School of Aerospace Engineering
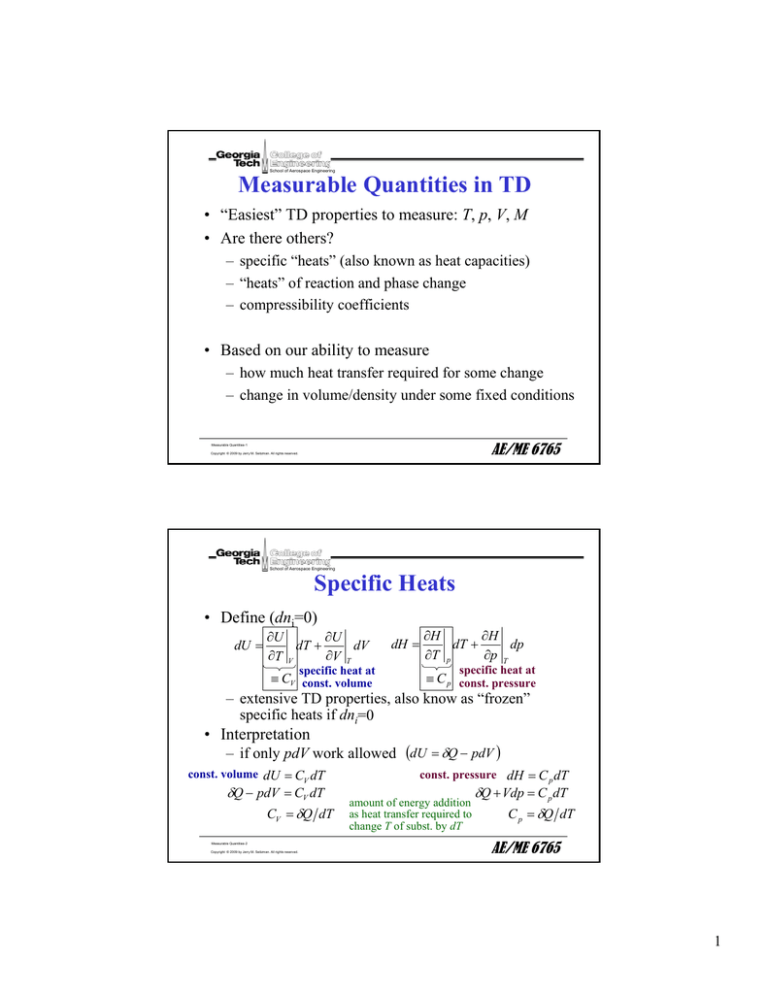
Measurable Quantities in TD
• “Easiest” TD properties to measure: T, p, V, M
• Are there others?
– specific “heats” (also known as heat capacities)
– “heats” of reaction and phase change
– compressibility coefficients
• Based on our ability to measure
– how much heat transfer required for some change
– change in volume/density under some fixed conditions
AE/ME 6765
Measurable Quantities-1
Copyright © 2009 by Jerry M. Seitzman. All rights reserved.
School of Aerospace Engineering
Specific Heats
• Define (dni=0)
dU =
∂U
∂U
dT +
dV
∂T V
∂V T
specific heat at
≡ CV const. volume
dH =
∂H
∂H
dT +
dp
∂T p
∂p T
specific heat at
≡ C p const. pressure
– extensive TD properties, also know as “frozen”
specific heats if dni=0
• Interpretation
– if only pdV work allowed (dU = δQ − pdV )
const. volume dU = CV dT
δQ − pdV = CV dT
CV = δQ dT
Measurable Quantities-2
Copyright © 2009 by Jerry M. Seitzman. All rights reserved.
const. pressure dH = C p dT
amount of energy addition
as heat transfer required to
change T of subst. by dT
δQ + Vdp = C p dT
C p = δQ dT
AE/ME 6765
1
School of Aerospace Engineering
Specific Heats
• So historically these properties were determined from
measuring temperature change for given heat addition (or
equiv. work by Joule) at fixed V or p
• From Maxwell Relations, already showed
∂S
∂S
∂U
∂S
similar approach C p = T
=T
⇒ CV = T
∂T V
∂T V
∂T V
∂T p
• Intensive versions
cp = Cp M
cv = CV M
ĉv = CV
∑n
i
ĉ p = C p
∑n
i
• Units: 1 BTU/lbmºF = 1 cal/gC = 4.187J/gK
defined historically for
H2O at room T
Measurable Quantities-3
Copyright © 2009 by Jerry M. Seitzman. All rights reserved.
air ~1 J/gK@300K
AE/ME 6765
School of Aerospace Engineering
Compressibility Coefficients
• Examine V=V(T,p), with (ni const.)
