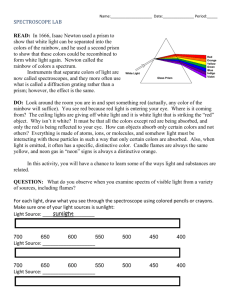
CD Light: An Introduction to Spectroscopy
advertisement

Instructor Side JCE Classroom Activity: #12 CD Light: An Introduction to Spectroscopy by the Journal’s Editorial Staff About These Activities perforated fold here and tear out This activity is based on CD-ROM Spectroscope: A Simple and Inexpensive Tool for Classroom Demonstrations on Chemical Spectroscopy (1), an article found in this issue of the Journal. The original directions for the construction of the spectroscope box have been expanded and modified in an attempt to make them easier for students to follow, and simple introductory exercises using common, household materials have been developed. This activity could be done by students outside of the laboratory, or in class. These safety considerations must be discussed with students prior to the activity: 1. A very sharp blade such as an X-acto knife, razor blade, or scalpel is needed to cut the slits in the box. Blades must be handled with extreme caution. They should be used only on a protected surface, such as a cutting board. An instructor or parent should be present when blades are used. 2. Do not observe strong sunlight through the spectroscope, as eye damage can result. If the reflected light in the spectroscope is difficult or painful to look at, immediately look away from the viewing window and move to a location where the light is less bright. Ordinary daytime indoor levels of light from the sun and incandescent or fluorescent light bulbs is sufficient to produce acceptable results in this activity. See the article (1) for information on the differences in the observed spectrum due to the light source. Any music CD or computer CD can be used in the spectroscope. Damaged CDs that no longer play or CD-ROMs distributed with unwanted trial software are ideal choices. However, if the CDs are handled carefully they will not be damaged in the activity. Lightweight cardboard such as that used in cereal boxes is recommended for construction of the spectroscope box. Cardboard should be easy to cut and fold, but stiff enough to hold its shape and thick enough to block light. The cardboard could be covered with black paint on one side to help keep unwanted light out. Measurements must be made carefully. Errors of 1–2 mm can result in the sides of the box not fitting together properly. In Part 2 of the activity, students first look at white light that has been separated into component colors by the spectroscope, and then look at light that has passed through a colored film—either a colored plastic sheet (such as a report cover) or a plastic sheet they have colored with a felt-tip pen (overhead transparencies and markers work well). The students will see that the complementary color is absorbed in each case. In Part 3, students will discover again that complementary colors are absorbed. With careful observation, they should be able to determine that the amount of absorbance depends on the concentration of the solution and the depth of solution in the container. Integrating the Activity into Your Curriculum This activity could be used as an introduction to those included in the article (1), or as a general introduction to spectroscopy. The activity is qualitative. Quantitative absorbance measurements require the use of a spectrometer. The student constructed spectroscope could be used in an activity studying line spectra from outdoor night lighting, as described by Jacobs (2). Because of the geometry and materials used in constructing the spectroscope, it should probably not be used to attempt to view line spectra from flame tests. If the concept of complementary colors has not been discussed in class, it can be introduced in the discussion of student data. In discussion of colors of light, the JCE Classroom Activity #7 (3) may be helpful, especially in reinforcing the idea that the familiar rules for combining pigments are not correct for combining colors of light. If the term absorbance is not familiar to students, it may be introduced to explain or describe the colors missing from the spectrum after light has passed through the colored plastic or solution. Based on the student’s observations in Part 3, Beer’s law could be deduced in a class discussion. References, Additional Activities, and Demonstrations 1. Wakabayashi, F.; Hamada, K.; Sone, K. J. Chem. Educ. 1998, 75, 1569–1570. 2. Jacobs, S. F. J. Chem. Educ. 1997, 74, 1070; http://jchemed.chem.wisc.edu/Journal/Issues/1997/Sep/art1070.html 3. How Many Colors in Your Computer? Discovering the Rules for Making Colors; J. Chem. Educ. 1998, 75, 312A. 4. Clarke, D. W. Shoebox Spectroscopy; Sci. Teach. 1998, 65(7), 29. 5. Gauger, R. Using laser refractometry to determine concentration; Sci. Teach. 