Document
advertisement

American Journal of Physics and Applications
2016; 4(1): 5-11
Published online February 17, 2016 (http://www.sciencepublishinggroup.com/j/ajpa)
doi: 10.11648/j.ajpa.20160401.12
ISSN: 2330-4286 (Print); ISSN: 2330-4308 (Online)
Electronic Electrical Conductivity in N-type Silicon
Abebaw Abun Amanu
Department of Physics, Haramaya University, Dire Dawa, Ethiopia
Email address:
meseret.abun@gmail.com
To cite this article:
Abebaw Abun Amanu. Electronic Electrical Conductivity in N-type Silicon. American Journal of Physics and Applications.
Vol. 4, No. 1, 2016, pp. 5-11. doi: 10.11648/j.ajpa.20160401.12
Abstract: The electrical conductivity of n-type silicon depends on the doping concentration which varies from1022-1026/m3
at a given temperature 300°K where ionized impurity scattering is the dominant scattering mechanism. This work founds that
the electrical conductivity of n-type silicon increases as the electron concentration increases as the result of doping. When the
electron concentration increases, the Fermi energy increases from the result of the Fermi level increment.
Keywords: Doping Concentration, Fermi Energy, Electrical Conductivity
1. Introduction
Semiconductors are materials at the heart of many
electronic devices, such as transistors, switches, diodes,
photovoltaic cells, etc… Silicon is widely used now a day
with several applications in light emitting diodes,
semiconductor lasers, microwaves lasers, and others
specialized areas [1].
Semiconductor is a material that has a conductance value
between that of an insulators and conductors. In addition,
their resistance between them. They are only different from
insulators because of conduction brought about by thermally
generated charge carries (extrinsic conduction) called
dopants in semiconductor devices only extrinsic conduction
is desirable, the charge carries are electrons and holes [2].
By adding the right kind of dopants it is possible to make
semiconductor materials, n-type materials and p-type
materials. If such impurities contribute a significant fraction
of the conduction band electrons and /or valance band holes,
one speaks of an “extrinsic semiconductors” [3].
The objective of this research is:
To show the relationship between Fermi energy the
electron concentration
To show the relationship between the electrical
conductivity and the electron concentration
To derive the mathematical expression for electrical
conductivity and to calculate the numerical values in ntype silicon for different doping concentrations in the
range 1016/cm3-1018/cm3.
The physical significance of this research is to understand
the electrical conductivity of the n-type silicon that has so
many applications in the electronic world.
2. Silicon
Silicon is the most widely used semiconductors [4], it is
there for important to consider atomic structure of silicon.
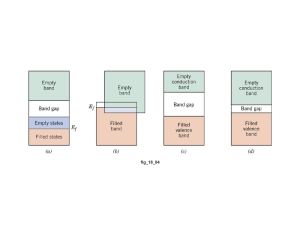
Silicon has a crystal structure like that of diamond, and as
in diamond an energy gap separates the top of its filled
valance band from an empty conduction band as show in the
figure below,
Figure 1. The valance band and conduction band.
The forbidden band in silicon, however, is only about 1ev
wide. At low temperature silicon is little better than diamond
as a conductor, but at room temperature a small number of its
valance electron have enough thermal energy to jump the
forbidden band and enter the conduction band. These
electrons though few, are still enough to allow small amount
of current to flow when an electric filed is applied.
6
Abebaw Abun Amanu: Electronic Electrical Conductivity in N-type Silicon
In intrinsic (pure) silicon, there are relatively few free
electrons, so neither silicon nor the other semiconductors is
very useful in intrinsic scale. Pure silicon is neither insulator
nor good conductor because current in a material depends
directly on the number of free electrons.
