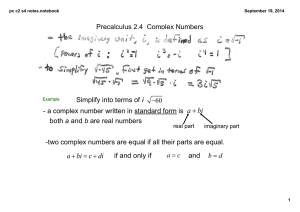
Working Backwards Hint If the Conjugate Base concentration is
advertisement
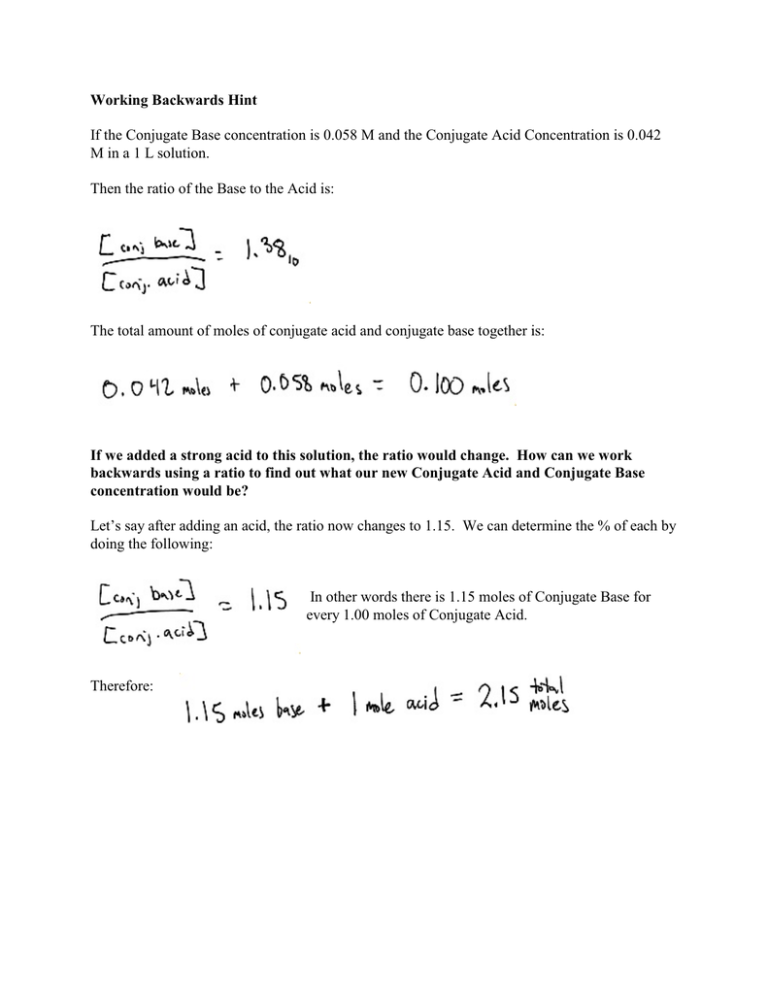
Working Backwards Hint If the Conjugate Base concentration is 0.058 M and the Conjugate Acid Concentration is 0.042 M in a 1 L solution. Then the ratio of the Base to the Acid is: The total amount of moles of conjugate acid and conjugate base together is: If we added a strong acid to this solution, the ratio would change. How can we work backwards using a ratio to find out what our new Conjugate Acid and Conjugate Base concentration would be? Let’s say after adding an acid, the ratio now changes to 1.15. We can determine the % of each by doing the following: In other words there is 1.15 moles of Conjugate Base for every 1.00 moles of Conjugate Acid. Therefore: Therefore if our original sample had 0.042 moles + 0.058 moles = 0.100 moles total. The New concentration of each would be: Let’s check our work to see if we calculated this right.