∂V
∂V
dV =
dT +
dp
∂T p
∂p T
1 ∂V
− 1 ∂V
Isobaric
Isothermal
α≡
κ≡
Compressibility
Compressibility
V ∂T p
V ∂p T
• Also
− 1 ∂V
Isentropic
Note: many texts reverse
Compressibility β ≡ V ∂p
defn. α↔β
S
– all intensive
– for fixed composition, can write all partials in terms of:
α, κ, cp, p, v, T
– strength materials: coeff. linear expansion = α/3
Young’s modulus of elasticity ∝ κ
– speed of sound → β
Measurable Quantities-4
Copyright © 2009 by Jerry M. Seitzman. All rights reserved.
AE/ME 6765
2
School of Aerospace Engineering
Compressibility Coeff: Perfect Gas
• Starting with PG state eqn.
∂V
∂T
nR T
V=
p
∂V
∂p
• So
α≡
1 ∂V
V ∂T
κ≡
p
− 1 ∂V
V ∂p
=
V
T
=
−V
p
p
T
T
κ =1 p
α =1 T
AE/ME 6765
Measurable Quantities-5
Copyright © 2009 by Jerry M. Seitzman. All rights reserved.
School of Aerospace Engineering
Compressibility Coefficients
• For gen’l. simple compressible substance (dni=0)
α≡
• and
1 ∂V
V ∂T
κ≡
p
− 1 ∂V
V ∂p
T
dV = αVdT − κVdp
1) From reciprocity
∂α
∂p
=
T
− ∂κ
∂T
p
T2
p2
dV
= ∫ αdT − ∫ κdp
V1 V
T1
p1
If α, κ constant
3) From cyclic rule
ln(V2 V1 ) = αΔT12 − κΔp12
∂p
α
∂p ∂T ∂V
=
= −1
∂T V ∂V p ∂p T
∂T V κ
2) Integrating
Measurable Quantities-6
Copyright © 2009 by Jerry M. Seitzman. All rights reserved.
V2
∫
AE/ME 6765
3
School of Aerospace Engineering
Specific Heats and Compress. Coeffs.
• Can develop relationship between these properties
∂S
∂p
• Start with dS = ∂S dV + ∂S dT
=
∂V
dS =
∂T
T
V
C
∂p
dV + V dT
∂T V
T
Showed from ∂V
Maxwell Relations
∂S
∂T
∂T
T
=
V
V
1 ∂U
T ∂T
V
• With similar methods (dH)
dS =
• Equate
dp =
Cp
− ∂V
dp +
dT
∂T p
T
C p − CV
T (∂V ∂T ) p
dT −
(∂p
(∂V
∂T )V
dV
∂T ) p
= (∂p ∂T )V
AE/ME 6765
Measurable Quantities-7
Copyright © 2009 by Jerry M. Seitzman. All rights reserved.
School of Aerospace Engineering
Specific Heats and Compress. Coeffs.
• So
∂p
∂T
• Showed
=
V
C p − CV
T (∂V ∂T ) p
∂p
∂T
• So
=
V
α
κ
α ∂V
T
κ ∂T
α
= Tα V
κ
α 2V
C p − CV = T
κ
C p − CV =
p
Perf. Gas
(1 T )2V = pV = nR
C p − CV = T
1 p
T
1) Turns out κ>0 for all stable substances
⇒Cp≥CV (or cp≥cv)
2) For α=0 ⇒ Cp=CV
3) As T→0 ⇒ Cp→CV (exper. show κ not →0)
4) Can also show C p CV = c p cv = κ β
Measurable Quantities-8
Copyright © 2009 by Jerry M. Seitzman. All rights reserved.
AE/ME 6765
4
School of Aerospace Engineering
Heats of Reaction and Phase Change
• Can examine change at specified conditions,
e.g., constant T and p
– phase change
– composition change
(liq)→(sol)
A+B→C
• Energy change (increase or decrease)
– for no work but pdV
saw in 1st Law
const.
Q = ΔU12
volume 12
const. Q = ΔH
12
pressure 12
Measurable Quantities-9
Copyright © 2009 by Jerry M. Seitzman. All rights reserved.
net heat transfer (heating) required
in phase change/reaction
related to energy difference
between phases or RHS vs LHS
AE/ME 6765
5



