© Copyright Hans J. Reich 2010
All Rights Reserved
University of Wisconsin
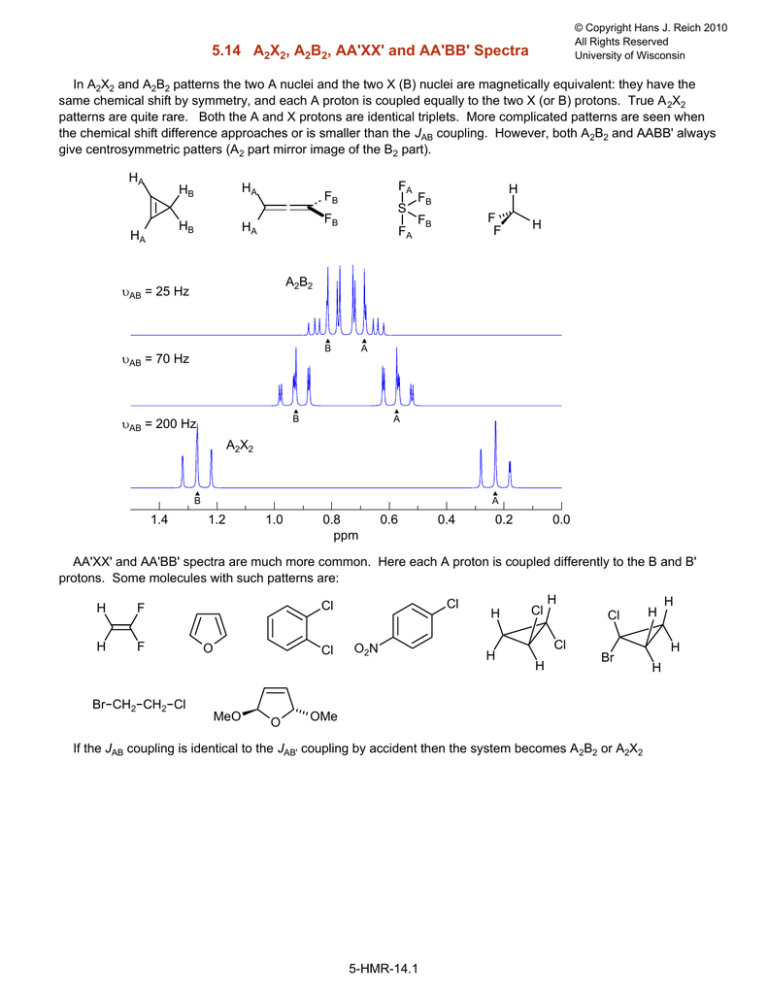
5.14 A2X2, A2B2, AA'XX' and AA'BB' Spectra
In A2X2 and A2B2 patterns the two A nuclei and the two X (B) nuclei are magnetically equivalent: they have the
same chemical shift by symmetry, and each A proton is coupled equally to the two X (or B) protons. True A 2X2
patterns are quite rare. Both the A and X protons are identical triplets. More complicated patterns are seen when
the chemical shift difference approaches or is smaller than the JAB coupling. However, both A2B2 and AABB' always
give centrosymmetric patters (A2 part mirror image of the B2 part).
HA
HA
HB
HB
HA
FA
FB
S
FB
HA
FA
H
FB
F
F
FB
H
A2B2
υAB = 25 Hz
B
υAB = 70 Hz
A
B
υAB = 200 Hz
A
A2X2
B
1.4
A
1.2
1.0
0.8
ppm
0.6
0.4
0.2
0.0
AA'XX' and AA'BB' spectra are much more common. Here each A proton is coupled differently to the B and B'
protons. Some molecules with such patterns are:
H
F
H
F
Br CH2 CH2 Cl
Cl
Cl
O
Cl
MeO
O
O2N
H
H
Cl
H
Cl
Cl
H
Br
OMe
If the JAB coupling is identical to the JAB' coupling by accident then the system becomes A 2B2 or A2X2
5-HMR-14.1
H
H
H
H
5.15 AA'XX' and AA'BB' Spectra
JAA' = -10
JXX' = -11
JAX = 12
JAX' = 2
AA'XX' spectra consist of a maximum of two identical 10-line
half spectra, each symmetrical about its midpoint, νA and νX,
respectively. See example A below.
