Separation of Fe and Sn--Cu Phases in an Fe--Sn--Cu-
advertisement

Materials Transactions, Vol. 47, No. 3 (2006) pp. 864 to 867
#2006 The Japan Institute of Metals
Separation of Fe and Sn–Cu Phases in an Fe–Sn–Cu–B System
Hideki Ono-Nakazato, Kenji Taguchi*1 , Daisuke Kawauchi*2 and Tateo Usui
Course of Materials Science and Engineering, Division of Materials and Manufacturing Science,
Graduate School of Engineering, Osaka University, Suita 565-0871, Japan
Scrap metal often includes a large amount of copper and tin. It is important to recover copper and tin from this scrap metal for recycling.
Separation into two liquid phases, namely Fe and Sn(–Cu) phases, has been investigated at 1523 K in Fe–Sn–B and Fe–Sn–Cu–B systems. In the
Fe–Sn–B system, the tin content of the Fe-rich phase and the iron content of the Sn-rich phase are 13.7 and 11.7 mass%, respectively, when
[mass%B](in Fe) ¼ 3:62. Boron widens the miscibility gap of the Fe–Sn binary system. The isothermal section diagram of the Fe–Sn–Cu–
2.66 mass%B quaternary system at 1523 K is described. In the Fe–Sn–Cu–B system, separation into two liquid phases, Fe-rich and [Sn–Cu]-rich,
is found over all ratios of [mass%Sn]/[mass%Cu]. The separation region is enlarged as the [mass%Sn]/[mass%Cu] ratio is decreased. By using
the separation into two liquid phases, iron can be enriched in the Fe-rich phase and copper and tin can be enriched in the [Sn–Cu]-rich phase. It is
possible to recover copper and tin effectively from Fe–Sn–Cu alloy.
(Received October 21, 2005; Accepted January 24, 2006; Published March 15, 2006)
Keywords: recycle, phase separation, shredder dust, scrap, boron, iron–tin–copper alloy
1.
Introduction
In recent years, interest in waste treatment and recycling
has increased, due to environmental concerns. In Japan, the
ratios of iron, copper and tin that are recycled are very low at
present, namely about 38, 47 and 8.3%, respectively.1) Most
of the rest of these resources are accumulated as scraps.
Accordingly, it is important to establish recycling technology
for these scraps and shredder dust.
The metal contained in automobile shredder residue (ASR)
is mainly composed of iron and copper. The amounts of iron
and copper in ASR generated in one year in Japan are
equivalent to approximately 0.15 and 4%, respectively, of the
annual domestic consumption of iron and copper. ASR is
expected to be mainly a copper resource, and it is desirable to
develop a recovery technique to this end.
An iron–copper binary system has a single liquid phase. It
has been reported that it separates into Fe-rich and Cu-rich
phases on the addition of C,2–4) P,5,6) Si7) or Co.8,9) In our
previous works,10,11) separation into two liquid phases was
also studied in Fe–Cu–B and Fe–Cu–B–C systems, and the
addition of boron was found to be more effective for the
phase separation than the addition of the other elements (C, P,
Si or Co). The tin content of scraps is considered to be
approximately one tenth of the copper content.12) It is difficult
to remove tin as well as copper from iron, and tin and copper
are well-known as tramp elements.
An iron–tin binary system has two liquid phases.13) On the
other hand, tin will be dissolved in copper because the
activity curve of the Sn–Cu binary system exhibits strong
negative deviation from ideality.14) Accordingly, it is
predicted that Fe and Sn–Cu phases are effectively separated
into two liquid phases in the Fe–Sn–Cu–B system. The phase
separation of the Fe–Sn–Cu–B system is investigated in the
present study.
*1JSPS
Research Fellow and Graduate Student, Graduate School of
Engineering, Osaka University
*2Graduate Student, Graduate School of Engineering, Osaka University
2.