By adding appropriate transformation to anew P’
coordinate system in which the constant energy surface
because spherical. The energy can be expressed in the form,
2.1. N-type Silicon
∗ ∗
is the density of the states
=
effective mass and Mv=6 number of equivalent energy
valleys. The number of quantum states in P-space in the
energy range E+dEis,
Where
A silicon atom with its four valance electrons shares an
electron with each of its four neighbours. This effectively
creates eight valance electrons. For each atom and produces a
state of chemical stability. To increase the number of
conduction band electrons intrinsic silicon, pentavalent
impurity atoms are add. This is known as n-type silicon (nstands for negative). Pentavalent atoms are atoms with five
valance electrons, such as arsenic (As), phosphorus (P), and
antimony (Sb). When a minute trace of the order of 1 part in
108 of such an element is added to pure silicon, the
conductivity is increased [5].
When an atom of a pentavalent element such as antimony
is introduce into a crystal of pure silicon, it enters into the
lattice structure by replacing one of the tetravalent silicon
atoms, but only four of the five valance electrons of the
antimony atom can join as covalent bond. Consequently, the
substitution of a pentavalent atom of silicon atom provides a
free electron. This states of affairs is represented that, the ion
of, say, antimony atom, carrying a positive charge of 5e, with
four of its valance electrons forming covalent bonds. With
fouradjacent atoms, and the unattached valance electron free
to wander at random in the crystal. This random movement,
however, is such that the density of these free or mobile
electrons remains constant through the crystal.
Once the pentavalent impurity atoms such as antimony are
responsible for introducing or donating free electron into the
crystal, they are termed donors; and crystal doped with such
impurity is referred as n-type (i.e. negative type) silicon.
The greater the amount of impurity in silicon, the greater is
the number of free electrons per unit volume and therefore
the greater is the conductivity of the silicon. The number of
impurity atom added to silicon can control the number of
conduction electrons.
2.2. Constant Energy Surfaces of Conduction Energy Band
Structure and the Quantum Density of States of N-type
Silicon
The system under consideration is n-type silicon. There are
six equivalent constant energy ellipsoids for electron in
silicon. These are six equivalents energy minimum along the
six {100} directions [3]. The constant energy surfaces as seen
by the {100} plane through the center of the first Brillion
zone in p-space with axis of symmetry in the x-axis will have
energy given by an expression of the form,
=
*
=
+
∗ +
∗
+
+
∗
(2.2)
∗
∗
√
=
−
(2.3)
If we measure energy from the bottom of conduction Ec=0,
then
can be expressed as,
∗
√
=
(2.4)
2.3. Fermi Dirac Statistics for N-type Silicon
The number of states per unit volume between and
in allowed band,
can be calculated from the
+
volume between and
in an allowed band,
can
be calculated from the volume between and
in k-space
divided by the volume of a single state in k-space. If the
shape of the energy surface in the k-spaceis known for a
given material, therefore,
can be calculated. If
is the probability that a state with energy will be occupied
states is given by an expression of the form,
=
∞
(2.5)
Where is the number of electrons in the conduction
band, now the function
, the profanity that a state with
energy will be occupied, is just the Fermi distribution
function [6,7]. For electron occupation of the conduction
band,
can be expressed as,
=
!"#
$%$&
*
'( )
(2.6)
Where + is the Fermi energy.
To derive the number of electrons in the conduction band,
use the above equations. Substitute eq. (2.4) and eq. (2.6)
into eq. (2.5), i.e.
=
3
=1
,
√
$%$&
!"#/*
.( )
∗
2
3
01
,
√
4
∗
$%$&
!"#/*
.( )
2
0
In addition, the normalized electron concentration
∗
(2.1)
Where m1 =ml=0.92m0 is the longitudinal effective mass
and m2*=m3*=mT=0.91m0 is the transverse effective mass.
=
5/
(2.7)
(2.8)
is,
(2.9)
We assume that the total mobile electron concentration in
the conduction band is equal to donor concentration Nd that
American Journal of Physics and Applications 2016; 4(1): 5-11
D"
varies from 1022-1026/m3 in our calculation.
3. Boltzmann Transport Equations
<=
?+
?+
=> @ +> @
?= A
?= B
(3.1)
where
<+
<=
?+
?+ <"
=> @ +
?= B
?" <=
+
?+ <C
?C <=
(3.2)
or
<+
<=
?+
=> @ +
?= B
?+
?+ <"
?" <=
?+
+
?+ <
(3.3)
?+
(3.4)
? <=
=> ?= @ + D" ?" + E" ?
