Theoretical Description of Electromagnetic Nonbonded Interactions
advertisement

J. Phys. Chem. C 2010, 114, 15315–15330
15315
Theoretical Description of Electromagnetic Nonbonded Interactions of Radical, Cationic,
and Anionic NH2BHNBHNH2 Inside of the B18N18 Nanoring
M. Monajjemi,*,†,# V. S. Lee,‡ M. Khaleghian,§ B. Honarparvar,† and F. Mollaamin⊥
Department of Chemistry, Science and Research Branch, Islamic Azad UniVersity, Tehran, Iran,
Computational Simulation and Modeling Laboratory (CSML), Department of Chemistry and Center for
InnoVation in Chemistry, Thailand Center of Excellence in Physics (ThEP), Faculty of Science, Chiang Mai
UniVersity, Chiang Mai, Thailand, Department of Chemistry, Islamshahr Branch, Islamic Azad UniVersity,
Islamshahr, Iran, and Department of Chemistry, Qom Branch, Islamic Azad UniVersity, Qom, Iran
ReceiVed: May 11, 2010; ReVised Manuscript ReceiVed: June 27, 2010
The electromagnetic nonbounded interactions of the NH2BHNBHNH2 molecule inside of the B18N18 ring
have been investigated with hybrid density functional theory (B3LYP) using the EPR-III and EPR-II basis
sets for a physicochemical explanation of electromagnetic nonbounded interactions within these nanosystems.
Optimized structures and hyperfine spectroscopic parameters such as total atomic charges, spin densities,
electrical potential, and isotropic Fermi coupling constants of radical, cationic, and anionic forms of the
NH2BHNBHNH2 molecule in different loops and bonds of the B18N18-NH2BHNBHNH2 systems have been
calculated. The correlations between structural, electronic, and spectral properties have been contributed to
identify the characteristics of hyperfine electronic structure. Besides structural characteristics, the lowest
unoccupied molecular orbital and the highest occupied molecular orbital for the lowest energy have been
derived to examine the structural stability of the B18N18-NH2BHNBHNH2 systems. We have also carried out
the calculation for the alanine-glycine amino acids coupled with the NH2BHNBHNH2 molecule inside of
the B18N18 ring (ALA-NH2BHNBHNH2-GLY) and obtained quantized transitional frequencies among the
forms of radical, anionic, and cationic. In a similar way, in B18N18-NH2BHNBHNH2, the three frequencies
have been yielded as νr-c ) 486948.498 GHz, νa-c ) 1792900.812 GHz, and νr-a ) 2507076.816 GHz. It
can be seen that all observed frequencies appeared in the IR and macrowave regions. It seems that the
B18N18-NH2BHNBHNH2 nonbonded system can be used for the measurement of rotational spectra related
to electrical voltage differences existing in a part of biomacromolecules. The radial coordinate of the dipole
moment vector (r) as well as the voltage differences (∆V) and relative energies (∆E) of the radical, anionic
and cationic forms of the NH2BHNBHNH2 in the B18N18-NH2BHNBHNH2 system exhibited Gaussian
distribution. The expectations of the ∆E and ∆V and r have been calculated from the Gaussian curves, which
have been fitted by various eigenvalues. In addition, the natural bond orbital (NBO) analysis has been performed,
which was informative to reveal some important atomic and structural features. Also, analysis of the NQR
hyperfine structure of the B18N18-NH2BHNBHNH2 system has been performed in terms of the electric field
gradient at each nitrogen nucleus, and the changes in the extent of electric charge distribution that accompanies
complex formation have been explored.
1. Introduction
Heterofullerenes became a subject of research interest soon
after the establishment of fullerene research itself.1-3 The
fullerenes containing boron and/or nitrogen atoms [refs 4-13
of ref 6] represent one distinguished class, though other elements
have been combined with the fullerenes too.4-6
Boron nitride (BN) is a synthetic III-V compound with
extraordinary mechanical, thermal, electrical, optical, and
chemical properties widely applied for technological purposes.1
Since BN units are isoelectronic with hexagonal BN possessing
a graphene-like layered structure, BN becomes the natural
* To whom correspondence should be addressed. E-mail: m_monajjemi@
cm.utexas.edu.
†
Science and Research Branch, Islamic Azad University.
‡
Chiang Mai University.
§
Islamshahr Branch, Islamic Azad University.
⊥
Qom Branch, Islamic Azad University.
#
Visiting Researcher: Department of Chemistry and Biochemistry,
Institute for Theoretical Chemistry, The University of Texas at Austin,
Austin, TX.
candidate to form heterofullerenes, which results in a certain
isomorphism. BN crystalline samples were synthesized at room
temperature and atmospheric pressure as structures containing
hexagonal sp2-bonded sheets isomorphic with graphene.7 BN
nanomaterials are expected in extentive application due to the
good stability at high temperatures with high electronic insulation in air.8 Despite the carbon nanotubes, BN nanotubes are
constant band gap materials and thus provide an attractive
opportunity for practical applications.9 The wide range of their
electronic properties from metallic to wide-gap semiconductors,
depending on their chemical composition, makes them suitable
candidates for nanosize electronic devices.10,11
Due to the similarity between B-N and C-C units, a lot of
effort has been devoted to BN fullerene-like materials in recent
years, which have excellent properties such as heat resistance,
insulation, and structural stability.12,13 Several studies have been
made on BN nanomaterials such as BN nanotubes, BN nanocapsules, and BN clusters since they have excellent properties
such as heat resistance in air and insulation, and these nano-
10.1021/jp104274z 2010 American Chemical Society
Published on Web 08/23/2010
15316
J. Phys. Chem. C, Vol. 114, No. 36, 2010
Monajjemi et al.
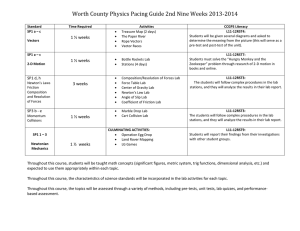
Figure 1. (a) The geometrical structure of the generation of our considered armchair nanotube (n ) m ) 6) through folding of a section of a
graphene sheet. (b) The optimized structure of the B18N18 ring at the B3LYP/EPR-III level of theory. (c) The optimized structure of alanineNH2BHNBHNH2-glysine at the B3LYP/EPR-III level of theory.
particles are expected to be useful as electronic devices, high
heat resistance semiconductors, and insulator lubricants.14-17
From the experimental standard formation enthalpy, the energies
of hybridized sp2 and sp3 B-N bonds are known to be stronger
in comparison with those of B-B and N-N bonds, namely,
4.00, 2.32, and 2.11 eV, respectively.18 Along with the
experimental efforts, extensive theoretical studies have also been
carried out on BN fullerenes to understand their relative stability
and size dependence of the properties.19-21 Several investigations
have dealt with the possibility of inorganic analogues of the
fullerene cages that would be constructed entirely of BN
pairs.22-25
Since the thermodynamic conditions for growth of BN
nanotubes from nuclei are still not well-defined, comprehensive
theoretical simulations on these nanotubes continue to attract
enhanced attention, and the lack of theoretical thermodynamic
data precludes a more detailed analysis.26
These nanotubes are found to be chiral or nonchiral; however,
a preference toward the armchair and zigzag configurations is
suggested. Electron energy loss spectroscopy yields a B/N ratio
of approximately 1 and a perfect chemical homogeneity.27 This
paper focuses on the tubes generated with the single-wall boron
nanotube (SWBNNT) from a MWNT ) 1 as an armchair
nanotube (n,m) with chirality n ) 6, m ) 6 and with a tube
NH2BHNBHNH2 Inside of the B18N18 Nanoring
length of 3 Å. The schematics of the generation of the
considered nanotube through folding of a section of a graphene
sheet and the optimized structure of the alanine-B18N18-glysine
are displayed in Figure 1, where C ) na1 + ma2 ) (n,m); a1
and a2 are the primitive lattice vectors of the graphene, and n
and m are integers.28,29
2. Computational Details
The geometry of the B18N18-NH2BHNBHNH2 system has
been optimized by Becke’s hybrid three-parameter exchange
functional and the nonlocal correlation functional of the Lee,
Yang,and Parr (B3LYP) method30,31 with the EPR-III and EPRII basis sets of Barone.32 The Gaussian quantum chemistry
package was used for all calculations.33 The optimization was
done along with a frequency calculation to verify that the
geometry was a real minimum without any imaginary frequency.
EPR-II is a double-ζ basis set with a single set of polarization
functions and an enhanced s part, (6,1)12,21 for H and (10,5,1)12,13,23
for B-F. EPR-III is a triple-ζ basis set including diffuse
functions, double d-polarizations, and a single set of fpolarization functions. Also in this case, the s-part is improved
to better describe the nuclear region, (6,2)10,13 for H and12,13,24,27
for B-F. Vibrational frequencies have been calculated at the
B3LYP/EPR-II level of theory to verify that the geometry was
a real minimum without any imaginary frequency and analyze
the thermochemical functions including enthalpies and Gibbs
free energies.34
In the current study, we have performed systematic firstprinciple calculations on the atomic and electronic nanostructures of the B18N18-NH2BHNBHNH2. Structure, stability, and
spectroscopic properties of this system have been explored. An
attempt is made to explain the anomalous nonbounded interactions of the NH2BHNBHNH2 molecule inside of the B18N18 ring
with a quantized nanospectrophotometer detection of various
quantized parameters of a given alanine-glysine amino acid.
In other words, a supposed picture of the electronic structure
of these magnetically unusual nanoparticles encouraged us to
imagine such a nanosystem as a quantized transition system
which would induce an electromagnetic field through electrostatic interaction of the NH2BHNBHNH2 molecule inside of
the B18N18 ring and also has the capability of detecting the
quantized parameters of the system considered as well as other
bimolecular amino acids which can be coupled with this system.
In other words, there is mutual electrostatic interaction between
the NH2BHNBHNH2 molecule and the B18N18 ring, which yields
the quantization of the radial component of the dipole moment
vector (r) as well as the voltage differences (∆V) and relative
energies (∆E) of the NH2BHNBHNH2 radical, cation, and anion.
The NH2BHNBHNH2 molecule moves among quantized coordinates of the radial component (r) of the dipole moment as
well as energy levels, and then, a specific spectrum would
appear. Therefore, when the NH2BHNBHNH2 is coupled with
two points of the amino acids inside of the B18N18 ring, different
radical, cationic, and anionic forms of the NH2BHNBHNH2 are
expected to appear due to the potential energy difference or
voltage caused by the NH2BHNBHNH2. Therefore, investigation
of the electrostatic interaction of the NH2BHNBHNH2 with its
surrounding ring along with exploring the variations of different
physicochemical properties such as dipole and quadropole
moments as well as NBO and NQR parameters of the B18N18NH2BHNBHNH2 system would be of great importance.
It has been demonstrated how this mechanistic question may
be addressed in the framework of modern electronic structure
methods, specifically with the B3LYP hybrid density functional
J. Phys. Chem. C, Vol. 114, No. 36, 2010 15317
method and EPR-III basis set. Natural bond orbital (NBO)
analysis has been employed to analyze the calculated electron
density in terms of localized Lewis structure and resonance
theoretical concepts.35 As a check on the quality of the calculated
geometrical parameters and their stability with respect to the
level of theory, the HOMO and the LUMO differences have
been explored.
In the course of determining hyperfine parameters and relating
them to the underlying electronic structure of the considered
system, anisotropic magnetic effects have been explained and
provided useful information on the interaction characteristics.26
The HOMO corresponds to a combination of lone pair orbitals
on the N atoms as well as the LUMO, which is characterized
by large contributions from vacant p orbitals on B atoms with
some admixture of N-based orbitals having been calculated.36
The NBO analysis has been performed by using NBO as
implemented in the Gaussian quantum chemistry package.35 The
asymmetry parameters as well as the quadrupole coupling
constant of nitrogen atoms involved in the B18N18NH2BHNBHNH2 system, which have been correlated with
atomic charges, have been computed.
The spin-spin magnetic hyperfine Hamiltonian as a part of
the molecular Hamiltonian can be presented as eq 1
HSS
hf )
µ0
gµ µ
4π S B N
∑
{
gR 3
i,R
f f
(Sfi · rf
iR)(IR · riR)
5
riR
-
(Sfi · IfR)
3
riR
+
}
8π
f f
f
× (Si · IR) · δ(3)(riR)
3
(1)
where gS and µB are the free electron g-factor and the Bohr
magneton, respectively, gS and µB are the nuclear g-factor and
the nuclear magneton, SfISi and IfR are “the spins of the electron
i and the nucleus R, and rf
IR represents the distance between an
electron i and nucleus R; i and R are referred to as electrons
and magnetic nuclei, respectively. This operator acts both in
the state space of the electrons and in the state space of the
nuclei. The anisotropic dipole-dipole interaction between the
electronic and nuclear spin magnetic moments is represented
by the first and the second parts of the considered equation.