Experimental
2.1 Sample preparation
High purity electrolytic iron (purity: 99.98%) and reagent
grade boron (purity: 99.8%) inserted in an alumina crucible
were inductively heated up to 1873 K in an Ar–33 vol%H2
atmosphere, and Fe–3.5 to 4.25 mass%B alloys were made.
Tin and Sn- up to 90 mass%Cu alloys were also prepared by
using a vertical SiC electric resistance furnace at 1373 K in an
argon atmosphere.
2.2 Procedure
The experimental apparatus consisted of an electric
resistance furnace which was connected to a proportional
integral and derivative action (PID) controller with a Pt–
6%Rh/Pt–30%Rh thermocouple. A mullite furnace tube
(60 mm o.d., 52 mm i.d., 1000 mm long) was used. In the
experiments with the Fe–Sn–B system, 10 g of the Fe–3.5 to
4.25 mass%B alloy and 10 g of the reagent grade tin were put
in an alumina crucible (15 mm o.d., 12 mm i.d., 100 mm
height). In the experiments with the Fe–Sn–Cu–B system,
13.3 g of the Fe–4.0 mass%B alloy and 6.7 g of the Sn–Cu
alloy were mixed in an alumina crucible (15 mm o.d., 12 mm
i.d., 100 mm height). The alumina crucible was inserted in a
carbon holder (42 mm o.d., 34 mm i.d., 150 mm height).
Then, the sample was held for over 5 h in an argon
atmosphere at 1523 K, and equilibrium was attained between
the Fe-rich and [Sn–Cu]-rich phases. After equilibrium was
attained, the sample was quickly withdrawn from the furnace.
The sample was cut vertically, and the immiscibility of the
liquid Fe and Sn–Cu phases was observed from the vertical
sections of samples in all the present experiments. The boron,
copper and tin contents of the Fe-rich phase and the boron,
copper, iron and tin contents of the [Sn–Cu]-rich phase were
analyzed by inductively coupled plasma (ICP) emission
spectrometry.
Separation of Fe and Sn–Cu Phases in an Fe–Sn–Cu–B System
865
Table 1 Experimental results for phase separation in Fe–Sn–B and Fe–Sn–Cu–B systems at 1523 K.
No.
Fe-rich phase
System
[mass%Fe]
[mass%B]
[mass%Sn]
[mass%Cu]
[mass%Fe]
[mass%B]
[mass%Sn]
1
(82.68)
3.62
13.7
—
11.7
<0:001
ð88.3Þ
—
2
(81.18)
3.42
15.4
—
10.5
<0:001
ð89.5Þ
—
3
Fe–Sn–B
[mass%Cu]
(82.23)
3.67
14.1
—
10.8
<0:001
ð89.2Þ
—
4
(82.23)
4.07
13.7
—
10.8
<0:001
ð89.2Þ
—
5
(82.98)
3.73
12.5
0.79
13.1
<0:001
76.8
(10.1)
6
(85.63)
3.42
9.57
1.38
13.4
<0:001
66.9
(19.7)
7
(87.09)
3.93
7.23
1.75
12.1
<0:001
62.1
(25.8)
8
(88.77)
3.82
5.29
2.12
10.5
<0:001
53.3
(36.2)
(89.79)
(90.25)
3.53
3.81
3.86
2.52
2.82
3.42
<0:001
<0:001
45.7
37.6
(45.8)
(56.3)
9
10
Fe–Sn–Cu–B
8.53
6.07
11
(90.97)
3.84
1.46
3.73
5.23
<0:001
29.1
(65.7)
12
(92.89)
3.90
0.56
2.65
3.77
<0:001
20.1
(76.1)
13
(90.92)
3.79
0.44
4.85
3.73
<0:001
10.5
(85.8)
Sn-rich
phase
Fig. 1
3.
