Set#5
advertisement
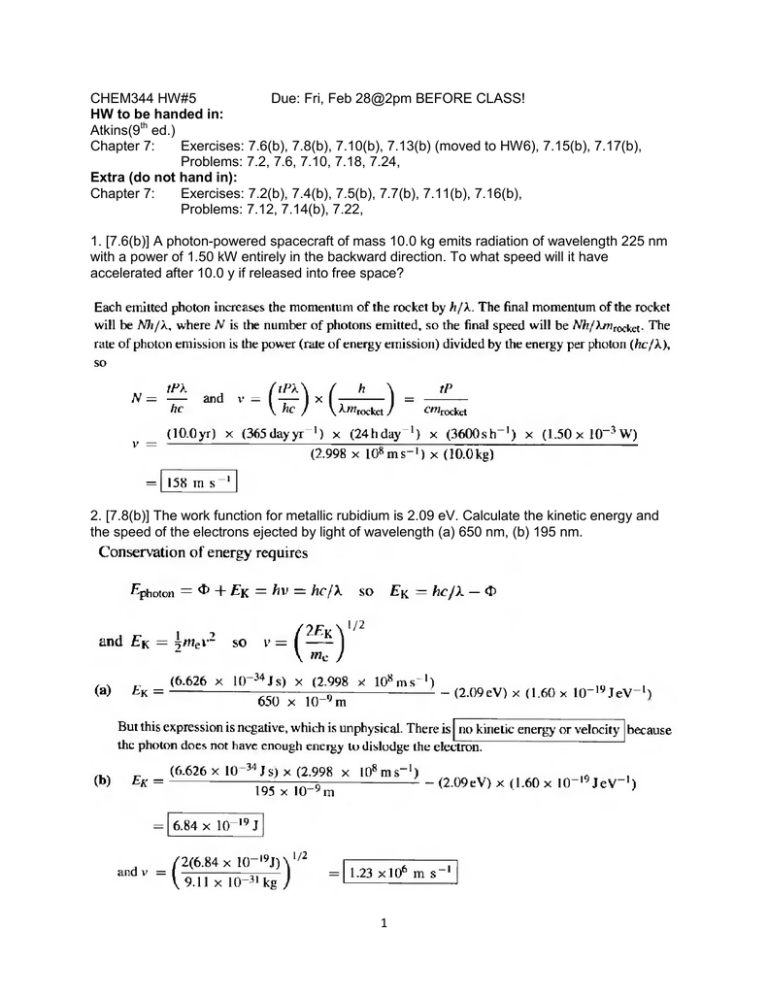
CHEM344 HW#5 Due: Fri, Feb 28@2pm BEFORE CLASS! HW to be handed in: Atkins(9th ed.) Chapter 7: Exercises: 7.6(b), 7.8(b), 7.10(b), 7.13(b) (moved to HW6), 7.15(b), 7.17(b), Problems: 7.2, 7.6, 7.10, 7.18, 7.24, Extra (do not hand in): Chapter 7: Exercises: 7.2(b), 7.4(b), 7.5(b), 7.7(b), 7.11(b), 7.16(b), Problems: 7.12, 7.14(b), 7.22, 1. [7.6(b)] A photon-powered spacecraft of mass 10.0 kg emits radiation of wavelength 225 nm with a power of 1.50 kW entirely in the backward direction. To what speed will it have accelerated after 10.0 y if released into free space? 2. [7.8(b)] The work function for metallic rubidium is 2.09 eV. Calculate the kinetic energy and the speed of the electrons ejected by light of wavelength (a) 650 nm, (b) 195 nm. 1 3. [7.10(b)] Calculate the de Broglie wavelength of an electron accelerated from rest through a potential difference of (a) 100 V, (b) 1.0 kV, (c) 100 kV. 4. [7.13(b)] For the system described in Exercise 7.11b, what is the probability of finding the electron between x = L/4 and x = L/2? Moved to Problem set #6 5. [7.15(b)] An electron is confined to a linear region with a length of the same order as the diameter of an atom (about 100 pm). Calculate the minimum uncertainties in its position and speed. 2 6. [7.17(b)] Determine the commutators of the operators a and a†, where a = ̂ ⁄ a† = ̂ ̂ . 3 ̂ ⁄ and 7. [7.2] For a black body, the temperature and the wavelength of emission maximum, λmax, are related by Wien’s law, λmaxT = 1/5c2, where c2 = hc/k (see Problem 7.12). Values of λmax from a small pinhole in an electrically heated container were determined at a series of temperatures, and the results are given below. Deduce a value for Planck’s constant. θ /°C 1000 1500 2000 2500 3000 3500 λmax/nm 2181 1600 1240 1035 878 763 Alternate: you could plot max vs. 1/T and slope would be hc/5k. Use values of k and c solve for h 8. [7.6] Atoms in a chemical bond vibrate around the equilibrium bond length. An atom undergoing vibrational motion is described by the wavefunction ψ(x) = Nexp(−x2/2a2), where a is a constant and −∞ < x<∞. (a) Normalize this function. (b) Calculate the probability of finding the particle in the range −a ≤ x ≤ a. Hint. The integral encountered in part (b) is the error function. It is defined and tabulated in M. Abramowitz and I.A. Stegun, Handbook of mathematical functions, Dover (1965) and is provided in most mathematical software packages. This is general N = [∫d]-½ but must use /N where = N () or more practically, () is the function you are asked to normalize 4 9. [7.10] A particle is in a state described by the wavefunction ψ(x) = (2a)1/2e−ax, where a is a constant and 0 ≤ x≤∞. Determine the expectation value of the commutator of the position and momentum operators. trivial since [px,x]=const, but if a function then the integral in numerator of <[]> will not be same as the denominator 10. [7.18] Determine which of the following functions are eigenfunctions of the inversion operator î (which has the effect of making the replacement x→−x): (a) x3 − kx, (b) cos kx, (c) x2 + 3x − 1. State the eigenvalue of î when relevant. 