Chapter 17: THE HYDROLOGIC CYCLE AND GROUNDWATER
advertisement

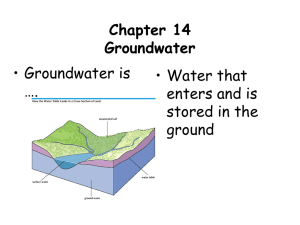
Chapter 17: THE HYDROLOGIC CYCLE AND GROUNDWATER What Makes water so important? p About the Hydrologic Cycle • Hydrology is the study of movements and characteristics of (ground)water. • The hydrologic cycle has a profound effect upon climate prediction. • Water is vital so we must understand where to find water and how water supplies cycle through the Earth. Flows and reservoirs ● Reservoirs R i include i l d all ll the th places l that water is stored in and on the Earth. ● Flows into a reservoir include inflows and outflows Flows and reservoirs • < 1% of world’s water is readily available for use. Globally, 70% of fresh water is used for agriculture, 20% for industry, 10% for residences. • If all the polar ice caps melts, then sea-level will rise by 75 m. Water: Residence times Flows and reservoirs ● The Th hydrologic h d l i cycle l ● precipitation i it ti ● infiltration i filt ti and d runoff ff ● evaporation, ti ttranspiration i ti ● groundwater flow The hydrologic cycle cycle-powered by solar energy Hydrology y gy and climate ● Key climatic factors ● relative humidity ● rainfall ● landscape ● Key tectonic factors ● ocean–land l d relationships l ti hi ● mountain rain shadows Hydrology and climate: the rain shadow effect Leeward slope Windward slope The hydrology of runoff ● Surface storage of water runoff ● lakes and reservoirs ● wetlands and swamps Groundwater ● Groundwater flow through soil and rock ● porosity p y and p permeability y ● groundwater d t ttable bl Groundwater • Porosity (φ): is the fraction of a porous material, which is void space φ = Vvoid/Vtotal Groundwater: porosity porosit and the amount of open space in various materials Groundwater ● Above and below the groundwater table ● unsaturated ((vadose)) zone ● saturated t t d (phreatic) ( h ti ) zone Groundwater: the water table Groundwater Two major zones of groundwater: •Vadose Zone: zone above the water table ((zone of aeration, unsaturated). •Zone of saturation: upper surface of saturation zone is [Groundwater] Water Table Table. Water Table mimics the ground topography, the height of which fluctuates with recharge/discharge. Groundwater ● Inflow and outflow of groundwater ● groundwater recharge (influent streams or losing g streams)) ● groundwater d t di discharge h ((effluent ffl t streams) Groundwater: effluent water headed for a stream Groundwater • Aquifer: any lithologic formation that stores sto es g groundwater ou d ate (gravel, (g a e , sa sand, d, limestone etc.). It not only stores but also transmits water at faster rates rates. • Aquiclude: is a formation that may contain water but does not transmit significant quantities (clays and shales). • Aquitard: is a formation with relatively low permeability. permeability Groundwater ● Types of aquifers ● unconfined – has an aquiclude below ● confined – has an aquiclude above and below ● Perched Types of Aquifer: Unconfined Aquifer qu e Confined Aquifer ● Characteristics of some confined aquifers ● artesian (flowing) wells ● artesian flow (under pressure) Groundwater: artesian conditions Potentiometric (peizometeric) surface: Imaginary level to which water rises due to hydrostatic y pressure. Groundwater ● Complex p g geological g environments ● perched h d water t ttables bl ● unpredictable flow conditions Groundwater: perched water t table Recharge of Groundwater ● Balancing recharge and discharge ● balance = stable water table ● excess recharge = rising water t bl table ● excess discharge = falling water table Groundwater: excess e cess discharge g and the cone of depression What is the consequence of over pumping (over draft)? ¾compaction and degradation of the aquifer ¾may trigger subsidence When the well is dry, we learn the worth of water" - Benjamin B j i Franklin F kli Groundwater: Excess discharge and The intrusion of salt water Q. What is the consequence off salt lt water t intrusion i t i into aquifers? Darcy’s y LAW: Darcy’s law provides an accurate description of the flow of ground water in almost all hydrogeologic environments. Head loss h1 - h2 determines flow rate Q – KA(dh/ Q= KA(dh/dL dL)) Q= – KA(dh/ Q KA(dh/dL dL)) Volumetric flow rate (Q) (m3/s or ft3/s) hydraulic conductivity (K) (m/s or ft/s) Area of cross section perpendicular to flow (A) Hydraulic gradient (dh/dL) dh =h hin - hout (difference (diff in i height h i ht off water t level l l in inlet and outlet peizometers) The minus signs on the right hand reflects that the hydraulic