Schrödinger Representation – Schrödinger Equation ∂ ∂
advertisement
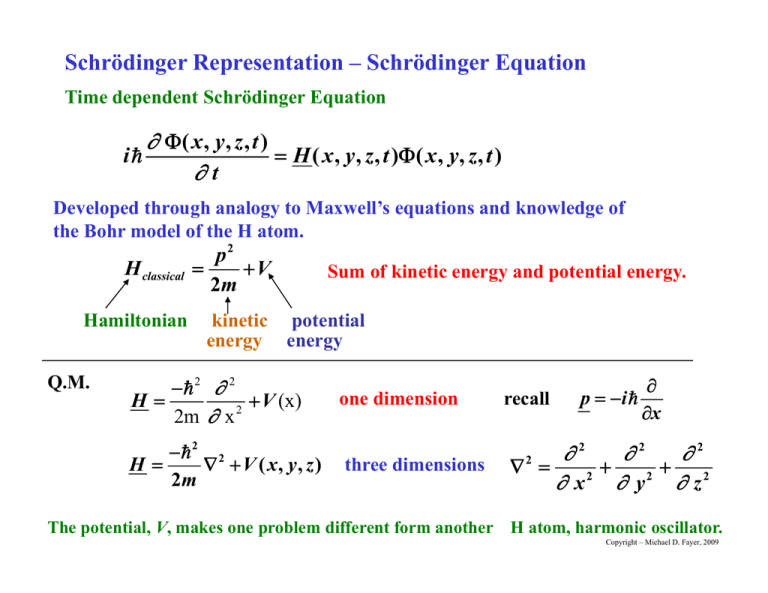
Schrödinger Representation – Schrödinger Equation Time dependent Schrödinger Equation i ( x , y, z , t ) H ( x , y , z , t ) ( x , y , z , t ) t Developed through analogy to Maxwell’s equations and knowledge of the Bohr model of the H atom. H classical Hamiltonian Q.M. p2 V 2m Sum of kinetic energy and potential energy. kinetic potential energy energy 2 2 H V ((x)) 2 2m x 2 2 H V ( x, y, z ) 2m 2m one dimension three dimensions recall p i x 2 2 2 2 2 x y z2 2 The potential, V, makes one problem different form another H atom, harmonic oscillator. Copyright – Michael D. Fayer, 2009 Getting the Time Independent Schrödinger Equation ( x , y, z , t ) wavefunction i ( x , y , z , t ) H ( x , y , z , t ) ( x , y , z , t ) t If the energy is independent of time H ( x, y, z ) Try solution ( x , y , z , t ) ( x , y , z )F ( t ) product of spatial function and time function Then i ( x , y , z )F ( t ) H ( x , y , z ) ( x , y , z )F ( t ) t i ( x, y, z ) F ( t ) F ( t ) H ( x , y , z ) ( x , y , z ) t independent of t divide through by F independent of x, y, z Copyright – Michael D. Fayer, 2009 dF ( t ) dt H ( x , y , z ) ( x , y , z ) F (t ) ( x, y, z ) i depends only on t depends only on x, y, z Can only be true for any x, x yy, zz, t if both sides equal a constant constant. Changing t on the left doesn’t change the value on the right. Changing x, x y, y z on right doesn’t change value on left. left Equal constant dF dt E H F i Copyright – Michael D. Fayer, 2009 dF dt E H F i Both sides equal a constant, E. H ( x , y , z ) ( x , y , z ) E ( x , y , z ) Energy eigenvalue problem – time independent Schrödinger Equation H is energy operator. Operate p on g get back times a number. ’s are energy eigenkets; eigenfunctions; wavefunctions. E Energy Eigenvalues Observable values of energy Copyright – Michael D. Fayer, 2009 Time Dependent Equation (H time independent) dF ( t ) dt E F (t ) ii i dF ( t ) E F (t ) dt dF ( t ) i E dt . F (t ) ln F i Et C Integrate both sides Take initial condition at t = 0, F = 1, then C = 0. F ( t ) e i E t / e i t Time dependent part of wavefunction for time independent Hamiltonian. Time dependent phase factor used in wave packet problem. Copyright – Michael D. Fayer, 2009 Total wavefunction E ( x , y , z , t ) E ( x , y , z )e i E t / E – energy (observable) that l b l state. labels Normalization E E *E E d E* e i E t / E e i E t / d E* E d Total wavefunction is normalized if time independent part is normalized. Expectation value of time independent operator S. S S *E S E d E* e i E t / S E e i E t / d S does not depend on t, e i E t / can be brought to other side of S. S E* S E d Expectation value is time independent and depends only on the ti time independent i d d t partt off the th wavefunction. f ti Copyright – Michael D. Fayer, 2009 Equation of motion of the Expectation Value in the Schrödinger Representation Expectation value of operator representing observable for state S A S A S example - momentum P S P S In Schrödinger g representation, p , operators p don’t change