|K| = |JAA' + JXX'|
"J" of one ab quartet
|L| = |JAX - JAX'|
"δ" of both ab quartets
|M| = |JAA' - JXX'|
"J" of other ab quartet
|N| = |JAX + JAX'|
"doublet"
1.2
1.1
1.0
νA
In the situation where JAX = JAX' (i.e. L = 0, A2X2) the spectrum
collapses to a triplet. In other words, the effective "chemical
shift" of each of the ab quartets is zero, and each gives a single
line at νA. This is more or less the situation with many
compounds of the X-CH2-CH2-Y type, provided that X and Y are
not too large, but cause very different chemical shifts. See
example C.
|L|
|JAA' - JXX'|
|M|
2R
(2P)2 - K2
(2R)2 - M2
F
Si(CH3)3
H
M2+L2
2R =
|L|
=
H
|
|
SeC(CH3)3
B
|
2P
K2+L2
|JAX-JAX'| = |L| =
Ph
|
|JAA' + JXX'|
|K|
In the situation where JAA' = JXX' (which is often approximately
the case with X-CH2-CH2-Y and p-disubstituted benzenes) the
second ab quartet collapses to two lines since M = 0. See
example A below.
A
0.8
|JAX + JAX'|
|N|
Each half-spectrum consists of a 1:1 doublet (intensity 50% of
the half spectrum), and two ab quartets, each with "normal"
intensity ratios, and apparent couplings (Jab) of |K| and |M| as
indicated. Unfortunately, K and M cannot be distinguished, the
relative signs of JAA' and JXX' are not known, nor is it known which
number obtained is JAA' and which is JXX'. The same ambiguity
occurs for JAX + JAX'.
H
0.9
C
F
OMs
MsO
200 MHz
60 MHz
140
120
100
4.5
4.0
3.5
ppm
5-HMR-15.1
3.0
30
20
10
0
5.15 The AA'BB' Pattern
As A and X of the AA'XX' spectrum move closer together, the lines of the 1:1 doublet each split into two lines, for
a total of twelve in each half-spectrum. The AA' and BB' parts are no longer centrosymmetric, as for the AA'XX', but
develop a mirror image relationship, so that the entire pattern is centrosymmetric. As is found for all AX to AB
transformations, "leaning" occurs, the inner lines increase and the outer lines decrease in intensity.
Typical molecular fragments which give AA'BB' patterns are:
X
HA
HA'
HB
X
HA
HA'
HB
Y
(a) AA'-Gem
HB'
HB
(b) AA'-Vic
HA
HA'
HB
HB'
R
HB'
Y
X
HA
R
HB'
Y
HA'
(c) ODCB
o-Dichlorobenzene
(d) p-Aromatic
(a) AA' Geminal (X-CH2CH2-Y). This is perhaps the most common type of AA'BB' pattern. The appearance can
range from essentially perfect triplets, to rather complicated patterns which cannot be easily analyzed. The two
spectral parameters which control appearance of the spectrum are νAB, and the difference between the two vicinal
coupling constants JAB and JAB'. If νAB is small (AA'BB'), then the pattern will always be complicated, no matter what
the coupling constants are. If JAB ≈ JAB' then the pattern will mimic A2X2/A2B2 and triplets will be seen if the the
chemical shift is large enough.
Changing chemical shifts while keeping coupling constants static
VAB = 27.00
JAA' = 15.00
JBB' = 15.00
JAB = 7.00
JAB' = 7.00
VAB = 50.00
JAA' = 15.00
JBB' = 15.00
JAB = 7.00
JAB' = 7.00
VAB = 110.00
JAA' = 15.00
JBB' = 15.00
JAB = 7.00
JAB' = 7.00
VAB = 210.00
JAA' = 15.00
JBB' = 15.00
JAB = 7.00
JAB' = 7.00
250
200
150
Hz
5-HMR-15.2
100
50
0
The key feature that determines the complexity of AA'BB' patterns is the relative size of JAB and JAB', which is
determined by the conformational properties of the X-CH 2CH2-Y fragment. For acyclic systems, if the X and/or Y
groups are small, then the populations of the anti and gauche conformations will be close to statistical (1:2). As can
be seen from the table and the simulated spectra below, the two averaged couplings become approximately equal
when there is 67% of gauche isomer, and the spectrum will mimic an A 2B2 pattern -- triplets if νAB is large enough
(νAB >> JAB). If X and Y are large, then the anti isomer will be favored and the pattern will be more complex. In
practice, adjacent CH2 groups often look like triplets, and thus the gauche conformation must usually be favored.
