels will start to be very close to, or even
completely degenerate, in energy. These energy levels are then said to form bands of energy.
According to the band theory, semiconductors will actually act as insulators at absolute zero.
Above this temperature and yet still staying below the melting point of the solid, the metal would act as a
semiconductor. Semiconductors are classified by the fully occupied valence band and unoccupied
conduction band. With the small band gap in between these two bands, it takes a certain amount of
energy to excite the electrons from the valence to conduction band. Thus it follows that the higher the
temperature, the more conductive the solid will be.
Band Energy: As stated previously, continuous bands of energy are formed due to the
combinations of molecular orbitals close in energy. Of course, due to the mass amounts of different
molecular orbital mixings, bands of varying energy will form. The difference between these band
energies is known as the band gap.
17
The band theory looks at the jump of electrons across the band gap. In particular, the jump of
electrons from their valence band to their conduction band across their Fermi energy level. This "jump"
dictates optical and magnetic properties of the solid.
Valence Band: The band of energy where all of the valence electrons reside and are involved in
the highest energy molecular orbital.
Conduction Band: The band energy where positive or negative mobile charge carriers exist.
Negative mobile charge carriers are simply electrons that had enough energy to escape the valence band
and jump to the conduction band. Here, they move freely throughout the crystal lattice and are directly
involved in the conductivity of semiconductors.
Positive mobile charge carriers are also referred to as holes. Holes refer to the lack of an electron
in the conduction band. In other words, a hole refers to the fact that within the band there is a place where
an electron can exist (i.e., negative mobile charge carrier), and yet the electron ceases to exist at that
particular location. Because the electron has the potential to be there and yet isn't there, it is referred to as
positive mobile charge carrier.
Fermi Level: This level refers to the highest occupied molecular orbital at absolute zero. It is
usually found at the center between the valence and conduction bands. The particles in this state each
have their own quantum states and generally do not interact with each other. When the temperature
begins to rise above absolute zero, these particles will begin to occupy states above the Fermi level and
states below the Fermi level become unoccupied.
Semiconductors: Semiconductors are defined to have conductivity in between an insulator and a
conductor. Due to this property, semiconductors are very common in every day electronics since they
likely will not short circuit like a conductor. They get their characteristic conductivity from their small
band gap. Having a band gap prevents short circuits since the electrons aren't continuously in the
conduction band. A small band gap allows for the solid to have a strong enough flow of electrons from
the valence to conduction bands in order to have some conductivity.
Electrons in the conduction band become free from the nuclear charge of the atom and thus can
move freely around the band. Thus, this free-moving electron is known as a negative charge carrier since
having the electron in this band causes electrical conductivity of the solid. When the electron leaves the
valence band, the state then becomes a positive charge carrier, or a hole.
Intrinsic Semiconductors: Pure semiconductors in which its properties are solely based off of the
material itself. Here, the number of electrons in the conduction band is equal the number of holes in the
valence band. Theses semiconductors are also known as i-types.
18
Extrinsic Semiconductors: Impure semiconductors that have been "doped" in order to enhance
its conductivity. There are two types of extrinsic semiconductors: p-type and n-type. A "dopant" atom is
added to the lattice in order to draw electrons from the valence band. This atom is referred to as an
acceptor. As more acceptors are added to the lattice, the number of holes will begin to exceed the number
of negative charge carriers, eventually leading to a p-type semiconductor. N-type semiconductors have a
large number of donors, "dopant" atoms that donate electrons to the conduction band.
ietchemistry@gmail.com
19
![Semiconductor Theory and LEDs []](http://s2.studylib.net/store/data/005344282_1-002e940341a06a118163153cc1e4e06f-300x300.png)