The last term, the isotropic Fermi contact term, arises from the
magnetic field inside of the nucleus, created by its magnetic
moment. The terms in the effective Hamiltonian are obtained
after integration over electronic spatial coordinates; each term
contains angular momentum operators and molecular parameters.37
Th isotropic Fermi contact constant bF (in MHz) is defined
by
bF )
2µ0
g g µ µ |Ψ(0)| 2
3h S N B N
where bF ) b + c/3. Thus, the basic quantities that determine
the HF interaction at the Nth nucleus are those in brackets and
|Ψ(0)|N2 . The ab initio calculated isotropic constant, bF ) (2µ0/
3h)gSgNµBµNPS(N), directly depends on the Fermi contact spin
density function per unpaired electron at a nucleus.36
3. Results and Discussion
The aim of this section is to first discuss the different aspects
of the electronic structure of the B18N18-NH2BHNBHNH2
15318
J. Phys. Chem. C, Vol. 114, No. 36, 2010
Monajjemi et al.
TABLE 1: Calculated Relative Corrected Interaction BSSE
Energy (kcal/mol) for Cationic, Radical, and Anionic Forms
of NH2BHNBHNH2 in the B18-N18-NH2BHNBHNH2 System
within Transition
∆E (kcal/mol)
B18N18-NH2
BHNBHNH2 transition (Å)
anion
cation
radical
0.0
0.4
0.8
1.2
1.6
2.0
2.4
2.8
3.2
3.6
4.0
0
0.4029
1.7404
3.2098
4.2482
4.6999
5.0443
5.2242
5.0070
4.2051
2.9775
0
0.3367
1.4300
2.4105
2.6559
2.2783
1.4876
0.7574
0.2040
0.1613
0.0923
0
0.1352
1.2544
2.4820
3.6512
3.8760
3.7206
3.3487
2.5572
1.5174
0.6075
system for further validation of theoretical results to increase
their usefulness in practical applications or for pre-experimental
modeling. Second, we have explored the electromagnetic nature
of the B18N18-NH2BHNBHNH2 system by calculating the
following parameters, which provide valuable information on
the interaction characteristics.
3.1. Relative Energies. To verify the structural stability of
our considered B18N18-NH2BHNBHNH2 system, we have
optimized the B18N18-NH2BHNBHNH2 system using DFT
method (B3LYP) with both EPR-II and EPR-III basis sets.
Undoubtedly, since we have focused on electromagnetic induction of NH2BHNBHNH2 inside of the B18N18 ring, employing
these employed basis sets seemed useful and helped us find
logical relationships between obtained data. The calculated
energy (Hartree), relative energy (kcal/mol), and BSSE (kcal/
mol) corrected interaction energy (kcal/mol) for cationic, radical,
and anionic forms of NH2BHNBHNH2 in the B18N18NH2BHNBHNH2 system within transition are compared in
Table 1.
Strikingly, despite the intrinsic linearity of NH2BHNBHNH2 in
different radical, cationic, and anionic forms, in this step, the
obtained optimization results confirmed the stability of the
B18N18-NH2BHNBHNH2 system, and the NH2BHNBHNH2 molecule was located strictly in the center of the B18N18 ring vertically.
According to the frequency calculation at the B3LYP/EPR-II level
of theory, observing no negative frequency as well as obtaining
thermochemical functions such as ∆G ) -67.7929888325 kcal/
mol and ∆H ) -124.401248337 kcal/mol confirmed the
structural stability of the B18N18 ring. This effect is probably
due to the large dipole moments of the B-N bonds, which
preferentially enhance the ring stability. Regarding the system’s
stability within transitions and rotations of radical, cationicm
and anionic forms of NH2BHNBHNH2, it is notable that the
obtained barrier energies for the radical, cationic and anionic
forms were 3.876, 2.655, and 5.224 kcal/mol, respectively. The
graphs of rotational and transition energy barriers of radical,
anionic, and cationic forms of NH2BHNBHNH2 in the
B18N18-NH2BHNBHNH2 system are displayed in Figure 2. To
account for these observations, two observed points are notable. First, for radical, anionic, and cationic forms of
NH2BHNBHNH2, the most stable condition has been observed
in the case that NH2BHNBHNH2 is located exactly in the center
of the B18N18 ring, that is, the coordination of nitrogen atoms
was (0,0,0). Second, the reported BSSE data revealed that
despite insignificant changes of barrier energies based on the
plotted graphs, the entire trend has not changed essentially from
that of the first energy calculations. These obtained results
Figure 2. The graphs of the rotational and transitional BSSE energy
barriers of NH2BHNBHNH2 in the B18N18-NH2BHNBHNH2 system.
motivated us to investigate the rotation of NH2BHNBHNH2.
Therefore, we have rotated the center of NH2BHNBHNH2
around one of its axes. In this case, the barrier energy for the
radical form was significant (48.5091 kcal/mol). On the basis
of such a considerably high barrier energy, we have observed
that the radical form of NH2BHNBHNH2 strongly resists under
this rotation and exhibits no tendency for rotation in the
horizontal state.
It has been understood that the only possible movement which
probably caused the system’s structural distortion was internal
rotation of the radical form of NH2BHNBHNH2 inside of the
ring. It is evident that with such a high barrier energy, we could
not expect any rotation.
According to the plotted rotational graph (Figure 1), it has
been found out that the energy barrier of the NH2BHNBHNH2
radical stands as the highest value, and the following trend could
be observed
NH2BHNBHNH2 (radical) > NH2BHNBHNH2 (anion) >
NH2BHNBHNH2 (cation)
3.2. HOMO-LUMO Gap of the System. The LUMOHOMO band gap is a gap between the LUMO (the lowest
unoccupied molecular orbital) and HOMO (the highest occupied
molecular orbital).38 BN nanotubes have a wide band gap (E)
of ∼6 eV and nonmagnetism independent of the tube diameters.
The large LUMO-HOMO gap is often regarded as a molecule
stability condition.39 More sophisticated treatment of large gaps
is seen to occur for systems with high relative stability.40 The
band gap of the B18N18-NH2BHNBHNH2 system as the relative
differences in the energy of the HOMO and the LUMO is reported
in Table 2. According to the results in Table 2, in anionic and
radical forms of the NH2BHNBHNH2 molecule, the system showed
the highest structural stability compared with the cationic state. In
other words, the obtained values for the anionic and radical forms
NH2BHNBHNH2 Inside of the B18N18 Nanoring
J. Phys. Chem. C, Vol. 114, No. 36, 2010 15319
TABLE 2: Band Gap of the B18N18-NH2BHNBHNH2
System As the Relative Differences in the Energy of the
HOMO and LUMO in Atomic Units
band gap (HOMO-LUMO) (Hartree)
coordinates
anion
cation
radical
0.0,0.0,0.0
0.0,0.0,10.0
0.0,0.0,30.0
0.0,0.0,50.0
0.0,0.0,70.0
0.0,0.0,90.0
0.0,0.0,110.0
0.0,0.0,130.0
0.0,0.0,150.0
0.0,0.0,170.0
27.07986
27.08589
27.0925
27.08153
27.08313
27.08313
27.08092
27.08205
27.08533
27.08011
0.02235
0.02186
0.0161
0.18398
0.02201
0.02128
0.0231
0.02202
0.02202
0.02355
26.86293
26.87004
26.86282
26.86311
26.86818
26.87556
26.86287
26.86627
26.86849
26.86282
were 26-27 Hartree, which were significantly different from those
of the cationic form (0.0161-0.18339 Hartree). In these anionic
and radical cases, especially in the anionic form at the 0,0,30
coordinate, the highest HOMO-LUMO was at 27.0925 Hartree.
After inspecting the highest HOMO-LUMO band gaps in all
three radical, anionic, and cationic forms, it seems that in all
three considered cases, the highest ∆(HOMO-LUMO) values
and the highest stability occurred in the center coordinates and
with the B18N18 ring. Therefore, in the cation, anion, and radical
at the 0,0,50, 0,0,30, and 0,0,90 coordinates, the highest
HOMO-LUMO band gaps were 0.18398, 27.0925, and 26.87556
Hartree, respectively.
It is understood that in the case of the anionic form at the
0,0,70 and 0,0,90 coordinates, for the cationic form at 0,0,130
and 0,0,150, and for the radical form at 0,0,30 and 0,0,170, the
same HOMO-LUMO band gaps could be observed.
3.3. Natural Bond Orbital (NBO) Analysis. The concepts
of natural atomic orbital (NAO) and NBO analyses are useful
for distributing electrons into atomic and molecular orbitals used
for the one-electron density matrix to define the shape of the
atomic orbitals in the molecular environment and then derive
molecular bonds from electron density between atoms.
The NAOs will normally resemble the pure atomic orbitals
and may be divided into a natural minimal basis, corresponding to the occupied atomic orbitals for the isolated atom,
and a remaining set of natural Rydberg orbitals based on the
magnitude of the occupation numbers. The minimal set
of NAOs will normally be strongly occupied, while the
Rydberg NAO usually will be weakly occupied. There are
as many NAOs as the size of the atomic basis set, and the
number of Rydberg NAOs thus increases as the basis set is
enlarged .The results of NBO analysis at the B3LYP/EPRIII level of theory are listed in Table 3.
At each considered coordination, the bonding and antibonding
coefficients of s and p orbitals of B-N bonds were 0.5 and
0.8. However, for both the B37-N38 and B39-N40 bonds,
the constant coefficients of 0.3 and 0.9 have been yielded. On
the basis of the constant values of the coefficients of a linear
combination of s and p orbitals of different bonds (0.5 and 0.8),
a specific voltage difference could be expected.
It is observed that the percent of s and p orbitals for different
bonds of the NH2BHNBHNH2 anion in the B18N18NH2BHNBHNH2 system at all coordinations refers to sp2
hybridization for B as well as sp3 hybridization for the N atom,
which is in agreement with the intrinsic sp2 hybridization of B
and N atoms. The obtained relationship between NBO and ∆V
values of different bonds of the B18N18-NH2BHNBHNH2
system revealed that in the case of the NH2BHNBHNH2 radical,
the closeness of the obtained ∆V values (55.245 au) derived by
EPR calculations was the lowest value of ∆V compared with
those of the NH2BHNBHNH2 cation and anion. In other words,
the average value of ∆V in the case of the NH2BHNBHNH2
radical low average (∆V ) 55.245 au) revealed the sharp
Gaussian distribution and could be related with the constant
bonding molecular orbital coefficients. Meanwhile, the opposite
behavior has been seen especially for the NH2BHNBHNH2
cation. It is notable that these values were in accordance with
the estimation of the sp2 hybridization of the B atom derived
by NBO analysis, while such a direct relationship has not been
observed for the NH2BHNBHNH2 cation and anion.
3.4. Nuclear Quadrupole Resonance Parameters. The
results obtained in the hitherto studies confirmed the usefulness
of NQR spectroscopy for determination of physical and chemical
properties of compounds and prediction of their chemical
activity. Moreover, the spectroscopic EPR and NQR parameters
characterizing the electronic effects are correlated with the
activity of the B18N18-NH2BHNBHNH2 system studied. The
information inferred from the NQR study on the local electron
density distribution together with analysis of the charge distribution by the density functional methods provided suitable
means for determination of reactive sites of the B18N18NH2BHNBHNH2 system and hence indicated possible promising
directions to be followed in nanodevices.41,42
The asymmetry parameters and quadrupole coupling constants
of nitrogen atoms of the B18N18-NH2BHNBHNH2 system at
different coordinates are listed in Table 4. It can be seen that
the coupling constants of nitrogen atoms of all different
coordinates increased from 0,0,0 up to a maximum point and
then decreased to the lowest value. As a whole, it is understood
that the maximum amount of charge density on the nitrogen
nuclei was concentrated at the edges and in the center of the
B18N18 ring, and at these regions, the lowest asymmetry
parameters could be observed. Another point is that among
nitrogen atoms, the N38 of the anionic form with χ ) 3.773
MHz and the N40 with χ ) 3.578 MHz yielded the highest
coupling constant values. It is notable that such a high value of
χ and, consequently, a high charge density corresponded to
nitrogen atoms of the NH2BHNBHNH2 molecule inside of the
ring and at the 0,0,50 and 0,0,30 coordinates for the radical
and cationic forms, respectively.