head always decreases in the direction off fflow. K represents the measure of the ability for flow (permeability) through porous media. • Darcy Velocity or Flux ( or specific discharge) [q]: volumetric flow per unit area q = Q /A = -K dh/dl Unit: L/T Darcy velocity is a fictitious velocity since it assumes that flow occurs across the entire cross-section of the soil sample. Flow actually takes place only through interconnected pore channels. The average pore water velocity is termed the seepage velocity (v) and is given by: v = q/φ = Q/(Aφ) where φ is the p porosity y of the p porous media ((varies from 0 to 1). Average g rate of groundwater g flow is ~ 15 m/day, y, mayy reach up to 125 m/day. Table. Typical Porosity and hydraulic conductivity of selected Earth Materials. Material Porosity y (%) Hydraulic Conductivity (K) in m/day Unconsolidated Clay 45 0.041 Sand 35 32.8 Gravel 25 205 Gravel & Sand 20 82 Sandstone 15 28.7 Limestone/ Shale 5 0.041 Granite 1 0.0041 Rock Numerical Problem Q Two Q: T wells ll are llocated d 100 feet f apart in i a sand d aquifer if with iha hydraulic conductivity of 0.04 feet per day and 35% porosity. The head of well 1 is 96 ft and the head of well 2 is 99 ft. Q What is the seepage velocity of water between the two Q. wells? Ans: v = q/φ = K/φ × dh/dl = (0.04ft/day × 1/0.35) × (0.03) = 0.0034 ft/day. Numerical Problem 4 km Sedimentary rocks Valley Well 1 1 km Well 2 Flo direction in valley Flow alle Sedimentary rocks The valley (blue) is 4 km wide, where 2 wells are drilled 1 km apart. y Porosity y The saturated zone below is 25 m thick,, with K = 100 m/day. (φ) of material is 30% (0.3). The elevation of water in wells 1 and 2 are 98 and 97 m respectively. 3 3 10,000 m /day Q What is the discharge, Q. discharge Q (m /day) of aquifer? Q. What is the time travel (T) of groundwater between wells 1 and 2? This helps in studying contaminant transport. 3000 days Erosion by yg groundwater ● Features of groundwater erosion ● caves and caverns ● stalactites and stalagmites ● karst features ((karst karst topography) ● sinkholes Erosion by yg groundwater: karst Satellite View — Karst K t Topography What are the Environmental concerns in karst terranes? ¾sinkhole collapse ¾Winter Park, Florida 1981: 100 m depression, 13 m deep, $2 million in damage ¾Susceptible to pollution from above ¾hard water problem: amount of Ca2+ and Mg2+ in water; difficulties with plumbing ¾thin residual soils, terra rosas, which makes waste burial or disposal management considerably difficult. difficult Erosion by y groundwater: Carlsbad Caverns, New Mexico Erosion by y groundwater: sinkhole in Winter Park, Park Florida 100 m wide, 13 m deep, $2 million in damage Groundwater Uses Why should we be concerned about groundwater pollution? ¾ Groundwater Pollution is difficult to detect. ¾ Groundwater has long-term residency (1000 yr), which means if polluted then it stays y there for a long g time. ¾ Difficult and expensive to clean aquifers. What steps can be taken to characterize groundwater pollution? • Geologic characterization (lithology structure) (lithology, • Hydrologic characterization: depth of water table, thickness of aquifer, direction and rate flow etc. etc • Contaminant (type & source) identification and understanding the transport mechanisms. mechanisms Aquifer Contamination ¾ Although unconfined aquifers are used for water supply, they are often contaminated by wastes and chemicals at the surface. ¾ Confined aquifers are less likely to be contaminated and thereby provide supplies of good quality. Water Quality ● Contamination Source of water supply l • • • • • • • • Oxygen-demanding waste Pathogenic organisms Nutrients Oil & Petroleum Products H Hazardous d Ch Chemicals i l Toxic Heavy Metals Radioactive Materials (Ra 222) Thermal Pollution A pollutant is any substance, excess exposure to which is known to be harmful to living organisms. Water Quality • Pathogenic organisms -Common C diseases are C Cholera, typhoid, hepatitis, dysentery etc. -Cryptosporidiosis Cryptosporidiosis (caused by Cryptosporidium, a protozoa infection in the gastrointestinal tract - standard measure of microbial pollution is to count f fecal l coliform lif bacteria b t i (a ( group off bacteria b t i found f d in i human & animal intestine & wastes). E. Coli ((usually y found in meat p products), ), a type yp of coliform bacteria causes illness and death. Acceptable limit for drinking water is 1 coliform per 100 mL of sample sample. Causes of Contamination: earthquakes, floods, storms etc., may damage sewer lines