HW 5 Solutions Problem 3.12 Setting an Energy balance, n Delta H
advertisement

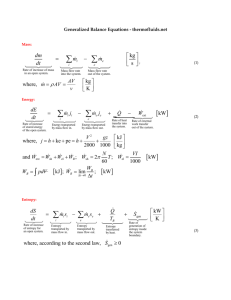
HW 5 Solutions Problem 3.12 Setting an Energy balance, n Delta H = Q + W, and solving for Q = - 2365 J/min Entropy balance, 0 = -n Delta S + Q/T + Sgen, solving for Sgen= n Delta S - Q/T Finding Sgen= n * [Cp*ln(T2/T1)-R*ln(P2/P1)] – Q/T = 3.2667 J/K Since Sgen>0 for total process, the proposed process is possible. Problem 3.13 a) Hout*Mout = Sum (Hin*min), solving for Tout and substituting all mass flows we have Tout = 300 K b) Entropy balance dS/dt = Sum (Sin*Nin ) - Sum (Sout*Nout ) + Q/T + Sgen We can get rid of Q/T, and set a reference state as SR = 0 at 300 K and 1 bar If we know that S = Cp ln (T/TR), Entropies SA and SB can be found. Plugging numbers we found SA = -5.3420 and SB = 0.9607. Sgen can be found by solving the entropy balance and assuming dS/dt = 0. Plugging the stream flows we have Sgen = 0.0919 J/s-K c) By energy balance Q = -Ws Using the entropy balance Q/T = 0.0919 J/s-K. Solving for Q = 27.1 J/s. Substituting into energy balance, Ws = - 27.1 J/s Problem 3.19 a) Assume isentropic process Sin = S’out Sin = 2.27 BTU/lb F (at 0.1013 MPa, - 240 F) Hin = 810 kJ/kg S’out = 2.27 BTU/lb F Pout = 0.4 MPa (given) From chart we have H’out = 920 kJ/kg W rev = H’out - Hin = 110 kJ/kg W actual = W rev / efficiency = 126 kJ/kg Work required per mole of methane = 126 (16/1000) = 2.016 kJ/mol Hout actual = Hin + W actual = 936 kJ/kg At H = 936 kJ/kg and P = 0.4 MPa, Sout actual is equal to 2.28 kJ/kg By entropy balance we have 0 = Sin - Sout + Sgen Solving for Sgen = m(Sout - Sin) = 11.72 kJ/hr–K Work in kW = 9.8 kW b) Methane is compressed Delta H’ = 1155-980 = 175. Since efficiency is 0.87, then Delta H = 201 Hout = 980 +201 = 1181 Work = Delta H = 201 kJ/kg Work in kW = 15.6 kW Sgen = n(Sout - Sin) = 17.6 kJ/kg Problem 3.30 a) Energy balance nAHA – nBHB – nCHC = 0 Set a reference state as T = 310 K and P = 5 bar Therefore HA = 0 HB = -1455 HC = 145.5 Use mass flow ratios to find nB and nC, we have nB = 2.909 mol/min nC = 0.2909 mol/min b) Entropy balance 0 = nASA – nBSB – nCSC + Sgen Reference state is condition A, therefore SB = Cp ln (TB/TA) – R ln (PB/PA) = 8.2624 J/mol K Similarly, SC = 13.846 J/mol K SA = 0 (Since it is the reference state… that’s the beauty of having arbitrary references) Solving for Sgen, we have 42.68 J/mol K c) For a reversible exchanger 0 = nBSB – nCSC – nB’SB’ - nC’SC’ Neglecting pressure drop through the exchanger, we have nBCp ln (TD/TB) + nCCp ln (TD/TC), solving for TD = 309.5 d) Energy balance 0 = - nBDelta HB – nCDelta HC + Ws. Use the temperature found above to obtain Delta H for B and C. Solve for Ws = -46.54 J/min = -0.776 Watts. Note that Sgen does not change. e) If mix directly nBDelta HB = nCDelta HC Cp cancels out and we can calculate the final temperature of the system, TD = 310 K (Sgen ) exchanger = nBDelta SB + nCDelta SC = 0.1345 J/K min (Sgen ) total = (Sgen ) exchanger + Entropy generated part b) = 42.81 J/K min