3.5. Nonbonded Interaction of NH2BHNBHNH2 with the
B18N18 Ring. In this section, the major point is embedded
in the investigation of the electrostatic interaction of
NH2BHNBHNH2 with its surrounding B18N18 ring, which forms
the basis for more detailed studies of other systems with
nonbounded interactions. To investigate the electrostatic interaction on NH2BHNBHNH2 with six different segments including
six loops and six connecting bonds of the B18N18 ring within
the vertical transition, first, the five hexagon loops have been
freezed, and the electrostatic interaction of NH2BHNBHNH2
with the one remaining active loop has been considered. Other
loops have been examined one by one in the same way, and
the changes of all of the following calculated quantities have
been explored. Next, we were focused on each bond of B18N18
individually and evaluated the interaction of NH2BHNBHNH2
with each of the six connecting bonds of the B18N18 ring and
repeated the calculations along each bond.
3.5.1. Analysis of Dipole Moments. The only known mechanisms for the creation of dipole moments are by current loops
or quantum mechanical spin since the existence of monopoles
has never been experimentally demonstrated.43-45 On the other
hand, dipole expansions are used in the study of electromagnetic
fields of charge and current distributions. The efficiency of such
B37-N38
B37-N38
B37-N41
N38-B39
B39-N40
B39-N40
B37-N38
B37-N38
B37-N41
N38-B39
B39-N40
B39-N40
B37-N38
B37-N38
B37-N41
N38-B39
B39-N40
B39-N40
B37-N38
B37-N38
B37-N41
B37-N41
N38-B39
B39-N40
B39-N40
B37-N38
B37-N38
B37-N41
N38-B39
B39-N40
B39-N40
B37-N38
B37-N38
B37-N41
N38-B39
B39-N40
B39-N40
B37-N38
B37-N38
B37-N41
B37-N41
N38-B39
B39-N40
B39-N40
B37-N38
B37-N38
B37-N41
N38-B39
B39-N40
B39-N40
bond
BD(1)
BD(2)
BD(1)
BD(1)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(1)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(1)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(1)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(1)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(1)
BD(1)
BD(2)
cation
0.5033*(sp2.23d0.01)B + 0.8641*(sp1.00)N
0.3792*(sp99.99d2.91)B + 0.9253*(sp99.99d0.20f0.30)N
0.5253*(sp1.76)B + 0.8509*(sp1.38)N
0.8641*(sp1.00)N + 0.5033*(sp2.23d0.01)B
0.3795*(sp99.99d1.28)B + 0.9252*(sp99.99d0.08f0.12)N
0.5250*(sp1.77)B + 0.8511*(sp1.39d0.01)N
0.5022*(sp2.23d0.01)B + 0.8648*(sp1.00)N
0.5264*(sp1.74)B + 0.8502*(sp1.39d0.01)N
0.8647*(sp1.00)N + 0.5022*(sp2.23d0.01)B
0.5265*(sp1.74)B + 0.8502*(sp1.39d0.01)N
0.5017*(sp2.24d0.01)B + 0.8650*(sp1.00)N
0.5275*(sp1.73)B + 0.8496*(sp1.41d0.01)N
0.8650*(sp1.00)N + 0.5018*(sp2.24d0.01)B
0.5275*(sp1.73)B + 0.8495*(sp1.41d0.01)N
0.5033*(sp2.23)B + 0.8641*(sp1.00)N
0.3792*(sp1.00)B + 0.9253*(sp99.99d1.34f1.99)N
0.5255*(sp1.75)B + 0.8508*(sp1.38d0.01)N
0.8641*(sp1.00)N + 0.5033*(sp2.22)B
0.3790*(sp1.00)B + 0.9254*(sp1.00)N
0.5022*(sp2.23d0.01)B + 0.8648*(sp1.00)N
0.5265*(sp1.74)B + 0.8502*(sp1.39d0.01)N
0.8647*(sp1.00)N + 0.5022*(sp2.23d0.01)B
0.5265*(sp1.74)B + 0.8501*(sp1.39d0.01)N
0.5018*(sp2.24d0.01)B + 0.8650*(sp1.00)N
0.5271*(sp1.74)B + 0.8498*(sp1.41)N
0.8650*(sp1.00)N + 0.5018*(sp2.24d0.01)B
0.5271*(sp1.74)B + 0.8498*(sp1.41d0.01)N
0.5033*(sp2.23)B + 0.8641*(sp1.00)N
0.3792*(sp99.99d4.91)B + 0.9253*(sp99.99d0.28f0.43)N
0.5254*(sp1.76)B + 0.8509*(sp1.38d0.01)N
0.8641*(sp1.00)N + 0.5033*(sp2.23)B
0.3790*(sp99.99d8.03)B + 0.9254*(sp99.99d0.55f0.84)N
0.5254*(sp1.75)B + 0.8509*(sp1.38d0.01)N
0.5021*(sp2.24d0.01)B + 0.8648*(sp1.00)N
0.5264*(sp1.74)B + 0.8502*(sp1.39d0.01)N
0.8648*(sp1.00)N + 0.5021*(sp2.24d0.01)B
0.5265*(sp1.74)B + 0.8502*(sp1.39d0.01)N
-
anion
0.5021*(sp2.12)B + 0.8648*(sp1.00)N
0.3275*(sp99.99d7.31)B + 0.9449*(sp1.00)N
0.5008*(sp1.97)B + 0.8656*(sp1.22)N
0.8648*(sp1.00)N + 0.5021*(sp2.12)B
0.5007*(sp2.00)B + 0.8656*(sp1.22)N
0.3549*(sp99.99d0.73)B + 0.9349*(sp99.99d0.03f0.07)N
0.5021*(sp2.12)B + 0.8648*(sp1.00)N
0.3276*(sp99.99d8.71)B + 0.9448*(sp1.00)N
0.5009*(sp1.97)B + 0.8655*(sp1.22d0.01)N
0.8648*(sp1.00)N + 0.5021*(sp2.12)B
0.5008*(sp1.99)B + 0.8656*(sp1.22d0.01)N
0.3542*(sp99.99d0.88)B + 0.9352*(sp99.99d0.03f0.08)N
0.5195*(sp2.10)B + 0.8544*(sp1.00)N
0.3527*(sp1.00)B + 0.9357*(sp1.00)N
0.5181*(sp1.88)B + 0.8553*(sp1.31)N
0.8544*(sp1.00)N + 0.5195*(sp2.10)B
0.3552*(sp99.99d4.50)B + 0.9348*(sp99.99d0.11f0.49)N
0.5180*(sp1.89)B + 0.8554*(sp1.31)N
0.5021*(sp2.12)B + 0.8648*(sp1.00)N
0.3275*(sp1.00)B + 0.9449*(sp1.00)N
0.5015*(sp1.97)B + 0.8652*(sp1.21)N
0.8648*(sp1.00)N + 0.5021*(sp2.12)B
0.5016*(sp1.98)B + 0.8651*(sp1.21)N
0.3544*(sp99.99d2.76)B + 0.9351*(sp99.99d0.11f0.28)N
0.5021*(sp2.12)B + 0.8648*(sp1.00)N
0.3276*(sp99.99d6.55)B + 0.9448*(sp1.00)N
0.5005*(sp1.97)B + 0.8657*(sp1.23d0.01)N
0.8648*(sp1.00)N + 0.5021*(sp2.12)B
0.5004*(sp2.00)B + 0.8658*(sp1.23d0.01)N
0.3548*(sp99.99d0.63)B + 0.9349*(sp99.99d0.02)N
0.5193*(sp2.10)B + 0.8546*(sp1.00)N
0.3522*(sp1.00)B + 0.9359*(sp1.00)N
0.5181*(sp1.88)B + 0.8553*(sp1.30)N
0.8546*(sp1.00)N + 0.5193*(sp2.10)B
0.3547*(sp99.99d11.73)B + 0.9350*(sp99.99d0.27f1.27)N
0.5181*(sp1.88)B + 0.8553*(sp1.31)N
0.5021*(sp2.12)B + 0.8648*(sp1.00)N
0.3275*(sp99.99d27.22)B + 0.9448*(sp1.00)N
0.5013*(sp1.97)B + 0.8652*(sp1.21)N
0.8648*(sp1.00)N + 0.5021*(sp2.12)B
0.5014*(sp1.98)B + 0.8652*(sp1.21)N
0.3543*(sp99.99d1.80)B + 0.9351*(sp99.99d0.07f0.18)N
0.5021*(sp2.13)B + 0.8648*(sp1.00)N
0.3278*(sp99.99d7.98)B + 0.9447*(sp1.00)N
0.5007*(sp1.98)B + 0.8656*(sp1.22)N
0.8648*(sp1.00)N + 0.5021*(sp2.12)B
0.5006*(sp2.00)B + 0.8657*(sp1.22)N
0.3543*(sp99.99d0.78)B + 0.9351*(sp99.99d0.02f0.07)N
radical
0.5020*(sp2.13)B + 0.8649*(sp1.00)N
0.3277*(sp99.99d5.16)B + 0.9448*(sp1.00)N
0.5009*(sp1.97)B + 0.8655*(sp1.23)N
0.8649*(sp1.00)N + 0.5020*(sp2.13)B
0.5007*(sp2.01)B + 0.8656*(sp1.23)N
0.3571*(sp99.99d0.52)B + 0.9341*(sp99.99d0.02f0.05)N
0.5020*(sp2.13)B + 0.8649*(sp1.00)N
0.3278*(sp99.99d7.24)B + 0.9448*(sp1.00)N
0.5014*(sp1.97)B + 0.8652*(sp1.23d0.01)N
0.8649*(sp1.00)N + 0.5020*(sp2.13)B
0.5013*(sp1.99)B + 0.8653*(sp1.23d0.01)N
0.3561*(sp99.99d0.76)B + 0.9344*(sp99.99d0.02f0.07)N
0.5019*(sp2.13)B + 0.8649*(sp1.00)N
0.3280*(sp99.99d25.66)B + 0.9447*(sp1.00)N
0.5019*(sp1.97)B + 0.8650*(sp1.23d0.01)N
0.8649*(sp1.00)N + 0.5019*(sp2.13)B
0.5019*(sp1.98)B + 0.8649*(sp1.23d0.01)N
0.3554*(sp99.99d1.56)B + 0.9347*(sp99.99d0.05f0.16)N
0.5020*(sp2.13)B + 0.8649*(sp1.00)N
0.3277*(sp1.00)B + 0.9448*(sp1.00)N
0.5018*(sp1.97)B + 0.8650*(sp1.21)N
0.8648*(sp1.00)N + 0.5020*(sp2.13)B
0.5019*(sp1.98)B + 0.8649*(sp1.21)N
0.3558*(sp99.99d2.18)B + 0.9346*(sp99.99d0.08f0.22)N
0.5020*(sp2.13)B + 0.8649*(sp1.00)N
0.3277*(sp99.99d5.09)B + 0.9448*(sp1.00)N
0.5009*(sp1.97)B + 0.8655*(sp1.24d0.01)N
0.8649*(sp1.00)N + 0.5020*(sp2.13)B
0.5007*(sp2.01)B + 0.8656*(sp1.24d0.01)N
0.3568*(sp99.99d0.51)B + 0.9342*(sp99.99d0.02f0.05)N
0.5019*(sp2.13)B + 0.8650*(sp1.00)N
0.3280*(sp1.00)B + 0.9447*(sp1.00)N
0.5021*(sp1.97)B + 0.8648*(sp1.22d0.01)N
0.8650*(sp1.00)N + 0.5019*(sp2.13)B
0.5022*(sp1.97)B + 0.8648*(sp1.22d0.01)N
0.3550*(sp99.99d3.43)B + 0.9349*(sp99.99d0.09f0.36)N
0.5020*(sp2.13)B + 0.8649*(sp1.00)N
0.3278*(sp99.99d19.81)B + 0.9448*(sp1.00)N
0.5016*(sp1.97)B + 0.8651*(sp1.21)N
0.8649*(sp1.00)N + 0.5020*(sp2.13)B
0.5017*(sp1.98)B + 0.8650*(sp1.21)N
0.3560*(sp99.99d1.41)B + 0.9345*(sp99.99d0.05f0.14)N
0.5020*(sp2.13)B + 0.8649*(sp1.00)N
0.3279*(sp99.99d5.89)B + 0.9447*(sp1.00)N
0.5011*(sp1.97)B + 0.8654*(sp1.23d0.01)N
0.8649*(sp1.00)N + 0.5020*(sp2.13)B
0.5009*(sp2.00)B + 0.8655*(sp1.23d0.01)N
0.3566*(sp99.99d0.59)B + 0.9342*(sp99.99d0.02f0.06)N
J. Phys. Chem. C, Vol. 114, No. 36, 2010
0.0,0.0,130.0
0.0,0.0,110.0
0.0,0.0,90.0
0.0,0.0,70.0
0.0,0.0,50.0
0.0,0.0,30.0
0.0,0.0,10.0
0.0,0.0,0.0
orientations
NBO analysis
TABLE 3: NBO Analysis of the B18N18-NH2BHNBHNH2 System Considering Radical, Cationic, and Anionic Forms of NH2BHNBHNH2 with Different Coordinates at the B3LYP/
EPR-III Level of Theory
15320
Monajjemi et al.
radical
0.5019*(sp2.13)B + 0.8650*(sp1.00)N
0.3282*(sp1.00)B + 0.9446*(sp1.00)N
0.5022*(sp1.97)B + 0.8647*(sp1.21)N
0.8649*(sp1.00)N + 0.5019*(sp2.13)B
0.5023*(sp1.97)B + 0.8647*(sp1.21)N
0.3550*(sp99.99d6.18)B + 0.9349*(sp99.99d0.16f0.66)N
0.5019*(sp2.13)B + 0.8649*(sp1.00)N
0.3280*(sp1.00)B + 0.9447*(sp1.00)N
0.5021*(sp1.97)B + 0.8648*(sp1.20)N
0.8649*(sp1.00)N + 0.5019*(sp2.13)B
0.5022*(sp1.97)B + 0.8648*(sp1.20)N
0.3553*(sp99.99d6.06)B + 0.9347*(sp99.99d0.19f0.64)N
NH2BHNBHNH2 Inside of the B18N18 Nanoring
J. Phys. Chem. C, Vol. 114, No. 36, 2010 15321
a fast method is superior if the system is clustered and has large
density fluctuation.44 Therefore, the lack of experimental
demonstration and its importance in theoretical simulations was
a motivation for us to investigate dipole moments from a
theoretical point of view.