g in time. Time dependence contained in wavefunction. Want Q.M. equivalent of ti time d derivative i ti off a classical l i l dynamical d i l variable. i bl P P classical momentum goes over to momentum operator P P ? want Q.M. operator equivalent of time derivative t Definition: The time derivative of the operator A , ii. e., e A is defined to mean an operator whose expectation in any state S is the time derivative of p of the operator p the expectation A . Copyright – Michael D. Fayer, 2009 Want to find d A dt Use S A S t H S i S t time dependent Schrödinger equation. S A S S A S S A S t t t product rule time independent – derivative is zero S. t (operate to left) S H i Use the complex p conjugate j g of the Schrödinger g equation q Then i S S H t i S H S t , and from the Schrödinger equation From Schrödinger eq. i S A S S H A S S AH S t From complex conjugate of Schrödinger eq. Copyright – Michael D. Fayer, 2009 Therefore d A dt This is the commutator of H A. i S H A AH S i A [ H , A] The operator representing the time derivative of an observable is i/ times the commutator of H with the observable. Copyright – Michael D. Fayer, 2009 Solving the time independent Schrödinger equation The free particle momentum problem P P p P p ( x) 1 2 eik x p k k p/ Free particle Hamiltonian – no potential – V = 0. 2 P H 2 2m Commutator of H with P 3 3 P P [P, H ] 0 2m 2m P and H commute P S PPP S 3 Simultaneous Eigenfunctions. Copyright – Michael D. Fayer, 2009 Free particle energy eigenvalue problem H P E P 2 2 H 2m x 2 U momentum Use t eigenkets. i k t 2 2 1 i k x H P e 2 2m x 2 2 2 2m 2m ( ik )2 e i k x 2k 2 1 i k x e 2m 2 p2 P 2m 2 p Therefore, E 2m energy eigenvalues i l Energy same as classical result. Copyright – Michael D. Fayer, 2009 Particle in a One Dimensional Box V= V= V=0 Infinitely high, thick, impenetrable walls -b 0 b x Particle inside box. Can’t get out because of impenetrable walls. Classically E is continuous. E can be zero. One D racquet ball court. Q.M. x p /2 H E E can’t be zero. Energy eigenvalue problem Schrödinger Equation 2 d 2 ( x ) +V ( x ) ( x ) E ( x ) 2 2m d x V ( x) 0 x b V ( x) x b Copyright – Michael D. Fayer, 2009 For x b 2 d 2 ( x ) E ( x) 2 2m d x Want to solve differential Equation Equation, but solution must by physically acceptable. Born Condition on Wavefunction to make physically meaningful. 1. The wave function must be finite everywhere. 2 The wave function must be single valued. 2. valued 3. The wave function must be continuous. 4 First derivative of wave function must be continuous 4. continuous. Copyright – Michael D. Fayer, 2009 d 2 ( x ) 2mE ( x) 2 2 dx Functions with this property Second derivative of a function equals a negative constant times the same function. sin and cos. d 2 sin(ax ) 2 a sin(ax ) 2 dx d 2 cos(ax ) a 2 cos(ax ) dx These are solutions provided 2m E a 2 2 Copyright – Michael D. Fayer, 2009 Solutions with any value of a don’t obey Born conditions. Well is infinitely deep. Particle has zero probability of being found outside the box. 0 = 0 for x b -b x 0 b Function as drawn discontinuous at x b To be an acceptable wavefunction sin and cos 0 at x b Copyright – Michael D. Fayer, 2009 will vanish at x b if n a an n is an integer 2b cos an x n 1, 3, 5... sin an x n 2, 4, 6... 