B
For the present, we want to avoid excessive complications
?+
by means of relaxation time approximations for > ?= @ . The
B
effect of collisions is always to restore a local equilibrium
situation described by the distribution function F, D, : . Let
us further assume that if the electron distribution is
distributed from the local equilibrium value , then the effect
of the collision is simply to restore to the local equilibrium
value exponentially with a relaxation time τ which is the
order of the time between electron collisions with ion.
?+
?+
?+
i.e.> ?= @ + D" ?" + E" ?
B
G
(3.5)
From the relations
H" =
∗
E"
(3.6)
Substitute eq.(3.6) into eq.(3.5)
<+
<=
?+
= > @ + D"
?= B
?+
?"
+
+G ?+
∗ ?
G
(3.7)
Where ∗ is the effective mass of an electron.
From the general relation of the electrical force and the
electric field, we get the below eq.
Where e=1.6x10-19C, electric charge and Ex is the electric
field in the x-direction.
<+
<=
?+
= > @ + D"
?= B
?+
?"
−
!4G ?+
∗ ?
G
(3.8)
For the steady state condition, the electron distribution is
<+
independent of time, i.e. = 0, eq. (3.8) becomes,
<=
?"
−
!4G ?+
∗ ?
G
?+
= −> @
?= B
(3.9)
Where in the relaxation time approximation
The conductivity of a substance is determined by the
concentration and mobility of charge carriers.
The probability of electrons occupying a unit volume of
phase space with the center at point (x, k) at the moment of
time t is
7, 9, : . [8] That is to say
7, 9, : is the
distribution function for no equilibrium state the distribution
function will change with time, the nature of change being
dependent on which process predominates; the change due to
the action of the electric field (F), and as a result of charge
carrier collision(C).
<+
?+
7
?+
> @ =
?= B
+J+K
(3.10)
τ
and
D"
?+
?"
−
!4G ?+
∗ ?
G
?+
= −> @ = −
?= B
+J+K
L
(3.11)
4. Electron Scattering Mechanism
There are different scattering mechanisms like acoustic
phonon scattering, ionized impurity scattering, carrier-carrier
scattering among others responsible for the resistivity of the
material [9, 10].
Conwell and Weisskopf have calculated the rate of change
of distribution function due to ionized impurity scattering by
using the following assumptions;
i. in the electron ionized impurity scattering only the
direction of electrons changes
ii. an electron gets scattered by a single ion at a time i.e.
by the one which is closest to it at that particular
instant of time.
Therefore one can express the number of electrons per unit
volume per second into a solid angle MN at O N ,P N as,
Q D, O, P R O, O N D M
(4.1)
Where Q is the number of electron per unit volume,
Q D, O, P M is the number of electrons per unit volume
with solid angle M.
R O, O N = >
S!
@
TUK UV WX Y ZJZ /
(4.2)
Is the Rutherford scattering cross-section and v is the
relative velocity between elelctron and ion and can be taken
as electron velocity.
The Conswell and Weisskopf formula for ionized impurity
relaxation time is,
[= [ >
4
\( ]
@ =[ ^
(4.3)
Where ε is the dimensionless kinetic energy.
Among varies scattering mechanisms responsible for
resistivity in the temperature range 77-3000K and for electron
concentration, ≥ 10 a / b the ionized impurity scattering
is the dominant scattering mechanism. We shall use the
above expression of relaxation time for ionized impurity
scattering in subsequent sections to obtain the explicit
expression for thermal conductivity.
5. Electrical Conductivity
Electrical conduction is transport processes resulting from
the motion of charge carriers under the action of internal or
external field.
8
Abebaw Abun Amanu: Electronic Electrical Conductivity in N-type Silicon
We are interested about the conductivity of n-type silicon
in which the conductivity is due to the excess electrons.
Current is defined as the time rate at which charge is
transported across a given surface in a direction normal to it,
the current will depend on both number of charges free to
move and the speeds at which they move.