The coefficients of angular coordinates of multipole moment
are defined as a sum of following spherical harmonics
∞
f(θ, φ) )
l
∑ ∑ ClmYlm(θ, φ)
(2)
l)0 m)-l
Therefore, the electromagnetic potential can be obtained as
∞
V ) (r, θ, φ)
l
∑ ∑ Clm(r)Ylm(θ, φ) )
0.5019*(sp2.24d0.01)B + 0.8649*(sp1.00)N
0.5268*(sp1.74)B + 0.8500*(sp1.39d0.01)N
0.8649*(sp1.00)N + 0.5019*(sp2.24d0.01)B
0.5269*(sp1.74)B + 0.8500*(sp1.39d0.01)N
0.5033*(sp2.23d0.01)B + 0.8641*(sp1.00)N
0.3790*(sp99.99d5.73)B + 0.9254*(sp99.99d0.33f0.52)N
0.5253*(sp1.76)B + 0.8509*(sp1.37d0.01)N
0.8641*(sp1.00)N + 0.5033*(sp2.23d0.01)B
0.3788*(sp99.99d15.10)B + 0.9255*(sp99.99d1.09f1.71)N
0.5253*(sp1.75)B + 0.8509*(sp1.37d0.01)N
0.0,0.0,170.0
cation
anion
0.5020*(sp2.13)B + 0.8649*(sp1.00)N
0.3280*(sp1.00)B + 0.9447*(sp1.00)N
0.5016*(sp1.97)B + 0.8651*(sp1.21)N
0.8649*(sp1.00)N + 0.5020*(sp2.13)B
0.5016*(sp1.98)B + 0.8651*(sp1.21)N
0.3530*(sp99.99d4.59)B + 0.9356*(sp99.99d0.13f0.50)N
0.5020*(sp2.13)B + 0.8649*(sp1.00)N
0.3277*(sp1.00)B + 0.9448*(sp1.00)N
0.5016*(sp1.97)B + 0.8651*(sp1.20)N
0.8649*(sp1.00)N + 0.5020*(sp2.13)B
0.5016*(sp1.98)B + 0.8651*(sp1.19)N
0.3533*(sp99.99d6.04)B + 0.9355*(sp99.99d0.20f0.64)N
B37-N38
B37-N38
B37-N41
N38-B39
B39-N40
B39-N40
B37-N38
B37-N38
B37-N41
B37-N41
N38-B39
B39-N40
B39-N40
BD(1)
BD(2)
BD(1)
BD(1)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(2)
BD(1)
BD(1)
BD(2)
bond
orientations
TABLE 3: Continued
0.0,0.0,150.0
NBO analysis
i)0 m)-l
∞
∞
l
∑∑ ∑
m
Dl,j
j
r
j)1 l)0 m)-l
Ylm(θ, φ)
(3)
The tailor expansion of V(r - R) around the r ) 0 is
V(r - R) ) V(R) -
∑
R)x,y,z
raVR(R) +
∑ ∑
1
2 R)x,y,z
rRrβVaβ(R) - ... + ... (4)
β)x,y,z
where
VR(R) )
(
∂V(r - R)
∂rR
)
(
and VRβ(R) )
∂2V(r - R)
∂rR∂rβ
)
r)0
(5)
Therefore, the above equation can be considered as the
differential of V in terms of r.
3.5.2. Interaction of Two NonoWerlapping Parts of
NH2BHNBHNH2 and B18N18. The total electrostatic interaction
energy of the considered system (UNH2BHNBHNH2-B18N18) between
the two charge distributions of two B18N18 and NH2BHNBHNH2
molecules is
UB18N18-NH2BHNBHNH2 )
∑
µ∈B18N18
qµqV
4πε0rµV
V∈NH2BHNBHNH2
(6)
∑
As a consequence of the electrostatic B18N18-NH2BHNBHNH2
interaction, the charge distribution of the NH2BHNBHNH2
molecule inside of the B18N18 ring polarizes the B18N18 charge
distribution and induces the electromagnetic field in the
B18N18-NH2BHNBHNH2 system.
Considering rXY ) rY - rX, it can be defined as
RB18N18-NH2BHNBHNH2 + rNH2BHNBHNH2,V + rVµ - rµ,B18N18 ) 0
(7)
Since the two distributions do not overlap
0.502
1.873
0.202
2.303
0.217
2.233
0.495
1.885
0.213
2.319
0.207
2.320
0.497
1.911
0.197
2.328
0.192
2.308
0.502
1.876
0.201
2.302
0.217
2.232
0.497
1.885
0.216
2.317
0.210
2.318
0.498
1.910
0.200
2.328
0.196
2.308
0.469
3.769
0.413
3.483
0.413
3.479
0.507
1.858
0.207
2.312
0.220
2.243
0.493
1.902
0.216
2.319
0.211
2.314
0.503
1.905
0.193
2.350
0.188
2.302
0.505
1.857
0.208
2.313
0.220
2.245
0.493
1.902
0.214
2.321
0.209
2.315
0.502
1.908
0.191
2.350
0.185
2.302
0.471
3.771
0.405
3.564
0.405
3.566
N4
N41
0.499
1.899
0.183
2.296
0.195
2.319
0.502
1.859
0.222
2.307
0.216
2.324
0.494
1.914
0.211
2.308
0.206
2.317
0.503
1.894
0.182
2.294
0.194
2.318
0.497
1.853
0.219
2.311
0.213
2.328
0.489
1.920
0.210
2.312
0.205
2.320
1
3.763
0.426
3.384
0.426
3.391
0,0,30
0.503
1.904
0.178
2.302
0.197
2.375
0.509
1.848
0.218
2.276
0.213
2.320
0.490
1.914
0.210
2.312
0.209
2.324
0.502
1.906
0.175
2.302
0.194
2.373
0.510
1.849
0.219
2.274
0.213
2.319
0.491
1.914
0.213
2.311
0.213
2.322
0.472
3.773
0.404
3.578
0.404
3.572
0,0,50
0.493
1.915
0.191
2.313
0.202
2.329
0.505
1.875
0.215
2.230
0.203
2.298
0.496
1.886
0.212
2.320
0.216
2.320
0.501
1.910
0.194
2.309
0.207
2.324
0.511
1.866
0.212
2.228
0.201
2.296
0.490
1.880
0.207
2.325
0.211
2.324
0.468
3.768
0.412
3.489
0.412
3.500
0,0,70
0.493
1.915
0.191
2.313
0.202
2.329
0.505
1.875
0.215
2.230
0.203
2.298
0.496
1.886
0.212
2.320
0.216
2.320
0.501
1.910
0.194
2.309
0.207
2.324
0.511
1.866
0.212
2.228
0.201
2.296
0.490
1.880
0.207
2.325
0.211
2.324
0.468
3.768
0.412
3.489
0.412
3.500
0,0,90
anionic
0.491
1.913
0.213
2.321
0.214
2.309
0.503
1.904
0.202
2.374
0.182
2.300
0.508
1.850
0.214
2.320
0.210
2.286
0.490
1.913
0.210
2.323
0.210
2.310
0.502
1.906
0.199
2.372
0.178
2.300
0.509
1.851
0.214
2.318
0.210
2.285
0.470
3.768
0.404
3.567
0.404
3.562
0,0,110
0.496
1.890
0.212
2.318
0.209
2.319
0.498
1.910
0.205
2.325
0.193
2.307
0.503
1.871
0.207
2.299
0.205
2.248
0.496
1.890
0.215
2.317
0.211
2.318
0.499
1.907
0.206
2.325
0.193
2.307
0.501
1.871
0.208
2.300
0.205
2.249
0.469
3.765
0.407
3.520
0.408
3.521
0,0,130
0.500
1.865
0.213
2.310
0.212
2.323
0.491
1.916
0.215
2.307
0.204
2.318
0.499
1.896
0.191
2.293
0.198
2.320
0.500
1.864
0.213
2.310
0.213
2.323
0.492
1.915
0.214
2.306
0.203
2.318
0.498
1.897
0.191
2.294
0.198
2.321
0.465
3.757
0.414
3.444
0.415
3.441
0,0,150
0.504
1.858
0.205
2.286
0.216
2.315
0.490
1.912
0.212
2.310
0.210
2.321
0.501
1.906
0.183
2.298
0.198
2.371
0.505
1.859
0.206
2.283
0.216
2.313
0.491
1.911
0.214
2.308
0.212
2.320
0.502
1.904
0.186
2.299
0.200
2.371
0.471
3.764
0.402
3.572
0.402
3.575
0,0,170
0.503
1.939
0.261
2.370
0.218
2.354
0.511
1.945
0.236
2.359
0.211
2.377
0.454
1.953
0.281
2.489
0.228
2.432
0.502
1.940
0.262
2.369
0.218
2.356
0.510
1.947
0.240
2.353
0.214
2.372
0.455
1.955
0.282
2.489
0.229
2.428
0.358
8.992
0.061
3.538
0.062
3.537
0,0,0
0.476
1.949
0.269
2.391
0.234
2.374
0.522
1.943
0.238
2.350
0.209
2.381
0.464
1.948
0.251
2.435
0.222
2.416
0.475
1.949
0.269
2.392
0.236
2.375
0.522
1.944
0.240
2.345
0.211
2.377
0.465
1.949
0.252
2.435
0.223
2.412
0.355
9.091
0.024
3.471
0.025
3.471
0,0,10
0.432
1.949
0.285
2.429
0.329
2.507
0.545
1.936
0.207
2.388
0.232
2.368
0.491
1.927
0.214
2.413
0.196
2.435
0.431
1.947
0.288
2.431
0.334
2.505
0.547
1.935
0.207
2.387
0.228
2.373
0.489
1.929
0.216
2.412
0.198
2.433
0.353
9.463
0.030
3.424
0.029
3.421
0,0,30
0.443
1.961
0.234
2.428
0.325
2.542
0.524
1.934
0.219
2.357
0.257
2.365
0.501
1.948
0.219
2.368
0.228
2.372
0.442
1.960
0.234
2.429
0.324
2.545
0.523
1.936
0.217
2.360
0.260
2.362
0.500
1.950
0.221
2.363
0.232
2.365
0.358
9.013
0.065
3.564
0.065
3.561
0,0,50
0.443
1.961
0.234
2.428
0.325
2.542
0.524
1.934
0.219
2.357
0.257
2.365
0.501
1.948
0.219
2.368
0.228
2.372
0.442
1.960
0.234
2.429
0.324
2.545
0.523
1.936
0.217
2.360
0.260
2.362
0.500
1.950
0.221
2.363
0.232
2.365
0.358
9.013
0.065
3.564
0.065
3.561
0,0,70
0.490
1.945
0.216
2.394
0.233
2.367
0.434
1.958
0.304
2.491
0.267
2.408
0.530
1.945
0.250
2.357
0.211
2.396
0.490
1.937
0.221
2.386
0.225
2.372
0.438
1.947
0.308
2.469
0.261
2.431
0.534
1.940
0.240
2.370
0.224
2.378
0.357
9.137
0.013
3.373
0.010
3.382
0,0,90
cationic
0.501
1.950
0.231
2.363
0.224
2.359
0.444
1.962
0.322
2.536
0.234
2.431
0.520
1.936
0.255
2.384
0.218
2.371
0.501
1.949
0.230
2.364
0.225
2.359
0.444
1.962
0.322
2.537
0.235
2.431
0.520
1.936
0.255
2.380
0.217
2.373
0.356
8.995
0.062
3.550
0.063
3.547
0,0,110
0.524
1.943
0.234
2.344
0.222
2.369
0.464
1.954
0.259
2.431
0.224
2.406
0.474
1.948
0.277
2.430
0.237
2.383
0.524
1.944
0.234
2.344
0.220
2.372
0.464
1.953
0.260
2.427
0.221
2.411
0.474
1.946
0.276
2.433
0.239
2.380
0.355
9.089
0.041
3.502
0.042
3.499
0,0,130
0.524
1.943
0.234
2.344
0.222
2.369
0.464
1.954
0.259
2.431
0.224
2.406
0.474
1.948
0.277
2.430
0.237
2.383
0.524
1.944
0.234
2.344
0.220
2.372
0.464
1.953
0.260
2.427
0.221
2.411
0.474
1.946
0.276
2.433
0.239
2.380
0.355
9.089
0.041
3.502
0.042
3.499
0,0,150
0.512
1.942
0.220
2.379
0.251
2.395
0.502
1.951
0.222
2.366
0.230
2.365
0.444
1.961
0.236
2.454
0.309
2.528
0.512
1.941
0.221
2.376
0.251
2.398
0.503
1.950
0.223
2.364
0.229
2.365
0.445
1.961
0.237
2.452
0.308
2.527
0.356
8.959
0.068
3.541
0.068
3.539
0,0,170
J. Phys. Chem. C, Vol. 114, No. 36, 2010
N40
N38
N36
N34
N32
N31
N29
N28
N25
N22
N20
N19
N17
N16
N13
N10
N8
N7
N5
0,0,10
0,0,0
atoms
χ (MHz)
η
TABLE 4: NQR Parameters of Nitrogen Atoms of B18N18-NH2BHNBHNH2 in the Anionic, Cationic, and Radical Forms at the B3LYP/EPR-III Level of Theory
15322
Monajjemi et al.