0 –bb b Integral number of half wavelengths in box. Zero at walls. Have two conditions for a2. 2 2 n 2mE 2 an 2 2 4b Solve for E. n 2 2 2 n 2 h2 Energy eigenvalues – energy levels, not continuous. En 2 8mb 8m L2 L = 2b – length g of box. Copyright – Michael D. Fayer, 2009 n2 2 2 n2 h2 En 2 8mb 8m L2 Energy levels are quantized. Lowest energy not zero. 1 2 n x 1 n ( x ) cos 2b b 1 2 n x 1 n ( x ) sin 2b b x b n 1, 3, 5 x b n 2, 4, 6 L = 2b – length of box. wavefunctions including normalization constants First st few ew wave wavefunctions. u ct o s. Quantization forced by Born conditions (boundary conditions) n =3 0 n =2 0 Fourth Born condition not met – first derivative not continuous. Physically unrealistic problem because the potential is discontinuous. n =1 0 -b 0 x b Like classical string – has “fundamental” and harmonics. Copyright – Michael D. Fayer, 2009 Particle in a Box Simple model of molecular energy levels. Anthracene L6 A L E2 S1 E E1 electrons – consider “free” in box of length L. Ignore all coulomb interactions. S0 m me 9 1031 kg 6 1010 m L6A Calculate wavelength of absorption of light. i in i box energy level formula f Form particle 3h2 E E2 E1 8 2 8mL E h h 6.6 1034 Js E / h 7.64 1014 Hz c / 393 nm blue-violet E 5.04 1019 J Experiment 400 nm Copyright – Michael D. Fayer, 2009 Anthracene particularly good agreement. Other molecules, naphthalene, benzene, agreement much worse. Important point Confine a particle with “size” of electron to box size i off a molecule l l Get energy level separation, light absorption, in visible and UV. Molecular structure, realistic potential give accurate calculation, but It is the mass and size alone that set scale. Big molecules Small molecules absorb in red. absorb in UV. Copyright – Michael D. Fayer, 2009 Particle in a Finite Box – Tunneling and Ionization Box with finite walls. Ti Time independent i d d tS Schrödinger h ödi E Eq. V(x)=V V(x)=V V(x)=0 -b 0 x 2 d 2 ( x ) +V ( x ) ( x ) E ( x ) 2 2m d x 2m V ( x) 0 x b V ( x) V x b b Inside Box V = 0 2 d 2 ( x ) E ( x) 2 2m d x d ( x) 2mE E ( x) 2 2 dx 2 Second derivative of function equals q negative constant times same function. Solutions – sin and cos. Copyright – Michael D. Fayer, 2009 Solutions inside box ( x ) q1 sin i 2mE x 2 or ( x ) q2 cos 22mE mE x 2 Outside Box d 2 ( x ) 2m ( E V ) ( x) 2 2 dx T o cases: Bo Two Bound nd states, states E < V Unbound states, E > V Bound States d 2 ( x ) 2m (V E ) ( x) 2 2 dx Second derivative of function equals positive constant times same function. Not oscillatory. Copyright – Michael D. Fayer, 2009 Try solutions d 2 e ax 2 a x a e 2 dx exp p ax Second derivative of function equals positive constant times same function function. Then, solutions outside the box 1/ 2 ( x) e 2 m (V E ) x 2 x b Solutions must obey Born Conditions ( x ) can’t blow upp as x Therefore,, 1/ 2 ( x ) r1 e 2 m (V E ) x 2 xb 1/ 2 ( x ) r2 e 2 m (V E ) x 2 Outside box Inside box exp. decays exp oscillatory x b Copyright – Michael D. Fayer, 2009 Outside