resulting in contamination of water supply. Eutrophication: Eutrophication p : Nutrients Acondition in aquatic ecosystem where high nutrient concentration stimulates growth of phytoplankton. Higher amounts of nutrients in a water body help grow algae & aquatic plants, which cloud water & block sunlight. Underwater grasses, which serves as food for aquatic creatures dies. When algae die & decompose, O2 is used up, resulting in depletion of dissolved Oxygen. • Hazardous Chemicals: – Toxic synthetic organic and inorganic compounds – oxygen additives dditi (Methyl (M th l Terbutyl T b t l Ether, Eth MTBE) in i gasoline in order to increase oxygen level of gasoline and decrease CO emissions emissions. MTBE is water soluble and commonly detected as Volatile Organic g Compound p (VOC) ( ) in groundwater. g Conc. Of 20-40 μg/L (ppb) can cause difference in taste & odor. – Common VOC are - gasoline, industrial chemicals such as benzene, toluene and xylene (a.k.a. BTX), formaldehyde (carcinogens) (carcinogens), and tetrachloroethylene, perchloroethylene (principal dry cleaning solvent) Oil & Petroleum Products • Oil discharge in to water involving oil-tanker accidents in sea. Case History: In 03/24/1989, the oil tanker, Exxon Valdez, spilled > 11 million gallons of crude oil in sea near Valdez, Al k ((world’s Alaska ld’ mostt pristine i ti & ecologically l i ll rich i h marine i environment). Out of this 50 % deposited on the shoreline, 20% evaporated, and only 14% was collected by waste recovery recovery. • • • In 1994 1994, N N. Russia Russia, pipe line fracture resulted in about 4-80 million gallons of crude oil spill contaminating land & water. Sept 16, 2008. A crack in the diesel pipeline of the Indian Oil C Corporation ((IOC) OC) has led to oil spillage in G Ganga Aug 22, 2009 - oil spill across a 100-km swath on the south Gujarat Guja at coast, threatens t eate s the t e marine a e bio-diversity b o d e s ty in tthe e National at o a Marine Park and Sanctuary. Two major oil refineries — Reliance and Essar. Toxic Substances • Heavy Metals: – Such as Pb, Hg, Zn, Cd, As are often deposited with natural sediments at the bottom of stream channels. Become incorporated into plants, crops, and thus animals and humans. – Inorganic ions of such metals are toxic – Hg (from volcanic emissions, natural deposits) contamination of aquatic systems. Methylation:- Change of inorganic Hg2+ to CH3Hg+ (methylmercury) due to bacterial activity. CH3Hg+ is more toxic than Hg2+. The conc. of CH3Hg+ increases higher in the food chain. Residence time of Hg in ocean = 80,000 yrs Source of Water Pollution Surface water Pollution • Point source pollution: – readily identifiable localized and/or confined sources such as industrial/municipal pipes, sewers that empty into streams or rivers. – These pollutants controlled by on on-site site treatment/ disposal and are regulated by permit. • Non Non-point point source pollution: – Diffused and intermittent pollution – Influenced by y factors such as climate,, hydrology, y gy, topography, vegetation, geology, urbanization etc. – Includes all sorts of pollutants entering the water system t from f any sources (e.g, ( i insecticides ti id from f plant l t is washed away by rain and added to rivers) Acid Mine Drainage – non non--point source pollution Oxidation of pyrite FeS2 + 3 3.75 75 O2 + 3.5 3 5 H2O ⇔ Fe(OH)3 + 2 H2SO4 Remediation ● Reversing contamination ● easier if recharge rate is fast ● usually costly and very slow ● decontamination after pumping ● in in--ground water treatments Groundwater Treatment • Treatment processes: – Extraction Wells: Pump out contaminated water and treat it by filtration, oxidation, reverse osmosis etc. – Vapor Extraction: Using vapor extraction well and treatment. – Bioremediation: using microorganisms to attenuate contamination. – Permeable treatment bed: contact treatment as contaminated water plume moved through beds, which neutralizes contaminants by chemical, physical and/or bi l i l processes. biological Control System for C t i t d Ground Contaminated G d Water Extraction Wells Water q quality y Factors that determine the quality of drinking water: • Acidity (pH) —should be around 7 • Salinity (TDS) —should be <500mg/L • Dissolved Di l d constituents tit t Organic Inorganic g • Pathogens • Taste (bad taste due to too much of some dissolved constituents such as Fe, Cu, S, Cl)) •Toxic metal and organic contaminant levels Quality Criteria of Indian Drinking Water Set by Indian Standards Institution and Indian Council of Medical Research (ICMR): ISI (IS:10500-1989) Sl. No. Substance or Characteristic (1) 1. 