0.476
1.973
0.269
2.350
0.234
2.284
0.522
1.976
0.238
2.353
0.209
2.356
0.464
1.988
0.251
2.382
0.222
2.358
0.475
1.973
0.269
2.350
0.236
2.284
0.522
1.975
0.240
2.352
0.211
2.356
0.465
1.988
0.252
2.383
0.223
2.358
0.355
3.760
0.024
3.429
0.025
3.429
0.498
1.965
0.258
2.350
0.276
2.283
0.490
1.975
0.254
2.358
0.251
2.354
0.471
1.988
0.258
2.415
0.243
2.360
0.498
1.965
0.258
2.350
0.276
2.283
0.490
1.975
0.254
2.358
0.252
2.354
0.471
1.988
0.258
2.416
0.243
2.360
0.463
3.762
0.394
3.532
0.394
3.531
N4
N41
N40
N38
N36
N34
N32
N31
N29
N28
N25
N22
N20
N19
N17
N16
N13
N10
N8
N7
N5
0,0,10
0,0,0
atoms
TABLE 4: Continued
0.432
1.968
0.285
2.318
0.329
2.348
0.545
1.979
0.207
2.350
0.232
2.360
0.491
1.990
0.214
2.358
0.196
2.441
0.431
1.968
0.288
2.317
0.334
2.348
0.547
1.979
0.207
2.350
0.228
2.360
0.489
1.990
0.216
2.358
0.198
2.440
0.353
3.761
0.030
3.540
0.029
3.539
0,0,30
0.462
1.989
0.237
2.364
0.265
2.446
0.510
1.960
0.267
2.306
0.258
2.352
0.485
1.979
0.252
2.352
0.248
2.362
0.463
1.989
0.237
2.364
0.266
2.445
0.510
1.960
0.267
2.306
0.258
2.352
0.485
1.979
0.252
2.352
0.248
2.361
0.464
3.766
0.392
3.558
0.393
3.556
0,0,50
0.474
1.988
0.244
2.360
0.263
2.380
0.485
1.968
0.276
2.284
0.258
2.348
0.495
1.976
0.250
2.357
0.253
2.354
0.474
1.987
0.244
2.360
0.263
2.379
0.485
1.968
0.276
2.284
0.258
2.348
0.496
1.976
0.251
2.357
0.253
2.354
0.460
3.760
0.396
3.447
0.397
3.447
0,0,70
0.481
1.984
0.244
2.362
0.257
2.351
0.460
1.986
0.268
2.380
0.250
2.354
0.508
1.968
0.253
2.357
0.257
2.340
0.481
1.984
0.244
2.362
0.257
2.350
0.460
1.986
0.268
2.380
0.250
2.354
0.508
1.968
0.253
2.357
0.257
2.340
0.456
3.754
0.404
3.341
0.404
3.342
0,0,90
radical
0.485
1.978
0.249
2.361
0.253
2.351
0.462
1.990
0.270
2.445
0.241
2.361
0.510
1.962
0.260
2.351
0.258
2.316
0.485
1.978
0.248
2.361
0.253
2.351
0.463
1.989
0.270
2.445
0.241
2.361
0.510
1.962
0.260
2.351
0.258
2.316
0.463
3.762
0.393
3.543
0.393
3.542
0,0,110
0.495
1.973
0.254
2.353
0.252
2.356
0.474
1.986
0.263
2.383
0.244
2.360
0.484
1.973
0.263
2.343
0.268
2.296
0.495
1.973
0.254
2.353
0.252
2.357
0.475
1.986
0.264
2.382
0.244
2.360
0.484
1.973
0.263
2.343
0.267
2.296
0.460
3.756
0.394
3.473
0.394
3.472
0,0,130
χ (MHz)
η
0.505
1.970
0.255
2.340
0.255
2.356
0.480
1.986
0.259
2.351
0.246
2.361
0.463
1.987
0.254
2.351
0.265
2.386
0.505
1.970
0.255
2.340
0.255
2.356
0.480
1.985
0.259
2.351
0.246
2.361
0.463
1.987
0.253
2.351
0.265
2.386
0.457
3.754
0.397
3.390
0.397
3.388
0,0,150
0.504
1.968
0.254
2.318
0.263
2.348
0.485
1.979
0.254
2.350
0.249
2.360
0.464
1.990
0.244
2.358
0.266
2.441
0.504
1.968
0.254
2.317
0.264
2.348
0.485
1.979
0.254
2.350
0.249
2.360
0.464
1.990
0.244
2.358
0.266
2.440
0.463
3.761
0.392
3.540
0.392
3.539
0,0,170
NH2BHNBHNH2 Inside of the B18N18 Nanoring
J. Phys. Chem. C, Vol. 114, No. 36, 2010 15323
15324
J. Phys. Chem. C, Vol. 114, No. 36, 2010
Monajjemi et al.
|RB18N18-NH2BHNBHNH2 | > |rB18BHNBHNH2,V - rB18N18,µ |
(8)
The Laplace expansion could be considered as
1
)
|rV - rµ |
1
)
|RB18N18-NH2BHNBHNH2 - (rB18N18,µ - rNH2BHNBHNH2,V)|
∞
L
∑ ∑
L)0 M)-L
(-1)MIL-M(RB18N18-NH2BHNBHNH2) ×
RLM(rB18N18,µ
(9)
- rNH2BHNBHNH2,V)
whereILM and RLM are irregular and regular solid harmonics,
respectively. The dipole moment can be measured by a variety
of experimental methods or computed with an atomic charge
distribution directly derived from molecular orbital calculations,
as well as the interaction energy of two B18N18 and
NH2BHNBHNH2 charge distributions at a distance of
RB18N18-NH2BHNBHNH2 apart. Since
B18N18+mNH2BHNBHNH2)
Il-(m
(RB18N18-NH2BHNBHNH2) )
B N +lNH BHNBHNH
18 18
2
[
2
4π
2lB18N18 + 2lNH2BHNBHNH2 + 1
]
1/2
×
B18N18+mNH2BHNBHNH2)
Yl-(m
(R̂B18N18-NH2BHNBHNH2)
B N +lNH BHNBHNH
18 18
2
2
NH2BHNBHNH2+1
RBlB1818NN1818+l
-NH2BHNBHNH2
(10)
A dipole moment which appears to be due to an electric charge
distribution usually involves powers (or inverse powers) of the
distance to the origin (r) as well as some angular dependence
(Θ and Φ), where Θ is the angle with the x and y axes and Φ
is the angle with the vertical axis inside of the ring.44,45 The
dipole moment converges under two conditions, (1) if the
charges are localized close to the origin and the point at which
the potential is observed is far from the origin where the
coefficients of the series expansion are called exterior dipole
moments or simply dipole moments and (2) if the charges
are located far from the origin and the potential is observed
close to the origin, namely, interior dipole moments. The
importance of this quantity is embedded in the fact that the
potential at a position within a charge distribution can often be
computed by combining interior and exterior dipoles.43-45 When
a single NH2BHNBHNH2 molecule is just supposed, the Θ )
Φ ) 0, that is, the dipole vector, is expected to be coincident
on the NH2BHNBHNH2 axis. According to obtained dipole
moments, it can be distinguished that the r component of the
dipole moment vector of each the radical, cationic, and anionic
forms of NH2BHNBHNH2 involved in the ring had the tendency
to rotate in three different cone surfaces. Therefore, it could be
realized that our observed dipole moment has been directed
linearly, and this observation supported the intrinsic linear form
of the NH2BHNBHNH2 molecule.
In this regard, it seems that if a biomolecule is being set in
the B18N18-NH2BHNBHNH2 system due to generation of
radical, anion, and cation forms of NH2BHNBHNH2, the
electrical current will cross along the ring that changes all
calculated atomic physicochemical properties. Here, it is notable
that the three emerged radical, cationic, and anionic forms of
NH2BHNBHNH2 generate frequently to each other, and if these
three species are imagined in the three quantized cone surfaces,
it can be deduced that the variation of the radial vector of
system’s dipole moment (r) would be quantized within crossing
of these three cone levels.
An induced dipole of any polarizable charge distribution F
of the NH2BHNBHNH2 molecule has been caused by an electric
field external to F that originated from an ion or polar molecule
in the vicinity of F .The strength of the induced dipole is equal
to the product of the strength of the external field and the dipole
polarizibility of F. Therefore, along with the variation of the
radial component (r), the two other remaining components of
the dipole moment, namely, Θ and Φ, will be changed and cause
the quantized rotation of the NH2BHNBHNH2 molecule due
to the electrical charge of NH2BHNBHNH2. Its induced
electrostatic interaction on the ring will be affected, and the
rotation of the B18N18 ring will also be expected to be quantized.
On the other hand, for a dipole moment (m), the energy of the
dipole interaction (U) is defined as43-45
U ) -m · B
(11)
Supposing eq 11, the logical variation of the dipole moment
at different rotational angles of the NH2BHNBHNH2 radical
was satisfactory. The average value of the dipole moment vector
(r) for anion, cation, and radical forms of NH2BHNBHNH2 has
been obtained as 10.842, 5.258, and 3.302 D, respectively. Along
with the high values of Θ and Φ, the r of the dipole moment
holds a Gaussian distribution; this fact can be observed in the
plotted Gaussian graphs of the dipole moment (r) versus the Θ
and Φ angles (Figure 3). Here, it is interesting that for each
radical, cation, and anion of NH2BHNBHNH2, three individual
expectation values of ⟨∆E⟩, ⟨∆V⟩, and ⟨∆r⟩ have been obtained,
and as a whole, it seems that the r component of the system’s
dipole moment, voltage differences, and relative energies is
quantized, and the system undergoes quantization through
rotation.
3.6. Electromagnetic Hyperfine Parameters. In this section,
the major point is embedded in the investigation of the
electrostatic interaction of NH2BHNBHNH2 with its surrounding
B18N18 ring, which forms the basis for more detailed studies of
other systems with nonbounded interactions. Total atomic
charges, spin densities, electric potential, and isotropic Fermi
coupling constants of cationic and anionic forms of
NH2BHNBHNH2 in different loops and bonds of the B18N18
system are reported in Table 5.