[Sn(–Cu)]-rich phase
Sn-Cu-rich
phase
Fe-rich
phase
Fe-rich
phase
(a) No.3
(b) No.6
Fe-rich
phase
Sn-Cu-rich
phase
(c) No.9
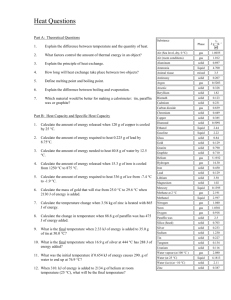
Images of separation into two liquid phases for several samples from Fe–Sn–B and Fe–Sn–Cu–B systems (Samples nos. 3, 6 and 9).
Results and Discussion
3.1 Observation of separation into two liquid phases
All the experimental samples were separated into two
liquid phases (Fe-rich and [Sn(–Cu)]-rich). The experimental
results are tabulated in Table 1. The values in parentheses
denote the values calculated from the analysed values of
other elements. It can be seen from Table 1 that almost all the
boron dissolves in the Fe-rich phase. Cross-sectional views
of several samples (Nos. 3, 6 and 9) are shown in Fig. 1.
In samples No. 1 to 6, the upper and bottom layers are
[Sn(–Cu)]-rich and Fe-rich phases, respectively, as shown in
Figs. 1(a) and (b). In the other samples, the upper and bottom
layers are changed to the Fe-rich and [Sn–Cu]-rich phases,
respectively [Fig. 1(c)]. This is caused by the variation in the
specific gravity of the [Sn(–Cu)]-rich phase with a changing
copper content.
3.2
Effect of boron on separation of Fe–Sn–B system
into two liquid phases
The effects of the boron content of the Fe-rich phase on the
tin content of the Fe-rich phase and the iron content of the Snrich phase at 1523 K are shown in Fig. 2. The iron content of
the Sn-rich phase is about 11 mass% and is independent of
the boron content of the Fe-rich phase. On the other hand, the
tin content of the Fe-rich phase decreases with an increasing
boron content in the Fe-rich phase. From the phase diagram
of the Fe–Sn binary system, the Fe–Sn alloy is separated into
two liquid phases, Fe–53 mass%Sn and Fe–79 mass%Sn
alloys, at 1523 K.13) In the present study, the tin contents of
866
H. Ono-Nakazato, K. Taguchi, D. Kawauchi and T. Usui
Fe
20
[m
ass
%C
u]
15
10
5
3
3.5
4
[mass%B](in Fe)
Cu
[mass%M](in Fe) (M=Cu, Sn)
[mass%Fe](in Sn–Cu)
60
Two liquid phases
separation
40
80
4.5
Fig. 2 Effect of boron content of the Fe-rich phase on tin content of the Ferich phase and iron content of the Sn-rich phase at 1523 K.
20
20
40
60
[mass%Sn]
80
Sn
Fig. 4 Isothermal section diagram of Fe–Sn–Cu–2.66 mass%B quaternary
system at 1523 K.
15
(a)
Sn
of the Fe-rich phase decreases and the tin content of the
Fe-rich phase increases as the tin content of the [Sn–Cu]-rich
phase increases. From Fig. 3(b), it can be seen that the
iron content of the [Sn–Cu]-rich phase increases with an
increasing tin content in the [Sn–Cu]-rich phase up to
[mass%Sn](in Sn{Cu) ¼ 70.
In the present experiments, the boron content of the whole
sample (Fe–Sn–Cu–B) is 2.66 mass%. The isothermal section
diagram of the Fe–Sn–Cu–2.66 mass%B quaternary system
at 1523 K for the experimental results is presented in Fig. 4.
In the Fe–Sn–Cu–B system, separation into Fe-rich and [Sn–
Cu]-rich phases is found over all ratios of [mass%Sn]/
[mass%Cu]. The separation region is enlarged as the ratio of
[mass%Sn]/[mass%Cu] is decreased.
10
Cu
5
0
15
(b)
10
5
Present work
11)
Taguchi et al.