Electrical conduction takes place as a result of the motion
of the free electrons under the action of an applied electric
field [11].
Derivation of electrical conductivity.
Current is defined as the time rate at which charge is
transported across a given surface in a direction normal to it,
the current will depend on both the number of charges free to
move and the speeds at which they move.
The electrical current density is given by,
! ∗
c" = −
d D"
! ∗
c" = −
De DS
d D"
b
(5.1)
D
(5.2)
Where can be expanded as = + D" " to the first
order approximation for weak/normal dc electric field.
c" = −
! ∗
d D"
+ D"
"
b
D
(5.3)
+ D" " ≈ D" " , since no current flows in equilibrium,
does not contribute to the electric field current.
Thus,
! ∗
c" = −
d D"
"
b
D
(5.4)
T
2k ∗b
=−
m
ℎb
=−
a! ∗
?+
? G
≈
?+K
? G
+ D"
∗ ?
G
=−
"
+J+K
g
=−
+G G
g
(5.5)
!4G ?+K
∗ ?
G
=
=
(5.6)
G ? G
(5.7)
Thus,
c" = −
!
∗
d D"
"
b
D
c" = −
c" = −
T
T
3
D"
"
(5.12)
=−
4k ∗b T 3
m m DnohO
ℎb
a! ∗
T
3
D
a
"
" noh
D hi O O D
Ohi O O D
(5.13)
By using the relations of the above equations, we candrive
the below equation.
c" = −
=−
a! ∗
4k
c" = −
a!
T
∗b
3
"
ℎb
!4G g ?+K
Da
T
3
∗
?4
T
noh Ohi O O D
k[ q
∗ q
m m Da
∗ 4
G
(5.14)
noh Ohi O O D
3
noh Ohi O O
3
m noh Ohi O O
Da[
?+K
?4
D
(5.15)
Using integration by substitution, we can integrate the
above equation, i.e. let
nohO = r, then– hi
O= r
Replacing the first thing in u, then;
i.e.t−
uW Z
b
c" = −
c" = −
(5.8)
(5.9)
Substitute this eq.(5.9) into eq.(5.7)
!
(5.11)
T
v =−
noh b − noh b 0 =
b
b
!
∗ 4
G
b
3
Da[
?+K
D
?4
(5.16)
!
b
∗ 4
G
3
?+K
D a [^
D
?4
(5.17)
From the relation of v and energy E
D = D" De DS = D hi O O P D
∗
D hi O O D
"
Substitute eq.(5.12) into eq.(5.11)
D
By using solid angle relations
b
D"
Substitute eq.(4.3) into eq. (5.16), then,
!4G g?+K
∗
3
Then
+
− G G
g
Then,
"
T
D hi O O D
"
D" = DnohO
, leaving the higher order terms in the expansion
of . From the above eq. (5.5) relations.
3
From the vector v and angle O relations
The Boltzmann transport equation in the presence of a d.c
electric field " in the x direction is calculated by;
!4G ?
T
P m m D"
D hi O O P D
(5.10)
>
∗
∗
D @=
D=
,D D =
(5.18)
<4
∗
(5.19)
Substitute eq.(5.19) into eq.(5.17)
c" = −
T!
b
∗ 4
G
3
D b >[ ^ @
?+K
?4
>
<4
∗
@
(5.20)
American Journal of Physics and Applications 2016; 4(1): 5-11
∗
4
D = ,D =
4
, :ℎk , D = w
∗
∗
ˆ
ƒ
K „…†‡>ƒ%ƒ @
&
Substitute eq.(5.21) into eq.(5.21)
T!
c" = −
=−
∗ 4
G
b
3
∗ 4 g
G K
5
∗
x√ T!
b
>
4
@ >[ ^ @
∗
3
?+K
^
?+K
?4
Change all the energies that are the equation becomes
dimensionless kinetic energy of an electron.