5 11. [7.24] Calculate the average linear momentum of a particle described by the following wavefunctions: (a) eikx, (b) cos kx, (c) exp(−ax2), where in each one x ranges from −∞ to +∞. Note: this has trivial solution since cos(x)sin(x) odd fct which when integrate over symmetric interval = 0 Again this is an even function, first derivative makes it odd, product of odd with even function is odd, symmetric integral is 0 6 Extra (do not hand in): 12. [7.2(b)] Calculate the linear momentum of photons of wavelength 350 nm. What speed does a hydrogen molecule need to travel to have the same linear momentum? 13. [7.4(b)] Calculate the energy per photon and the energy per mole of photons for radiation of wavelength (a) 200 nm (ultraviolet), (b) 150 pm (X-ray), (c) 1.00 cm (microwave). 7 14. [7.5(b)] Calculate the speed to which a stationary 4He atom (mass 4.0026mu) would be accelerated if it absorbed each of the photons used in Exercise 7.4b. 15. [7.7(b)] A laser used to read CDs emits red light of wavelength 700 nm. How many photons does it emit each second if its power is (a) 0.10 W, (b) 1.0 W? 16. [7.11(b)] An unnormalized wavefunction for an electron in a carbon nanotube of length L is sin(2πx/L). Normalize this wavefunction. Moved to Problem Set #6 8 17. [7.16(b)] In an X-ray photoelectron experiment, a photon of wavelength 121 pm ejects an electron from the inner shell of an atom and it emerges with a speed of 56.9 Mm s−1. Calculate the binding energy of the electron. 18. [7.12] Derive Wien’s law, that λmaxT is a constant, where λmax is the wavelength corresponding to maximum in the Planck distribution at the temperature T, and deduce an expression for the constant as a multiple of the second radiation constant, c2 = hc/k. 9 19. [7.14(b)] Prior to Planck’s derivation of the distribution law for black-body radiation, Wien found empirically a closely related distribution function that is very nearly but not exactly in agreement with the experimental results, namely ρ = (a/λ5)e−b/λkT. This formula shows small deviations from Planck’s at long wavelengths. (a) By fitting Wien’s empirical formula to Planck’s at short wavelengths determine the constants a and b. (b) Demonstrate that Wien’s formula is consistent with Wien’s law (Problem 7.12) and with the Stefan–Boltzmann law (Problem 7.13). 10 20. [7.22] A particle is in a state described by the wavefunction ψ = (cos χ)eikx + (sin χ)e−ikx, where χ (chi) is a parameter. What is the probability that the particle will be found with a linear momentum (a) +k ħ, (b) −k ħ? What form would the wavefunction have if it were 90 per cent certain that the particle had linear momentum +k ħ? 11