2. 3. 4. 5 5. 6. 7. 8. 9. (2) Colour, Hazen Units Odour Agreeable Turbidity Di l d solids, Dissolved lid mg/l /l pH value Total hardness (as CaCO3), mg/l Calcium (as Ca), mg/l Magnesium (as Mg), mg/l 10. 10 11. 12. 13. 14. 15. 16. 17. Copper (as Cu), Cu) mg/l 0 05 0.05 Iron (as Fe), mg/1 0.3 Manganese(as Mn),mg/1 0.1 Chlorides (as Cl), mg/l 250 Sulfate (as SO4), mg/l 150 Nitrate (as NO3), mg/l 45 Fluride (as F), mg/I 0.6 to 1.2 Phenolic Compounds (as 0.001 C6H5OH),mg/I Mercury (as Hg),mg/l. 0.001 Cadmium(as Cd),mg/l 0.01 Selenium (as Se),mg/l 0.01 Arsenic (as As),mg/l 0.05 Cyanide(as CN), mg/l. 0.05 Lead (as Pb),mg/1. 0.10 Zinc (as Zn), mg/l 5 Chromium (as Cr6+), mg/1 0.05 P ti id Pesticides Ab t Absent Radio-active materials: a) Alpha emitters uc**** per ml, 10-8 Max. b) Beta emitters uc per ml, Max 10-7 18. 19. 20. 21. 22. 23. 24. 26. 30 30. 31. Prescribed by ISI Prescribed by ICMR Requirement (Desirable Highest Desirable Level Limit) Max. Permissible level (3) (4) 10 5 units Unobjectionable Unobjectionable Unobjectionable Unobjectionable 10 NTU 5JTU 500 500 6.5 to 8.5 7.0 to 8.5 300 300 75 75 30 Not more than 50 mg/I Maximum Permissible level (5) 25 units Unobjectionable Unobjectionable 25 JTU 1500* 6.5 to 9.2 600 200 00.05 05 0.1 0.1 200 200 20 1.0*** 0.001 11.55 1.0 0.5 1000 400 ** 1.5 0.002 ------------ 0.001 0.01 0.01 0.05 0.05 0.10 ---3 pci/l***** -- 30 pci/1 Oct. 2008 World-wide Hydrological Mapping and Assessment Programme (WHYMAP); United Nations Educational, Scientific and Cultural Organization (UNESCO) & German Federal Institute for Geosciences and Natural Resources (BGR) A map of aquifer zones in India (1,2, 3: aquifer zones zones)) 1. Alluvial aquifers of IndoGangetic plains in the northern and eastern India. Also, Narmada and Tapi river alluvium, coastal alluvium, coastal sand dunes and coastal limestone aquifers. 2. Aquifers in the alluvium, li limestone, t sandstone d t etc. t in i th the inland sedimentary basins of central and southern India. 3. Hard rock aquifers in the Basalt (Deccan Traps), Metamorphic rocks and Basement complex (granite and gneiss). Source: groundwater resources of the world and their use; United Nations Educational, Scientific and Cultural Organization (UNESCO) report 2004. Hydrological map of India and Yield Potential Central C t l Ground G d Water W t Board (Ministry of Water Resources Government of India) India)http://cgwb.gov.in Groundwater Resources of IndiaIndia- Recharge The map shows groundwater changes in India during 2002-08, losses in red and gains in blue, based on GRACE satellite observations. Estimated rate of depletion of groundwater in northwestern India (Jaipur and New Delhi) is 33 cm (1 ft) of water per year over the past decade. Increases in groundwater in southern India are due to recent above-average rainfall, whereas rain in northwestern India was close to normal during the study period. Loss is almost entirely due to human activity. Gravity Recovery and Climate Experiment (GRACE) satellite data Credit: II. Velicogna/UC Irvine published in the August 20 issue of Nature Watershed of India Central Ground Water Board (Ministry of Water Resources Government of India)http://cgwb.gov.in -Watershed atlas of India -Basin wise watershed maps available Thought g questions q for this chapter p 1 If the Earth warmed 1. warmed, causing evaporation from the oceans to increase greatly, how would the hydrologic cycle of today be altered? 2. If you lived near the seashore and started to notice that your well water had a slightly salty taste, how would you explain the change in water quality? 3. Why would you recommend against extensive development and urbanization of the recharge area of an aquifer that serves your community? 4. Why should communities ensure that septic tanks are maintained in good condition? Thought g questions q for this chapter p 5. Why are more and more communities in cold climates restricting the use of salt to melt snow and ice on highways? 6. You are exploring a cave and notice a small stream flowing on the cave floor. Where could the water be coming i from? f ? Key y terms and concepts p Aquiclude Aquifer Artesian flow Darcy’s Darcy s law Discharge Groundwater Groundwater table Hydraulic gradient Hydrologic cycle Hydrology yd ot e a water ate Hydrothermal Infiltration Karst topography Meteoric water Permeability Key y terms and concepts p Potable water Precipitation Rain shadow Recharge Relative humidity Reservoir Runoff Saturated zone Sinkhole Unsaturated zone