0
0
50
[mass%Sn](in Sn–Cu)
100
Fig. 3 Effect of tin content of the [Sn–Cu]-rich phase on copper and tin
contents of the Fe-rich phase and iron content of the [Sn–Cu]-rich phase at
1523 K.
the Fe-rich and Sn-rich phases are 13.7 and 88.3 mass%,
respectively, when [mass%B](in Fe) ¼ 3:62 (No. 1). It is
found that boron widens the miscibility gap of the Fe–Sn
binary system.
3.3
40
60
[mass%Sn](in Fe)
[mass%Fe](in Sn)
80
e]
%F
ass
[m
[mass%M]
20
Separation of Fe–Sn–Cu–B system into two liquid
phases
The effects of the tin content of the [Sn–Cu]-rich phase
on the copper and tin contents of the Fe-rich phase and the
iron content of the [Sn–Cu]-rich phase at 1523 K are shown
in Fig. 3. The experimental results for the Fe–Cu–B
system ([mass%Sn](in Sn{Cu) ¼ 0)11) are also plotted in
Fig. 3. It can be seen from Fig. 3(a) that the copper content
3.4
Enrichment of iron in the Fe-rich phase, and of
copper and tin in the [Sn–Cu]-rich phase, on
addition of boron
The enrichment of iron, copper and tin is considered by
using the separation into two liquid phases, the Fe-rich and
[Sn–Cu]-rich phases. To enhance the separation, 2.66 mass%
boron was added to Fe–Sn–Cu alloys at 1523 K. The
enrichment ratio of the element M(= Fe, Cu, Sn), RM , is
defined by
mM
RM ð%Þ ¼ 100 (M = Fe, Cu, Sn)
ð1Þ
mM
where mM
and mM denote the mass of the element M in the
initial Fe–Sn–Cu alloy and in the M-rich phase after
separation with the addition of 2.66 mass% boron. The
compositions of the Fe-rich and [Sn–Cu]-rich phases in the
Fe–Sn–Cu–2.66 mass%B system after the separation into two
liquid phases can be determined from Fig. 4, and the
enrichment ratio of an element M can be calculated from
eq. (1). The results are shown in Fig. 5. It can be seen from
Fig. 5 that high enrichment ratios of iron, copper and tin can
be obtained when the alloy is separated into Fe-rich and [Sn–
Cu]-rich phases by boron addition. In particular, after the
Separation of Fe and Sn–Cu Phases in an Fe–Sn–Cu–B System
RM > 90%
RM > 80%
Fe
80
40
60
[mass%Sn]
80
Sn
Cu
]
Cu
ss%
60
20
20
40
60
[mass%Sn]
(a) RFe
80
60
[m
a
ss%
[m
a
40
80
40
e]
%F
ass
20
60
60
20
80
[m
80
]
Fe
40
40
e]
%F
ass
60
%
ass
40
Cu
]
20
[m
Cu
]
20
[m
[m
ass
%
80
60
Cu
RM > 70%
Fe
Fe
20
867
40
80
Sn Cu
(b) RCu
20
20
40
60
[mass%Sn]
80
Sn
(c) RSn
Fig. 5 Enrichment ratios of iron, copper and tin from Fe–Sn–Cu alloy resulting from the addition of 2.66 mass% boron at 1523 K.
addition of boron, the enrichment ratios of copper and tin are
high and the iron contained in the [Sn–Cu]-rich phase can be
easily removed by oxidation, which suggests that copper and
tin can be recovered effectively by using this method.
On the other hand, it is difficult to reuse the Fe-rich phase
as a ferrous resource after the separation, because a few
mass% of copper and tin are contained in the Fe-rich phase,
which has harmful effects on the mechanical properties of
steel. For practical use of the Fe-rich phase, it is necessary to
remove the copper and tin from steel or to dilute the copper
and tin with a virgin metal such as pig iron. On the other
hand, boron in steel can be removed by oxidation, in
principle. Moreover, boron greatly improves the mechanical
properties of steel, even with an ultra-low boron content.