box Inside box 0 -b 0 x exp. decays oscillatory The wavefunction and its first derivative continuous ti att walls ll – Born B Conditions. C diti b Expanded view For a finite distance into the material finite p probability y of finding gp particle. x b Classically forbidden region V > E. 0 probability 0 Copyright – Michael D. Fayer, 2009 Tunneling - Qualitative Discussion Classically forbidden region. Wavefunction not zero at far side of wall. 0 Probability of finding particle small but finite outside box. X A particle placed inside of box with not enough energy to go over the wall can Tunnel Through the Wall. Formula derived in book mass = me E = 1000 cm-1 V = 2000 cm-1 e 2 d [2 m (V E ) / 2 ]1/2 Ratio probs - outside Wall thickness (d) 1 Å probability 0.68 vs. inside edges of wall. 10 Å 0.02 100 Å 3 10-17 Copyright – Michael D. Fayer, 2009 Electron in a rectangular potential exact result. Infinite wall at x = 0. Finite wall between 4 Å and 6 Å. barrier height 25 eV electron energy 17 eV No longer truly discreet single states. Energy continuous with peaks. 2Å energy no longer delta function density o of states ampllitude 1.0 0.5 00 0.0 -0.5 -1.0 0 5 10 15 20 energy amplitu ude 1.0 Peaks in density of states near particle in the box energies. Higher energy – wider. 0.8 0.6 0.4 0.2 0.0 -0.2 2 3 4 5 6 7 8 9 10 11 Prof. Pat Vaccaro – analytical solution Daniel Rosenfeld – program, numerical calculations x (Å) Copyright – Michael D. Fayer, 2009 Chemical Reaction not enough energy to go over barrier Temperature dependence of some chemical reactions shows to much product at low T. V > kT . V reactants products q D Decay off wavefunction f ti iin classically l i ll forbidden f bidd region i for f parabolic b li potential. t ti l d (2mV / 2 )1/ 2 e- tunneling parameter distance mass light particles tunnel barrier height Copyright – Michael D. Fayer, 2009 Methyl Rotation H C H H Methyl groups rotate even at very low T. Radioacti e Deca Radioactive Decay some probability outside nucleus repulsive Coulomb interaction nuclear attraction strong interaction Copyright – Michael D. Fayer, 2009 Unbound States and Ionization E large enough - ionization If E > V - unbound states V( ) = V V(x) bound states E<V -b b b Inside the box (between -b and b) V=0 2mE ( x ) q1 sin x 2 2mE ( x ) q2 cos x 2 S l ti Solutions oscillatory ill t d 2 ( x ) 2m ( E V ) ( x) 2 2 dx Outside the box (x > |b|) E>V 2m ( E V ) ( x ) s1 sin x 2 ( x ) s2 cos 2m ( E V ) x 2 S l ti Solutions oscillatory ill t Copyright – Michael D. Fayer, 2009 unbound state -b b To solve (numerically) Wavefunction and first derivative equal q at walls,, for example p at x = b In limit 2m ( E V ) 2mE s sin b q1 sin b 1 2 2 q1 s1 E V (E V ) E Wavefunction has equal amplitude everywhere. Copyright – Michael D. Fayer, 2009 For E >> V ( x ) sin 2mE x 2 for all x As if there is no wall. Continuous range of energies - free particle Particle has been ionized. 2mE 2 2mp 2 2 2m 2m ( x ) sin k x 2k 2 k 2 Free p particle wavefunction Copyright – Michael D. Fayer, 2009 In real world potential barriers are finite. Tunnelingg Ionization Copyright – Michael D. Fayer, 2009