^=
4
\( ]
,
= ^yz {
∗
x√ T!
b
c" = −
∗
x√ T!
b
∗
x√ T!
c" =
4G gK
b
3
^yz {
4G gK
3
4G gK
3
^yz { (5.25)
? U\( ]
^yz {
^yz {
?+K
^
(5.26)
?+K
^
(5.27)
^
?U
^ −
?U
By using integration by parts we can solve the above
complex mathematical equation. So,
3 b
D=
−
?+K
?U
rD − m D r =
^
^, D =
,E
}−^ b ~3
+ 3m
−
?+K
^
?U
(5.28)
, r = ^b, E
3
^
r = 3^
^ = 0 + 3m
^ (5.29)
3
^
^
By substituting,
=
*•€• U*U&
=3
U <U
*•€• UJU&
(5.30)
Finally,
c" = −
c" = −
∗
x√ T!
x√ T!
b
∗
4G g \( ]
4G g \( ]
3
3
3
From the general relation of c" = R"
R" =
R" =
R" =
∗
x√ T!
√ T!
∗
gK
gK
\( ]
\( ]
∗
‚G
U <U
*•€• UJU&
U <U
*•€• UJU&
(5.31)
(5.32)
"
4G
(5.33)
3
! gK
U <U
*•€• UJU&
3
U <U
*•€• UJU&
(5.36)
ƒ Vƒ
„…†‡ ƒ%ƒ&
(5.37)
ˆ
ƒ
K „…†‡>ƒ%ƒ @
&
=
A U&
A U&
(5.38)
This eq.(5.38) is known as the normalized electrical
conductivity.
6. Numerical Calculation
?+K
^
‰G
Š ‹K
Œ∗
3
(5.24)
Substitute eq.(5.24) int0 eq.(5.23)
c" = −
∗
R"< =
(5.23)
ˆ
! gK K
R" =
(5.22)
?4
∗
! gK
R" =
(5.21)
9
U <U
*•€• UJU&
Substitute eq.(2.8) into eq. (5.35), i. e.
(5.34)
(5.35)
6.1. Numerical Calculation of Electrical Conductivity
To calculate numerical values of the normalized Fermi
energy ^+ and the dimensionless electrical conductivity R"<
for the given electron concentration, we use the formula for
electron concentration.
=,
T
∗
√
3
0
, yz {
=,
T
@ yz {
!"#-
$%$&
/*
.( )
0 (6.1)
\( ]
energy.
T
\( ]
$
<-. )/
(
{ = 300 y, ∗ = 1.18
Ži:ℎ
=
4
^=
is the dimensionless kinetic
Where
9.11710Jb 9•, E
=,
>
4
∗
∗
3
√
0
√
0 yz {
- yz { ^
3
<U
/
(6.2)
<U
/
(6.3)
*•€• UJU&
-^
*•€• UJU&
By substituting the numerical values of the constants, we
will got,
n= 3.62
H# ^+ =
3
3
-^
-^ #
<U
*•€• UJU&
<U
*•€• UJU&
/
/
(6.4)
This integral is known as Fermi integral.
To get the dimensionless Fermi energy using the given value
of normalized electron concentration those are shown in table
1. we use eqn. (2.9) for normalized doping concentration
and the integral equation (6.3). The integral equation (6.3) for
electron concentration is difficult to evaluate because the
normalized Fermi energy ^+ is unknown.
We use iteration method in such a way that for a given
arbitrary value of ^+ the left side of the integral equation (6.3)
can be evaluated by using a Mathematica software. The value
of the normalized electron concentration obtained by this
numerical calculation will be compared with the known
Abebaw Abun Amanu: Electronic Electrical Conductivity in N-type Silicon
Table 1. A data of normalized electron concentration corresponding to
dimensionless Fermi energy and normalized electrical conductivity.