The expectation values of the quantized radical coordinate
of the dipole moment, voltage differences (au), and relative
energies of B18N18-NH2BHNBHNH2 systems are displayed in
Figure 3. Also, the relative energies (∆E), radial coordinate of
the dipole moment (r), as well as the voltage differences (∆V)
and transition of the B18N18-NH2BHNBHNH2 and B18N18-AlaNH2BHNBHNH2-Gly systems are given in Tables 6 and 7,
respectively.
The voltage differences of the anionic form of the
NH2BHNBHNH2 molecule for each bond were scattered
compared with those of the NH2BHNBHNH2 cationic and
radical forms and yielded the highest values (78.62-183.41 au).
In the case of the cationic form of NH2BHNBHNH2, the bonding
∆V values were close together and were between those of
anionic and radical forms (70.90-82.91 au).
NH2BHNBHNH2 Inside of the B18N18 Nanoring
J. Phys. Chem. C, Vol. 114, No. 36, 2010 15325
Figure 3. The Gaussian distributions and expectation values of the quantized radical coordinate of the dipole moment, voltage differences (au) and
relative energies of B18N18-NH2BHNBHNH2 systems at the B3LYP/EPR-III level of theory.
The bonding ∆V of the NH2BHNBHNH2 radical was lower
than those of the anionic and cationic forms, and the negative
values have been found for bond 6 and bond 3 (-8.77 and
-72.24 au).
The graphs of ∆V values of the anion, cation, and radical
versus θ are exhibited in Figure 4. In each case, linear
relationships have been found between ∆V and θ values. An
approximate coincidence has been observed between the cationic
and radical forms, and it is notable that at θ ) 88.32 and 95.15,
which correspond to the negative bonding voltages (∆V ) -8.77
and 12.24 au, respectively), the two figures crossed each other.
However, in the case of the NH2BHNBHNH2 anion, the
variation of θ had no effect on the bonding ∆V for the cation’s
two broadened picks (at θ ) 90.79 and ∆V ) 76.79 au) and
for the radical’s single broad Gaussian curve (at θ ) 0.99 and
∆V ) 148.05). A similar trend with a minimum pick could be
observed for the NH2BHNBHNH2 radical and cationic forms,
and conversely, the maximum belonged to the NH2BHNBHNH2
anion.
The graphs of the isotropic Fermi constants versus the spin
densities in each loop of the B18N18-NH2BHNBHNH2 system
are exhibited in Figure 5a and b. The two distinct trends among
the various loops of the B18N18-NH2BHNBHNH2 anion could
be observed. In more detail, dished and bulged points could be
distinguished for even and odd loops, respectively.
B(1)
B(2)
N(4)
N(34)
B(35)
N(36)
B(3)
N(4)
B(3)
N(5)
B(6)
N(7)
N(8)
B(9)
N(8)
B(11)
N(10)
B(11)
B(12)
N(13)
B(14)
N(16)
B(15)
N(16)
B(15)
N(17)
B(18)
N(19)
N(20)
B(21)
N(20)
B(23)
N(22)
B(23)
B(24)
N(25)
B(26)
N(28)
B(27)
N(28)
B(27)
N(29)
B(30)
N(31)
N(32)
B(33)
N(32)
B(35)
0.675284
0.591562
-0.89845
-0.98373
0.695921
-1.02612
0.071167
-0.92833
0.690514
-1.00033
0.730436
-1.12252
-0.90231
0.656619
-0.95917
0.195710
-1.04827
0.694175
0.688249
-1.06352
0.675016
-0.90220
0.091168
-0.90299
0.695482
-0.98443
0.675554
-1.02635
-0.89783
0.591673
-0.93487
0.079622
-1.00051
0.690371
0.730313
-1.12344
0.657002
-0.90102
0.104765
-0.89361
0.694157
-1.04696
0.688494
-1.06391
-0.90326
0.675468
-0.88202
0.093133
total
atomic
charges
0.087011
0.109841
-0.02246
0.138450
0.530512
0.146993
0.595556
0.529990
0.557910
0.128953
0.078658
0.146106
-0.01073
0.093774
0.834190
0.389747
0.182759
0.520889
0.075684
0.180201
0.077424
-0.03989
0.813937
0.366837
0.530718
0.139249
0.086817
0.147012
-0.02278
0.109707
0.624585
0.498282
0.129061
0.557756
0.078699
0.146824
0.093860
-0.01166
0.756039
0.452083
0.520844
0.181703
0.075560
0.180575
-0.03906
0.077323
0.470138
0.734027
-11.6155
-11.6164
-18.5947
-18.6168
-11.5953
-18.6171
-11.6261
-18.6167
-11.5945
-18.6170
-11.6136
-18.6150
-18.5958
-11.6152
-18.5751
-11.5970
-18.6240
-11.6032
-11.6211
-18.6237
-11.6215
-18.6018
-11.6189
-18.6071
-11.5952
-18.6168
-11.6154
-18.6170
-18.5946
-11.6164
-18.6150
-11.6266
-18.6170
-11.5946
-11.6136
-18.6150
-11.6152
-18.5958
-11.6176
-18.5850
-11.6034
-18.6242
-11.6212
-18.6239
-18.6019
-11.6216
-18.5885
-11.6160
82.70281
81.19617
16.06927
50.92149
279.8979
49.02775
143.2242
-4.95392
275.1061
48.08880
77.92125
48.91770
13.49543
77.78502
2.86549
89.36184
49.39768
274.2737
78.46085
49.18305
78.54765
14.45283
191.4932
-8.36072
279.8553
50.89899
82.66915
49.03377
16.05581
81.16706
-2.44963
128.2253
48.08775
275.0559
77.92315
48.91758
77.79908
13.49334
174.5651
-7.01310
274.2811
49.39048
78.46328
49.17238
14.45320
78.55123
-6.91405
166.3131
electric isotropic Fermi
potential coupling MHz
∆V )
V2 - V1
total
atomic
charges
0.99
156.18
0.99
125.27
0.99
95.96
0.99
64.99
0.99
37.38
0.99
6.35
0.99
24.25
0.99
54.71
0.99
80.59
0.99
115.07
0.99
143.73
0.99
173.71
φ
145.79
131.68
165.93
148.05
78.62
183.41
0.725051
0.677126
-0.70608
-0.71460
0.860079
-0.71373
0.530478
-0.34314
0.849106
-0.74044
0.785697
-0.82325
-0.71234
0.745644
-0.30546
0.593936
-0.73598
0.851300
0.755150
-0.74603
0.747836
-0.67543
0.525317
-0.32706
0.860698
-0.71431
0.725552
-0.71364
-0.70382
0.677482
-0.33832
0.520910
-0.73996
0.848913
0.785423
-0.82326
0.745692
-0.71286
0.562499
-0.35556
0.861636
-0.72340
0.761249
-0.72938
-0.64668
0.756490
-0.28129
0.599537
-
-11.2592
-11.2593
-18.2721
-18.2563
-11.1753
-18.2417
-11.1522
-18.1693
-11.1789
-18.2528
-11.2643
-18.2485
-18.2928
-11.2664
-18.1058
-11.1302
-18.2611
-11.1799
-11.2597
-18.2671
-11.2601
-18.2460
-11.1600
-18.1688
-11.1746
-18.2557
-11.2584
-18.2411
-18.2700
-11.2584
-18.1658
-11.1564
-18.2525
-11.1790
-11.2644
-18.2483
-11.2666
-18.2934
-11.1594
-18.1566
-11.1662
-18.2449
-11.2442
-18.2473
-18.2201
-11.2445
88.72
2.79
89.43
62.10
90.18
48.67
90.30
141.79
93.10
104.45
93.40
156.41
90.79
170.50
90.80
111.32
90.46
124.13
89.51
38.98
86.81
76.07
86.63
24.23
φ
θ
θ
total
isotropic
atomic
Fermi
spin
electric coupling
densities potential MHz
dipole
orientation
cation
dipole
orientation
82.91
70.90
75.11
76.79
78.01
73.97
∆V ) V2 V1
0.737816
0.684236
-0.73264
-0.76079
0.850185
-0.77635
0.563853
-0.57495
0.855647
-0.77442
0.784624
-0.87397
-0.72616
0.739976
-0.51690
0.518013
-0.77216
0.835288
0.745643
-0.78105
0.740220
-0.75089
-0.47333
-1.46360
0.849841
-0.76056
0.738039
-0.77664
-0.73258
0.684131
-0.60438
0.538637
-0.77442
0.855627
0.784467
-0.87399
0.740291
-0.72597
0.517304
-0.51677
0.835479
-0.77218
0.745799
-0.78116
-0.75136
0.740700
-1.44748
-0.54586
total
atomic
charges
0.000109
0.000227
0.000013
0.002103
-0.00091
0.000010
-0.07237
0.056951
-0.00016
-0.00046
-0.00088
0.002307
-0.00001
0.000664
-0.15202
0.152977
0.000160
-0.00077
-0.00092
0.000223
-0.00084
0.000921
-0.03485
-0.02805
-0.00087
0.002112
0.000077
0.000045
0.000036
0.000198
0.040675
-0.09240
-0.00046
-0.00015
-0.00090
0.002309
0.000662
-0.00005
0.153492
-0.15241
-0.00083
0.000149
-0.00096
0.000235
0.000977
-0.00088
-0.01138
0.004108
total
atomic
spin
densities
-11.3898
-11.3911
-18.3875
-18.4059
-11.3118
-18.4040
-11.2783
-18.3886
-11.3107
-18.4021
-11.3897
-18.4048
-18.3896
-11.3903
-18.3357
-11.2997
-18.4092
-11.3170
-11.3956
-18.4095
-11.3956
-18.3941
-12.0626
-19.3815
-11.3117
-18.4059
-11.3898
-18.4039
-18.3875
-11.3911
-18.4230
-11.3021
-18.4022
-11.3107
-11.3897
-18.4048
-11.3903
-18.3896
-11.3005
-18.3362
-11.3171
-18.4094
-11.3957
-18.4096
-18.3942
-11.3958
-19.3868
-12.1017
-0.02108
-0.04224
0.01514
0.16408
-0.24277
-0.02153
-63.0528
3.58130
-0.35774
-0.04320
0.00752
0.25368
-0.03083
-0.07111
-10.2540
156.7020
0.00450
-0.14478
-0.01670
-0.04216
0.00792
-0.01188
-11.3461
-1.08112
-0.24631
0.15719
-0.02223
-0.01368
0.01571
-0.04268
2.69101
-68.1557
-0.04749
-0.35715
0.00799
0.25980
-0.07093
-0.03105
156.9968
-10.1730
-0.14620
0.00258
-0.01819
-0.04513
-0.01150
0.00741
-1.11873
8.76202
isotropic
electric Fermi coupling
potential
MHz
radical
88.32
160.46
89.72
157.65
88.40
26.47
97.19
150.32
95.22
23.62
95.75
87.45
95.15
17.51
90.43
23.56
91.75
154.58
82.88
29.17
82.58
139.04
84.29
124.15
φ
θ
dipole
ordination
-8.77
57.32
51.46
-12.24
57.28
51.98
∆V ) V2 V1
J. Phys. Chem. C, Vol. 114, No. 36, 2010
bond 6
loop 6
bond 5
loop 5
bond 4
loop 4
bond 3
loop 3
bond 2
loop 2
bond 1
loop 1
B18N18 NH2BHNBHNH2
total
atomic
spin
densities
anion
TABLE 5: Total Atomic Charges, Spin Densities, Electric Potential, and Isotropic Fermi Coupling Constants of Cationic, Anionic, and Radical Forms of NH2BHNBHNH2 in
Different Loops and Bonds of the B18N18- NH2BHNBHNH2 System with the EPR-III Basis Set
15326
Monajjemi et al.