For cyclic systems (e.g., N-cyanomorpholine) the ring constrains the -CH 2CH2- fragment to mostly the gauche
conformation, and clean triplets are not usually seen.
%anti %gauche
The coupling constants were calculated
using the simple Karplus equations
below:
100
0
JAB = 2.3
JAB' = 12
JΘ = Jo cos2 Θ
Jo = 12 Hz for Θ > 90°
Jo = 9 Hz for Θ < 90°
JAA' = JBB' = -13 Hz
JAB = 3.2
Y
HB'
HA
HA'
Z
HB
HB'
Y
Y
HB'
HB
Z
HA'
HB
gauche
gauche
anti
20
JAB' = 10.1
60
40
JAB = 4.2
HA
HA
HA'
Z
80
JAB' = 8.1
JAB = 5.5
CN
33
67
N
JAB' = 5.5
O
300 MHz (CDCl3)
Source: ASV
JAB = 6.2
JAB' = 4.2
20
80
Line width
= 1.5 Hz
3.8
3.6
ppm
3.4
3.2
0
100
JAB = 7.1
JAB' = 2.3
It is a common misconception that the equalization of coupling constants (and hence the appearance of triplets)
is a consequence of free rotation around the CH 2-CH2 bond. In fact, there is free rotation around almost all such
bonds in acyclic molecules at accessible temperatures. The appearance of more complicated patterns is the result
of a preference for the anti conformation over the gauche (or vice versa), and has nothing to do with the rate of
rotation.
New 35-01
5-HMR-15.3
X
AA'BB' Spectra X-CH2-CH2-Y
A
A' B'
B
Y
Br
Br-CH2-CH2-Cl
60 MHz
200 MHz
δ 3.4
δ 3.1
Ph
O
O
100 MHz
O
OMe
H
100MHz
3.5
3.0
H
2.4
2.5
2.2
2.0
1.8
1.6
SeC(CH3)3
Ph
PhS
Si(CH3)3
H
200 MHz
SeCH3
200 MHz
2.2
2.1
0.7
0.6
0.5
5-HMR-15.4
2.7
2.6
2.5
(b) AA' Vicinal. This type of AA'BB' pattern is much less common than type (a). It appears principally in 1,1disubstituted cyclopropanes, 2,2-disubstituted-1,3-dioxolanes and other similar structures. The patterns are rarely if
ever triplets because JAB is invariably quite different from JAB'.
HA
X
HA'
HA
HB'
Y
HA'
X O
Y
O
HB
HBH
B'
O
O
100 MHz
100 MHz
1.2
1.0
0.8
0.6
1.2
0.4
1.0
0.8
Br
O
O
200 MHz
4.0
3.9
3.8
270 MHz
3.7
4.0
3.9
O
3.8
O
Br
C
200 MHz
4.3
4.2
4.1
4.0
5-HMR-15.5
3.9
3.8
(c) o-Dichlorobenzene (ODCB) Type. This kind of AA'BB' pattern is often very complex because JAB (ortho
coupling) is usually much larger than JAB' (meta coupling). It is seen in symmetrically 1,4-disubstituted dienes and
ortho disubstituted benzenes. Note that for all AA'BB' systems the A and B patterns are identical (although they
have a mirror-image relationship). This is in spite of the fact that the coupling relationships are often quite different,
seen in the molecules on thi page.
HA
HB
300 MHz, CDCl3
HB'
HA'
7.9
7.8
7.7
ppm 7.6
7.5
7.4
7.3
Cl
S
300 MHz, CDCl3
300 MHz, CDCl3
Cl
7.5
7.4
ppm 7.3
7.2
7.3
7.1
H H
H
H
7.0
6.8
7.2
6.6
7.35
HA
HA'
HA
HX'
7.0
HX
6.8
2.10
OAc
2.13
5.95
H
100 MHz
7.2
HX
7.1
H
H
100 MHz
AcO
7.2
Ph Ph
Ph
Ph
H
ppm
60 MHz
OAc
AcO
(HX shown)
JOC-68-2835
60 MHz
7.10
5.87
HX'
HA'
(HX shown)
JOC-68-2835
JAX = 12.5
JAX' = -0.7
JXX' = 11.5
JAA' = 0.8
JAX = 6.7
JAX' = -1.3
JXX' = 11.0
JAA' = 1.6
6.3
6.1
5.9
ppm
5.7
6.0
5.8
ppm
5.6
Rev 40-01
5-HMR-15.6
(d) p-Disubstituted Benzene Type. These usually resemble an AB quartet, with extra lines and additional
splitting. When the chemical shift between A and B becomes small (as in p-bromochlorobenzene below), then the
extra lines become more pronounced.