Normalized electron
concentration(nn)
0.0462845
0.12039
0.1605
0.240398
0.5095
0.750925
1.0009
1.50085
2.0008
2.50075
3.0007
3.50065
4.0006
4.50055
5.0005
5.50045
6.0004
6.50035
7.0003
7.50025
8.0002
8.50015
9.0001
9.50005
10
Dimensionless Fermi
energy
-4.23354
-3.26945
-2.97748
-2.56469
-1.80185
-1.36966
-1.05497
-0.59533
-0.25354
0.023557
0.259635
0.46734
0.65422
0.825143
0.983438
1.131475
1.27101
1.403375
1.529607
1.65025
1.766795
1.878955
1.987462
2.092687
2.19496
Normalized electrical
conductivity
4.528399
4.55227
4.565182
4.590962
4.675266
4.756477
4.837995
5.002002
5.167141
5.333409
5.500866
5.669359
5.838826
6.009266
6.180707
6.352981
6.526144
6.700136
6.874981
7.050141
7.226896
7.407064
7.581702
7.760182
7.939213
6.2. Analysis and Discussion on the Result
The numerical values are used to draw the graph of
normalized Fermi energy ^+ vs normalized electron
concentration nn.
Again, the numerical values are used to draw the graph of
dimensionless electrical conductivity ( R"< ) vs normalized
electron concentration nn.
When we see the figure 2, the normalized Fermi energy
increases as the doping concentration or the normalized
electron concentration nn increases.
When we increase the normalized electron concentration,
by doping it from time to time, the Fermi energy level
increases with it. We get negative Fermi energy when the
location of the Fermi level is below the bottom of the
conduction band and a positive Fermi energy when the
location of the Fermi level is above the bottom of the
conduction band.
The graph of normalized electrical conductivity (R"< ) vs
normalized electron concentration nn shows that the electrical
conductivity of the semiconductor increases by increasing the
electron concentration in the conduction band as a result of
doping.
B
3
2
Dimensionless fermi energy(Ef)
initial value =0.04628. I continue my calculation until I get
the precise value of the normalized Fermi energy ^+
corresponding to the given normalized electron concentration
=0.04626. Therefore, we get the value on the right side of
eqn.(6.3) which must be approximately equals to the value of
on the left side of eqn.(6.3) with an error in the order of
10-3. This iteration method is used again to get the other
values of the normalized Fermi energy ^+ corresponding to
the given electron concentration
in the table. These values
are used to calculate dimensionless electrical conductivity R"<
corresponding to the given value of the normalized electron
concentration
as shown in table 1.
1
0
-1
-2
-3
-4
-5
0
2
4
6
8
10
Normalized Electron concentration
Figure 2. Dimensionless
concentration.
Fermi
energy
vs
normalized
elelctron
B
8.0
Normalized electrical conductivity
10
7.5
7.0
6.5
6.0
5.5
5.0
4.5
0
2
4
6
8
10
Normalized electron concentration(nn)
Figure 3. Normalized electrical conductivity vs normalized electron
concentration.
7. Conclusion
In my investigation of in this research how the electrical
conductivity of n-type silicon depends on the doping
concentration which varies from 1022-1026/m3 at a given
temperature 300°K where ionized impurity scattering is the
dominant scattering mechanism. I found that the electrical
conductivity of n-type silicon increases as the electron
concentration increases, the Fermi energy increases from the
result of the Fermi level increases.
American Journal of Physics and Applications 2016; 4(1): 5-11
References
[1]
Lionel Warne’s, Electronic and electrical engineering, 1995.
[2]
LK Maheshwari MMS Anand, Laboratory manual for
Introductory electronic experiments, 2000.
[3]
Neil W. Ascfroft, Solid state physics, 1976.
[4]
Yu, Peter, Fundamentals of Semiconductors, Berlin: SpringerVerlag. ISBN 978-3-642-00709-5(2010).
[5]
Floyd, electronics fundamentals, 4th edition. 1998.
11
[6]
R. P. Feynman, Statistical Mechanics (Perseus, Reading, MA,
1998.
[7]
R. V. Chamberlin, J. V. Vermaas, and G. H. Wolf, Eur. Phys.
J.B 71, 1 (2009).
[8]
F. Reif, Foundamental of statistical and thermal physics, 1985.
[9]
Sharma S K, IEEE Trans Electron Devices, 36 (1989) 768.
[10] Tesfaye G, Indian J Pure & Appl Phys, 43 (2005) 104.
[11] R. B Adler, A. C Smith, and R. L Longini, Introduction to
semiconductor physics, 1964.