3.5806
2.9537
40.0817
3.8248
2.9454
39.9291
Radical
3,4-NH2BHNBHNH2
8,11-NH2BHNBHNH2
15,16-NH2BHNBHNH2
20,23-NH2BHNBHNH2
27,28-NH2BHNBHNH2
32,35-NH2BHNBHNH2
73.97205049
78.01360121
76.79666404
75.11142022
70.90336144
82.9184318
183.4181297
78.62189996
148.051523
165.9353767
131.689849
145.7998385
∆V
4.7405
6.2301
7.4082
5.3907
7.5771
6.9203
16.6847
16.2562
16.6709
17.4159
15.738
14.736
-296.8050257
-296.7959073
-296.797529
-296.8034243
-296.7950223
-296.7947496
-297.3011035
-297.2936626
-297.290901
-297.3004458
-297.2918986
-297.2802135
∆E
∆V
-297.1875912
-297.1979642
-297.1277353
-297.1870679
-297.1517731
-297.1277108
∆E
∆V
72.32344707
65.13896048
47.34353246
71.83137729
49.45362824
48.8526457
0.0,0.0,30.0
2.1383
2.777
2.2536
1.8909
2.2835
15.868
r
∆V
r
1.9242
2.1117
20.3486
4.7775
3.3971
20.3695
∆E
0.0,0.0130.0
-297.1875912
-297.1977986
-296.9256791
-297.1269522
-297.1444916
-296.9240049
∆V
72.32344707
65.54155464
58.08217054
42.387377
52.22628573
58.59084503
r
2.2429
3.0891
2.3907
5.1867
2.9732
2.5548
∆E
0.0,0.0150.0
-297.1450826
-297.1462957
-297.147889
-297.1244024
-297.1482337
-297.161963
-296.8038289
-296.807387
-296.8037056
-296.7610122
-296.7823038
-296.803554
-297.1474922
-297.1489281
-297.1495568
-297.1879735
-297.1503905
-296.9907382
∆E
68.35679026
57.8263628
52.09335543
70.13849244
58.54643388
60.41087509
73.51607556
84.04250436
73.01431413
-296.802514
-296.7962275
-296.8046914
-297.1726014
-297.1541433
-297.1415442
-297.1760215
-297.1550241
-297.1567218
71.97336897
48.92890526
-296.803642
-296.7676678
-297.1702861
-297.1784423
-297.1911411
-297.1708904
-297.1786703
-297.1532399
-296.8017205
-296.8032711
-296.8052086
-296.7956764
-296.8031459
-296.8038468
Expectation Values
2.3374
1.7027
2.0341
2.3013
1.6931
2.3872
6.456
4.6703
4.796
8.0526
4.3875
4.9813
∆V
5.2756
6.3592
6.1544
5.7624
5.8356
6.6779
2.4593
14.0059
20.8958
2.2822
15.2442
20.9007
r
20.9704
7.1532
2.6492
20.7221
4.8733
2.6715
7.0468
5.187
8.2456
4.6699
5.0065
6.1772
16.1405
2.6757
2.9831
15.8837
3.8413
1.7474
r
∆V
-296.8040474
-296.8001824
-296.7966976
-296.8015843
-296.8024018
-296.7894125
-297.1549528
-297.018061
-296.9441091
-297.1487567
-296.996191
-296.9399447
∆E
0.0,0.0170.0
-296.9371613
-297.1176102
-297.1665714
-296.9512951
-297.1324423
-297.166202
-296.7958404
-296.8036949
-296.7813171
-296.8035966
-296.8043561
-296.7957145
73.74027904
77.61282593
84.56040114
74.96797687
73.75105752
89.30435916
60.7514843
35.429805
77.43602829
70.66747623
35.57209468
76.99326403
∆V
75.65692711
40.28868267
59.37449087
74.96052491
41.17942609
58.89083873
86.79411624
74.77131846
88.72476631
73.33083002
76.23866152
72.24042018
-297.0409236 -58.17047422
-297.1570842 63.62028381
-297.154413
55.044642
-297.0459962 -67.00710058
-297.1490317 65.83730602
-297.1783253 68.64014268
∆E
0.0,0.0,70.0
62.83373298 2.4598 -297.1492541 59.13620372 2.4707 -297.1563446 60.75084961
70.14386203 5.614 -297.1142335 37.34912396 2.8901 -297.1562986 58.00286999
72.37388193 42.0227 -296.2197214 -22.38555944 42.1078 -296.1765601 -27.92069574
63.53973678 3.0308 -297.1461258 55.30022789 3.519 -297.1426821 53.30695143
70.07053118 5.6166 -297.1143962 37.25622377 2.887 -297.1561308 57.96220763
62.85021458 42.5942 -296.2104798 -15.71696066 38.4851 -296.2870601 -43.31491394
75.63219803 5.1701 -296.8060198 67.56358837
73.27719339 25.8853 -296.3983032 -30.77592463
76.20112339 5.1912 -296.8013138 79.9498708
87.35570152 6.9389 -296.7939101 76.3099308
69.90327296 4.5586 -296.8038023 68.66707051
77.53129068 4.9186 -296.8005993 78.72639013
60.35677766
47.12537767
58.94451464
39.23730295
56.65773872
57.0762871
∆V
63.62420113
57.59232279
60.8797449
39.7132071
58.24810879
61.06999512
75.88571607
71.52631217
67.90773977
47.14508532
50.38895636
66.4678763
64.60133812
60.93193854
61.86538644
71.93795616
69.56791474
37.69673537
0.0,0.0,50.0
-297.0025552
32.39551179
9.4995 -297.110477
48.07805648 2.3158 -297.1506274
-296.8153581 -276.4829909
2.0027 -297.1959388 67.98781537 6.6596 -297.1308942
-297.1482892
64.26236827 12.9243 -297.0992434 135.6071855
2.8734 -297.1551363
-297.0096335
30.89087891 10.0484 -297.1042342 48.36342398 5.1745 -297.1295553
-296.2654734
-9.953363119 1.9827 -297.1957241 67.99681967 13.2866 -297.0723132
-297.1552991
60.37678398
2.3703 -297.1534902 62.80676482 3.0128 -297.153698
∆E
0.0,0.0,110.0
1.9242
2.1376
7.1652
1.9075
4.5738
7.4477
r
76.10088034 5.0226 -296.800844 79.82303135 4.3851
72.2204997
5.9668 -296.7990902 72.95049491 4.6953
89.40983079 11.2603 -296.7763455 172.1375715 4.6808
75.77623471 3.9444 -296.8037652 74.12505376 4.3045
82.11742053 5.3894 -296.8045112 68.96993479 3.8949
77.97468385 6.9677 -296.7963587 85.92898481 4.4462
243.1076945
118.8282092
102.5799102
164.3267807
80.78881521
73.43455808
0.0,0.0,10.0
⟨r⟩ ) 10.84223413 (Debye), ⟨∆E⟩ ) -297.0637411 (Hartree), ⟨∆V⟩ ) 61.51723858 (au)
⟨r⟩ ) 5.258401161 (Debye), ⟨∆E⟩ ) -296.7915971 (Hartree), ⟨∆V⟩ ) 70.97727363 (au)
⟨r⟩ ) 3.302491855 (Debye), ⟨∆E⟩ ) -296.7176833 (Hartree), ⟨∆V⟩ ) 55.24589482 (au)
νr-c ) 486948.498 GHz, νa-c ) 1792900.812 GHz, νr-a ) 2507076.816 GHz
1.8915
2.7614
3.6452
1.7376
2.6768
2.4845
Radical
3,4-NH2BHNBHNH2
2.2631 -297.1913582 66.8362876
8,11-NH2BHNBHNH2
5.6415 -297.1153161 37.30608467
15,16-NH2BHNBHNH2 19.4425 -296.9611761 80.35437573
20,23-NH2BHNBHNH2 2.3362 -297.1470172 61.42955212
27,28-NH2BHNBHNH2 6.2378 -297.1146633 39.43972611
32,35-NH2BHNBHNH2 20.9495 -296.9150134 169.9426868
anion
cation
radcal
5.5134
4.0205
5.0334
5.7488
5.9348
5.2324
-296.7798467 114.1707175
-296.8036527 73.83518335
-296.7959193 74.83415426
-296.8014382 76.44540798
-296.800476
79.86470916
-296.8049135 68.7983481
r
Cation
3,4-NH2BHNBHNH2
8,11-NH2BHNBHNH2
15,16-NH2BHNBHNH2
20,23-NH2BHNBHNH2
27,28-NH2BHNBHNH2
32,35-NH2BHNBHNH2
∆V
13.9565
26.2414
5.0326
12.8136
40.5363
2.4729
∆E
Anion
3,4-NH2BHNBHNH2
12.9769 -297.0627642 44.61822651
2.0029 -297.1889383 71.50579434
8,11-NH2BHNBHNH2
15,16-NH2BHNBHNH2 20.7711 -296.9585247 249.2417773
20,23-NH2BHNBHNH2 2.2257 -297.1494632 62.45019908
27,28-NH2BHNBHNH2 2.0705 -297.1881211 69.48339394
32,35-NH2BHNBHNH2 22.012 -296.9228108 618.7871587
9.4218
3.8956
6.3126
5.7933
5.0741
5.3348
r
-297.15112
51.98515419 2.3914 -297.1530876 63.49325747
-297.1524553 57.28597702 2.4235 -297.1475956 59.82793766
-296.3332429 -12.24219803 40.0044 -296.2839468 -7.864325557
-297.1506857 51.46525481 14.724 -297.0677137 138.4187253
-297.1526004 57.32731152 2.9955 -297.1534705 56.25370184
-296.2671761 -8.776486474 40.0227 -296.2840582 -8.178705774
-296.8056319
-296.8016614
-296.8001619
-296.8056343
-296.8031029
-296.7919488
-297.3017142
-297.2893226
-297.290822
-297.3011118
-297.291813
-297.2923238
∆E
0.0,0.0,0.0
0.0,0.0,90.0
5.054
6.9205
5.333
4.9366
5.4775
7.2961
Cation
3,4-NH2BHNBHNH2
8,11-NH2BHNBHNH2
15,16-NH2BHNBHNH2
20,23-NH2BHNBHNH2
27,28-NH2BHNBHNH2
32,35-NH2BHNBHNH2
r
16.5939
15.1347
15.7928
16.8942
15.4523
15.3515
r
Anion
3,4-NH2BHNBHNH2
8,11-NH2BHNBHNH2
15,16-NH2BHNBHNH2
20,23-NH2BHNBHNH2
27,28-NH2BHNBHNH2
32,35-NH2BHNBHNH2
B18N18-NH2BHNBHNH2
TABLE 6: Part of the Quantitative Expectation Values of Data Including Relative Energies (∆E), the Radial Coordinate of the Dipole Moment (r), As Well As the Voltage
Differences (∆V) and Transition of the B18N18-NH2BHNBHNH2 System
NH2BHNBHNH2 Inside of the B18N18 Nanoring
J. Phys. Chem. C, Vol. 114, No. 36, 2010 15327
-68.52583119
-61.80784132
-70.9732831
25.26434969
-
-
-
-
-
-
-903.1897052
-903.1839202
-903.1863449
-903.0327035
-1062.692251
-1062.685977
-1062.691185
-1062.691814
-1062.674445
-1062.686254
1.8499
4.5518
4.4671
16.7289
2.3371
4.0649
4.1171
3.8230
2.1848
1.2576
15.6987
16.7144
16.0366
13.6583
14.9210
14.6633
17.6144
19.7195
18.9285
17.4394
15,16-A-NH2BHNBHNH2-G
20,23-A-NH2BHNBHNH2-G
27,28-A-NH2BHNBHNH2-G
32,35-A-NH2BHNBHNH2-G
1,2,4,34,35,36-A-NH2BHNBHNH2-G
3,5,6,7,8,9-A-NH2BHNBHNH2-G
10,11,12,13,14,16-A-NH2BHNBHNH2-G
15,17,18,19,20,21-A-NH2BHNBHNH2-G
22,23,24,25,26,28-A-NH2BHNBHNH2-G
27,29,30,31,32,33-A-NH2BHNBHNH2-G
Note: The frequencies calculated are defined as: ν(GHz) ) [(⟨∆E⟩ × 627.5095 × 4.184 × 1000)/(6.023 × 1023 × 6.62 × 10-34)] × 10-9.