Br
60 MHz
Cl
7.5
7.4
7.3
S
7.2
7.1
S
MeO
OMe
300 MHz (CDCl3)
7.4
7.3
7.2
ppm
7.1
7.0
6.9
6.8
(d) Miscellaneous.
Here the 4JHH (AA' and AB')are large enough that the expected first-order AB quartet is not seen, but the more
complicated pattern shown
HA
HB
Ph
OH
Ph
HA' HB'
Reich (Wesley)
3.40
3.35
3.10
3.05
Rev 39-05
5-HMR-15.7
Origin of Complexity in Patterns of the AA'XX'... Type
There are two situations where spin systemes containing AA'XX' type do not show unusual complexity. One is
where JAX = JAX', in which case the pattern becomes first order A 2X2.
The second is systems in which there is no coupling between both of the chemical shift equivalent protons, i.e.,
JAA' = JXX' = 0. In such cases the degeneracy between spin states is no longer present, and first order systems
result. Consider two examples. A monosubstituted benzene is nn AA'BB'C or AA'MM'X system. A simulated
spectrum is shown below
R
HA
HA'
HM
HM'
HX
JAM = JA'M' = 7
JMX = JM'X = 7
JAA' = 2
JMM' = 2
JAX = JA'X = 2.0
JAM' = JA'M = 0.5
2.04
2.04
1.00
Simulation
8.0
7.5
7.0
6.5
ppm
If we recalculate the spectrum after setting JAA' =0 and JMM' = 0 then the spectrum becomes essentially first order
(it would be completely first order if the chemical shifts between A, M and X were made larger).
Simulation
2.5
JAA' = 0
JMM' = 0
2.0
1.5
ppm
1.0
0.5
For this reason, some spin systems which are formally of the AA'XX' type, but in which there is no spin-spin
coupling between the equivalent protons show first order spectra. For example, the fairly common spin system
below of the AA'BB'X type shows no unusual complexity (beyond that of normal ABX patterns) because there is no
coupling between the AA' and BB' protons.
HA HB HA' HB'
R
R
R' HX
5-HMR-15.8
Contrast this with the AA'MM'XX' system below. In this case, although there is no significant coupling between A
and A' or M and M', there is coupling between X and X', making the whole system a highly second order one.
O O
HA
HM'
S
HA'
HM
Br
HX'
HX
Br
HX
δA = 3.7 JAM = JA'M' = -13
δB = 4.03 JMX = JM'X' = 5
δX = 4.40 JAA' = 0
JMM' = 0
JAX = JA'X' = 8.0
JAM' = JA'M = 0
JXX' = 6
Simulated spectrum
HM
HA
JXX' = 6 Hz
4.5
4.4
4.3
4.2
4.1
4.0
3.9
3.8
3.7
3.6
If we remove the XX' coupling, the system becomes essentially first order (two isolated AMX patterns). We could
better describe it as an (AMX)2 rather than an AA'MM'XX' spin system.
Simulated spectrum
JXX' = 0
4.5
4.4
4.3
4.2
4.1
4.0
3.9
3.8
3.7
3.6
Actual Spectrum
250 MHz, CDCl3
4.85
4.80
Hz
4.75
4.10
4.05
4.00
Hz
5-HMR-15.9
3.95
3.60
3.55
Hz
3.50
Note the extra peaks - these are not impurities or bad
tuning. The additional complexity arises because we
have an AA'BB'XX' system here. Even though only X
and X' are significantly coupled, the AB signals are
complicated
300 MHz 1H NMR spectrum in CDCl3.
Source:Charlie Fry/Reich
O
A
MeO
OMe
B
X'
X
B'
MeO
OMe
A'
O
2.8
2.7
2.6
2.5
ppm
AA'BB'XX'
This is also an AA'BB' system
6.8
6.7
ppm
8.00
3.45
6.17
5.80
3.40
ppm
3.35
3.90
2.11
8
7
6
5
4
3
ppm
5-HMR-15.10
2
1
0