Recently, the active use of boron has been tried in steel
production,15) and much research on the use of boron in steel
has been reported. For example, boron in steel is effective in
suppressing the surface hot shortness due to copper.16)
Accordingly, it is desirable to reuse the Fe-rich phase by
combining the removal and dilution of copper and tin with
improvements in the quality of steel by using an element such
as boron as an additive.
4.
Conclusions
Separation of Fe–Sn–B and Fe–Sn–Cu–B systems into two
liquid phases, an Fe phase and a Sn(–Cu) phase, has been
investigated at 1523 K. The following conclusions were
reached:
(1) In the Fe–Sn–B system, the tin content of the Fe-rich
phase and the iron content of the Sn-rich phase are 13.7
and 11.7 mass%, respectively, when [mass%B](in Fe) ¼
3:62. Boron widens the miscibility gap of an Fe–Sn
binary system.
(2) The isothermal section diagram of the Fe–Sn–Cu–
2.66 mass%B quaternary system at 1523 K is described.
In the Fe–Sn–Cu–B system, separation into Fe-rich and
[Sn–Cu]-rich phases is found over all mass ratios of
[mass%Sn]/[mass%Cu]. The separation region is en-
larged as the mass ratio of [mass%Sn]/[mass%Cu] is
decreased.
(3) By using the separation into two liquid phases, iron can
be enriched in the Fe-rich phase and copper and tin can
be enriched in the [Sn–Cu]-rich phase. The enrichment
ratios of copper and tin are higher than that of iron. It is
possible to recover copper and tin effectively from Fe–
Sn–Cu alloy.
REFERENCES
1) Yearbook of iron and steel, non-ferrous metal, and fabricated metals
statistics, eds. by Research and Statistics Department, Economic and
Industrial Policy Bureau, Ministry of Economy, Trade and Industry,
Tokyo, (2003).
2) K. Yamaguchi and Y. Takeda: Metall. Rev. MMIJ 15 (1998) 26–37.
3) S. E. Amara, A. Belhadj, R. Kesri and S. H. Thibault: Z. Metallkd. 90
(1999) 116–123.
4) K. Marukawa, T. Tanaka and S. Hara: Eng. Mater. 43 No. 3 (2000) 62–
65.
5) M. Yamaguchi and T. Takeda: JPN Published Patent Application,
P2004-83962A, (2004).
6) T. Yoshida, S. Ueda and K. Yamaguchi: CAMP-ISIJ 17 No. 4 (2004)
P3.
7) M. Hino, T. Nagasaka and T. Washizu: J. Phase Equilib. 20 (1999)
179–186.
8) A. Munitz: J. Mater. Sci. 30 (1995) 2901–2910.
9) A. Munitz, R. Abbachian, C. Cotler and C. Shacham: High Temp.
Mater. Process. 15 (1996) 187–194.
10) K. Taguchi, H. Ono-Nakazato and T. Usui: ISIJ Int. 46 (2005) 29–32.
11) K. Taguchi, H. Ono-Nakazato and T. Usui: ISIJ Int., submitted.
12) M. Uemoto and T. Nagasaki: Proceedings of the Annual Conference of
Tokyo Metropolitan Industrial Technology Research Institute, Tokyo,
Japan, (2004). (http://www.iri.metro.tokyo.jp/event/present/yousisyuu/
pdf/16/16-2-3.pdf)
13) Binary Alloy Phase Diagrams, 2nd ed., ASM International, (The
Materials Information Society, Material Park, Ohio, 1996), CD-ROM.
14) The 140th Committee of Japan Society for Promotion of Science:
Handbook of Physico-chemical Properties at High Temperature, eds.
by Y. Kawai and Y. Shiraishi, (ISIJ, Tokyo, 1988), 49.
15) K. Shibata: Tetsu-to-Hagané 89 (2003) 321.
16) C. Nagasaki, H. Uchino, K. Shibata, K. Asakura and M. Hatano: Tetsuto-Hagané 89 (2003) 322–328.