-67.90846566
-903.230826
1.3108
14.2874
8,11-A-NH2BHNBHNH2-G
Figure 4. Graph of the bonding voltage at different dipole coordinates.
a
-71.67513456
-903.229604
2.9221
11.7354
3,4-A-NH2BHNBHNH2-G
-903.3405631
-205.98009
4.9936
-902.8585749
-64.53652
νr-c ) 2444361.716 GHz, νr-a ) 731005.1317 GHz, νa-c ) 3175366.848 GHz
-903.342549
-138.147929
5.7416
-902.8248283
-64.62006
νr-c ) 2674736.927 GHz, νr-a ) 736037.7502 GHz, νa-c ) 3410774.677 GHz
-902.125349
4.897124
36.8571
-901.8410913
4.648348
νr-c ) 8884748.358 GHz, νr-a ) 7012041.771 GHz, νa-c ) 1872706.587 GHz
-903.335254
-140.822952
2.5053
-902.8423343
-73.55501
νr-c ) 2250388.168 GHz, νr-a ) 996996.0498 GHz, νa-c ) 3247384.218 GHz
-903.3360877
-121.742020
28.7027
-902.4849156
296.53608
νr-c ) 4621057.829 GHz, νr-a ) 986514.4475 GHz, νa-c ) 5607572.277 GHz
-903.3300934
-130.383929
67.0407
-901.6791866
7.2015672
νr-c ) 8917049.613 GHz, νr-a ) 1959222.299 GHz, νa-c ) 10876271.91 GHz
-1062.7829098
3.1764
-1062.3720006
νr-c ) 2109828.629 GHz, νr-a ) 597265.5513 GHz, νa-c ) 2707094.18 GHz
-1062.7833884
4.0142
-1062.3684315
νr-c ) 2092008.587 GHz, νr-a ) 641752.0806 GHz, νa-c ) 2733760.668 GHz
-1062.7816206
3.8731
-1062.3697555
νr-c ) 2117596.61 GHz, νr-a ) 595795.0964 GHz, νa-c ) 2713391.706 GHz
-1062.7809104
3.7538
-1062.3721402
νr-c ) 2106029.954 GHz, νr-a ) 586972.3674 GHz, νa-c ) 2693002.321 GHz
-1062.7680881
3.8988
-1062.3571579
νr-c ) 2090306.233 GHz, νr-a ) 616926.2967 GHz, νa-c ) 2707232.529 GHz
-1062.7765492
2.0335
-1062.3647987
νr-c ) 2117766.582 GHz, νr-a ) 594870.1329 GHz, νa-c ) 2712636.714 GHz
∆V (au)
radical
∆E (Hartree)
∆V (au)
cation
∆E (Hartree)
r (Debye)
∆V (au)
∆E (Hartree)
r (Debye)
anion
Monajjemi et al.
r (Debye)
J. Phys. Chem. C, Vol. 114, No. 36, 2010
B18N18-Ala-NH2BHNBHNH2-Gly
TABLE 7: Relative Energies (∆E), Radial Coordinate of the Dipole Moment (r), As Well As the Voltage Differences (∆V) and Quantized Transitional Frequencies (∆ν) of the
B18N18-Ala-NH2BHNBHNH2-Gly Systema
15328
In other words, the two maximum picks have been observed
for the loops with odd numbers (loops 1, 3, 5), and the two
minimum picks are seen for loops with even numbers. The
negative spin densities in the ∆V range of 13.49334-16.069
au correspond to loops 5 and 1, respectively.
In the case of the NH2BHNBHNH2 radical (Figure 5c), similar
trends were obvious for loops of the B18N18-NH2BHNBHNH2
system. The graphs of total atomic charges versus isotropic
Fermi coupling in different loops of (a) the NH2BHNBHNH2
anion even loops and (b) the NH2BHNBHNH2 anion odd loops
in Figure 5, and (c) the NH2BHNBHNH2 radical are exhibited
in Figure 6. The same results have been obtained in these graphs
for both the NH2BHNBHNH2 anion and the NH2BHNBHNH2
radical forms.
4. Conclusion
The procedures discussed in this study place much emphasis
on the importance of electronic structure properties of boron
nitride rings (BN)n and their electromagnetic nonbonded interaction with the NH2BHNBHNH2 molecule and other biological
amino acids to examine the capability of a quantized transition
of the NH2BHNBHNH2 molecule inside of the B18N18 ring.
Indeed, the NH2BHNBHNH2 inside of the B18N18 ring is
supposed as a quantized nanospectrophotometer detector of
various quantized parameters of a given biomolecule coupled
with this system.
Optimized structures, relative stability, HOMO-LUMO band
gaps, nuclear quadrupole resonance (NQR), and hyperfine
spectroscopic parameters of radical, cationic, and anionic forms
of B18N18-NH2BHNBHNH2 systems including total atomic
charges, spin densities, electric potential, and isotropic Fermi
coupling constants of radical, cationic, and anionic forms of
NH2BHNBHNH2 in different loops and bonds of considered
system have been compared. The information inferred from
NQR study on the local electron density distribution together
with analysis of the charge distribution provided logical means
for determination of reactive sites and indicated possible
promising directions to be followed in the design of (BN)n
nanodevices.
It has been observed that the radial coordinate of the dipole
moment vector (r) as well as the voltage differences (∆V) and
relative energies (∆E) exhibited Gaussian distributions. We have
obtained a relationship between dipole moments and the voltage
differences and the system’s energy.
Moreover, the calculation has been repeated for the alanineglycine (Ala-NH2BHNBHNH2-Gly) amino acid coupled with
NH2BHNBHNH2 Inside of the B18N18 Nanoring
J. Phys. Chem. C, Vol. 114, No. 36, 2010 15329
Figure 5. Graphs of the total atomic spin densities versus isotropic Fermi coupling in different loops of the (a) NH2BHNBHNH2 anion even loops,
(b) NH2BHNBHNH2 anion odd loops, and (c) NH2BHNBHNH2 radical.
Figure 6. Graphs of the total atomic charges versus the isotropic Fermi coupling in different loops of the (a) NH2BHNBHNH2 anion even loops
and (b) NH2BHNBHNH2 anion odd loops.
the NH2BHNBHNH2 molecule inside of the B18N18 ring, and
the quantized frequencies in different cationic, radical, and
anionic forms of NH2BHNBHNH2 have been obtained. Therefore, it seems that these B18N18-NH2BHNBHNH2 systems can
be used for the measurement of rotational spectra aroused by
electrical voltage differences existing in these amino acids. For
further structural information, the LUMO and the HOMO
differences, namely, band gaps, have been reported to explore
the capability of the suitable NH2BHNBHNH2 candidate which
makes a stable B18N18-NH2BHNBHNH2 system.
The obtained results confirmed the structural stability of the
B18N18 ring and quantized characteristics of radial coordinate,
voltage differences (∆V), and relative energies (∆E) which
showed Gaussian distribution. Our current analysis is a prerequisite to better clarify their role and to calculate a wide spectrum
of ring properties. Indeed, such a considered nanodevice can
serve as a nanospectrophotometer detector and supplies a
sufficient impetus for further experimental research on the B/N
cluster system.
References and Notes
(1) Curl, R. F.; Smalley, R. E. Science. 1988, 242, 1017.
(2) Kroto, H. Science. 1988, 242, 1139.
(3) Weltner, W.; Van Zee, R. J Chem. ReV 1989, 89, 1713.
15330
J. Phys. Chem. C, Vol. 114, No. 36, 2010
(4) Locke, I. W.; Darwish, A. D.; Kroto, H. W.; Prassides, K.; Taylor,
R.; Walton, D. R. M. Chem. Phys. Lett. 1994, 225, 186.
(5) Behrman, E. C.; Foehrweiser, R. K.; Myers, J. R.; French, B. R.;
Zandler, M. E. Phys. ReV. A 1994, 49, 1543.
(6) Kaxiras, E.; Jackson, K.; Pederson, M. R. Chem. Phys. Lett. 1994,
225, 448.
(7) Haubner, R.; Wilhelm, M.; Weissenbacher, R.; Lux, B. High
Performance Non-Oxide Ceramics; Springer: New York, 2002; Vol. 102,
pp 1-45.
(8) Naruhiro, K.; Takeo, O. Solid State Commun. 2004, 131, 121–
124.
(9) Blasé, X.; Rubio, A.; Louie, S. G.; Cohen, M. L. Europhys Lett.
1994, 28, 335.
(10) Blasé, X.; Charlier, J. C.; de Vita, A.; Car, R. Appl. Phys. Lett.
1997, 70, 197.
(11) Miyamoto, Y.; Rubio, A.; Cohen, M. L.; Louie, S. G. Phys. ReV.
B 1994, 50, 4976.
(12) Chopra, N. G.; Luyken, R. J.; Herrey, K.; Crespi, V. H.; Cohen,
M. L.; Louie, S. G.; Zettl, A. Science. 1995, 269, 966.
(13) Blasé, X.; Rubio, A.; Louie, S. G.; Cohen, M. L. Europhys. Lett.
1994, 28L, 335.
(14) Chopra, N. G.; Luyken, R. J.; Cherrey, K.; Crespi, V. H.; Cohen,
M. L.; Louie, S. G. Science. 1995, 269, 966.
(15) Golberg, D.; Bando, Y.; Stephan, O.; Kurashima, K. Appl. Phys.
Lett. 1998, 73, 2441.
(16) Stephan, O.; Bando, Y.; Loiseau, A.; Willaime, F.; Shramchenko,
N.; Tamiya, T. Appl. Phys. A 1998, 67, 107.
(17) Takeo, O.; Atsushi, N.; Ichihito, N. Physica B 2004, 351, 184–
190.
(18) Pokropivny, V. V.; Skorokhod, V. V.; Oleinik, G. S.; Kurdyumov,
A. V.; Bartnitskaya, T. S.; Pokropivny, A. V.; Sisonyuk, A. G.; Sheichenko,
D. M. J. Solid State Chem. 2000, 154, 214–222.
(19) Fowler, P. W.; Heine, T.; Mitchell, D.; Schmidt, R.; Seifert, G.
J. Chem. Soc., Faraday Trans. 1996, 92, 2197.
(20) Seifert, G.; Flower, P. W.; Mitchell, D.; Porezag, D.; Frauenheim,
T. Chem. Phys. Lett. 1997, 268, 352.
(21) Alexandre, S. S.; Chacham, H.; Nunes, R. W. Appl. Phys. Lett.
1999, 75, 61.
(22) Stankevich, I. V.; Chistyakov, A. L.; Galpern, E. G. Russ. Chem.
Bull. 1993, 42, 1634.
(23) Tang, A. C.; Li, O. S.; Liu, C. W.; Li, J. Chem. Phys. Lett. 1993,
201, 465.
(24) Jensen, F.; Toftlund, H. Chem. Phys. Lett. 1993, 201, 417.
(25) Sun, M. L.; Slanina, Z.; Lee, S. L. Chem. Phys. Lett. 1995, 233,
279.
Monajjemi et al.
(26) Zhukovskii, Y.; SergeiPiskunov, N.; Baiba, B.; Laima, T.; Stefano,
B. J. Phys. Chem. Solids 2009, 70, 796–803.
(27) Loiseau, A.; Willaime, F.; Demoncy, N.; Schramchenko, N.; Hug,
G.; Colliex, C.; Pascard, H. Carbon 1598, 36, 743–752.
(28) Iijima, S.; Ichihashi, T. Nature 1993, 363, 603.
(29) Ajayan, P. M. Chem. ReV. 1999, 99, 1787.
(30) Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
(31) Lee, C.; Yang, W.; Parr, R. G. Phys. ReV. B 1998, 37, 785.
(32) Golberg, D.; Bando, Y.; Stephan, O.; Kurashima, K. Appl. Phys.
Lett. 1998, 73, 2441–2443.
(33) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scusera, G. E.; Robb,
M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A.; Stratmann,
R. E., Jr.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin,
K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi,
R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.;
Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.;
Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz,
J. V.; Baboul, A. G.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.;
Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham,
M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill,
P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez,
C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian 98, revision
A.7; Gaussian, Inc.: Pittsburgh, PA, 1998.
(34) Zhang, R. B.; Huyskensd, T. Z.; Ceulemeans, A.; Nguyen, M. T.
Chem. Phys. 2005, 316, 35–44.
(35) Branda, M. M.; Peralta, J. E.; Castellani, N. J.; Contreras, R. H.
Surf. Sci. 2002, 504, 235–243.
(36) Dumitrescu, S.; Sowerby, D. B. Inorg. Chem. 1992, 32, 3755.
(37) Jerosimic, S. V. J. Mol. Spectrosc. 2007, 242, 139–149.
(38) Zhu, H. Y.; Klein, D. J.; Seitz, W. A.; March, N. H. Inorg. Chem.
1995, 34, 1377.
(39) Rubio, A.; Corkill, J. L.; Cohen, M. L. Phys. ReV. B 1994, 49,
5081.
(40) Seifert, G.; Fowler, R. W.; Mitchell, D.; Porezag, D.; Frauenheim,
Th. Chem. Phys. Lett. 1997, 268, 352–358.
(41) Latosinska, J. N. J. Pharm. Biomed. 2005, 38, 577–587.
(42) Lee, V. S.; Nimmanpipuga, P.; Mollaamin, F.; Kungwana, N.;
Thanasanvorakunc, S.; Monajjemi, M. Russ. J. Phys. Chem. A 2009, 83,
2288–2296.
(43) Henrik, G.; Kjaergaard, A.; Bryan, R. H. Mol. Phys. 1994, 83, 1099–
1116.
(44) Rosmusb, P.; Vladimir, G.; Tyutere, V. Chem. Phys. Lett. 2000,
331, 317–322.
(45) Fan, J.-F.; Wang, Q.; Qi-Ying, X.; Graaf, V. Chin. J. Struct. Chem.
2002, 21, 139–141.
JP104274Z