1995, 62(3), 21. This Activity Sheet may be reproduced for use in the subscriber’s classroom. JChemEd.chem.wisc.edu • Vol. 75 No. 12 December 1998 • Journal of Chemical Education 1568A JCE Classroom Activity: #12 Student Side CD Light: An Introduction to Spectroscopy by the Journal’s Editorial Staff White light from the sun or a light bulb is a mixture of many colors of light. You have probably separated white light into colors using a prism or diffraction grating, and seen this happen in nature as a rainbow. You may have noticed rainbow colors on compact discs, such as computer or music CDs. The regularly spaced tracks on the CD act as a diffraction grating. You can use a CD to build a simple spectroscope to investigate how different colors of light interact with colored matter. Try This You will need: cardboard such as a flattened cereal box or 24-can soda box; scissors; sharp blade such as an X-acto knife*, cutting board, metric ruler; straightedge; adhesive tape; compact disk (CD or CD-ROM); light source; transparent colored plastic or colorless transparent plastic and colored felt tip markers; colorless, transparent glass or plastic cup or a petri dish; water; and food color. *Warning: Use caution in handling sharp blades. Let your instructor or parent know when you are using the blade. Cut cardboard only on a protected surface or a cutting board. 1. Building a Spectroscope: Cut a cardboard rectangle 39 × 26.3 cm. Place the cardboard on a table or desk top. As shown in Figure 1, use a pencil to draw a line 7.3 cm from the left (shorter) side, draw a second line 11.7 cm from the first line, and a third line 7.3 cm from the second line. Draw lines 6.4 cm from the top and bottom edges (long sides). Cut out the rectangles shaded in Figure 1 to form the shape in Figure 2. Erase the horizontal pencil lines, shown as dashed lines in Figure 2. Draw lines 1 cm from the top, bottom, and right edges of the horizontal portion, as shown in Figure 2. Cut off the corners shown shaded in Figure 2. Use a sharp blade to cut the three openings shown in Figure 3. If you have difficulty cutting a 1-mm slit, cut it wider, then tape a piece of cardboard to cover part of the slit leaving a 1-mm opening. Lightly score the remaining pencil lines with the blade. —6.4— Entrance slit: 1 mm wide, 3 cm long, center in panel 1 cm from top line CD slit: 2 mm wide, across panel 6.5 cm from top line — 7.3 — — 11.7 cm — — 12.7 cm — — 7.3 — — 6.4 — Figure 1 Viewing window: 6 mm wide, 2 cm long, center in panel 1.2 cm from top line Figure 2 Figure 3 Fold the cardboard along the scored lines to form a box. Secure the edges with tape. Insert light Entrance slit Viewing window a CD in the long slit at an angle so that you can see the shiny side through the viewing window. It should fit tightly and not fall out if you turn the box over. See Figure 4. 2. Colors: Hold your spectroscope so that light can shine through the entrance slit. Look though the viewing window. Warning: Looking at the reflection from strong sunlight CD can damage your eyes. If the reflected light is painful or difficult to look at, look away at Figure 4 once. Use a less intense light source. What do you see? Place a piece of transparent colored plastic over the entrance slit. How does what you see change? Repeat with several colors such as red, blue, green, yellow, purple, and orange. Colored markers can also be used to tint colorless plastic. 3. Solutions: Place a cup or petri dish of water over the entrance slit. Try to keep the container as still as possible. What do you see through the viewing window? Repeat with a solution made by mixing one drop of food color with about 100 mL of water. Add another drop of food color, mix the solution, and repeat the procedure. Has there been a change? Add additional drops of food color. What happens to the spectrum? Pour out about half of the solution and repeat the procedure. Does the depth of solution make a difference? Questions Name three pairs of complementary colors. How are complementary colors related to the absorbance of light by colored objects? On what factors does the amount of light absorbed by a colored solution depend? Information from the World Wide Web 1. About light: http://antoine.fsu.umd.edu/chem/senese/101/quantum/index.shtml; http://www.sfu.ca/chemcai/QUANTUM/ Quantum_Primer.html; http://www.qmw.ac.uk/~zgap118/index.html 2. More about building and using spectroscopes: http://129.82.166.181/CD_Spectroscope.htm; http://www.exploratorium.edu/ spectroscope/; http://asd-www.larc.nasa.gov/edu_act/simple_spec.html; http://genchem.chem.wisc.edu/labdocs/modules/spectro/spectros.htm This Activity Sheet may be reproduced for use in the subscriber’s classroom. 1568B Journal of Chemical Education • Vol. 75 No. 12 December 1998 • JChemEd.chem.wisc.edu