VIEW - Infobatt
advertisement

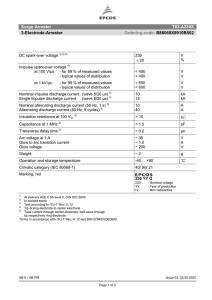
NiCd Battery Basics Agenda • Electrochemical reaction • NiCd battery characteristics • Typical failure modes • Different technologies • Main advantages/disadvantages Electrochemical reaction • Positive electrode (the nickel-hydroxide electrode): During discharge the Ni3+ ions are reduced to Ni2+ according to the (simplified) equation – NiOOH + H2O + e- => Ni(OH)2 + OH- • Negative electrode: This reacts via solution similar to the lead electrode, during discharge follows: – Cd + 2 OH- => Cd(OH)2 + 2 e- Discharge 2 Ni(OOH) + 2 H2O + Cd 2 Ni(OH)2 + Cd(OH)2 Recharge NiCd characteristics • Electrolyte – The electrolyte is alkaline – a solution of potassium hydroxide (KOH) – The electrolyte doesn’t change during charge & discharge – it retains the ability to transfer ions between cell plates – The specific gravity (nominal 1.20 g/cm3 at 20°C) remains the same regardless of the state of charge – Stratification does not occur NiCd characteristics • Plates – structural components are made of steel – Positive active material: Nickel hydroxide – Negative active material: Cadmium hydroxide • Alkaline electrolyte doesn’t react with steel - the plate integrity is maintained throughout the life of battery Typical “pocket plate” NiCd assembly NiCd characteristics • Voltage – The open circuit voltage of the NiCd system is 1.2 to 1.3 V (recognized nominal voltage is 1.2 V) – Requires more cells than lead acid batteries – Ex) 92 cells needed for substation battery string floating at 133V • Float current – Typical float current for pocket type NiCd cells is 100mA / 100 Ahr • Factors to consider when determining # of cells / string – Min/Max string voltages the system can handle – Must consider the float charge voltages NiCd characteristics • The equilibrium potential of the nickel-hydroxide electrode is slightly above that of water decomposition, so as in lead-acid batteries, oxygen evolution cannot be avoided at the positive electrode; however: – Corrosion of the nickel substrate in the + electrode is negligible – The reduction by oxygen at the nickel substrate of the negative cadmium electrode is faster than in lead-acid batteries - higher rate of self discharge compared to Lead Acid – The difference is self discharged NiCd cells can be recharged without loss in capacity – this is one of the major difference between NiCd and lead acid batteries Thermal characteristics • The reversible heat effect: – During discharge, the heat is generated – about 10.5 % of the discharged energy – During the charging process, the reversible heat effect causes cooling Thermal characteristics • Charge acceptance – Charge acceptance is significantly reduced at elevated temperatures, for example at the end of discharge, or after electrolyte filling at commissioning – Thus, it is necessary or recommended that batteries are allowed to cool before recharging (specially important for capacity witness testing) – Low temperatures also affect charging – compensate by raising the float voltage Failure modes for NiCd Cells • Main mechanism is Cadmium dendrite penetration through the separator, creating “soft shorts”, leading to full short circuits in time • Secondary mechanism is poisoning of the Positive active material by Iron (Fe) coming from the steel structure of the electrodes (mostly Nickel plated, but some small exposed areas) • Contamination of electrolyte from refilling water chlorine and carbonate Range of Plate technologies • There are 5 main technology types of NiCd plate available in the market today – – – – – Pocket Plate fibre (FNC) foam Sintered Sintered / plastic bonded Comparison of plates Performance classification Thickness (mm) Substrate / carrier Porosity of substrate / carrier (%) Capacity (Ah/cm3) Pocket Fibre Sintered L,M,H L-X 1.9 – 4.3 0.7 – 2.0 2 layer Ni-plated steeltape, perforated Ni-plated PP-felt 18 - 21 0.2 H-X Plasticbonded M, H, X Foam M, H, X 0.5 – 1.0 0.5 – 0.9 0.3 – 2.0 Single Single Ni-plated layer layer Polyurethane Nickel Nickel foam plated plated punched punched steel plate steel + sintered plate nickel > 90 80 95 > 90 NPPS: 50 0.25 – 0.4 0.4 0.5 – 0.6 0.2 - 0.6 NiCd sizing • NiCd sizing follows IEEE-1115 (latest revision) • Must have the following parameters – – – – – – Operating voltage window (min / max) Operating temperature End of discharge voltage Aging factor Design margin Load profile (# of steps, duration, and amount of current for each step) Configuration Options Mounting as compact blocks Assembling in stainless steel crates or battery trays Assembling in plastic crates Main advantages of NiCd • • • • • • Very good high power rating Reduced loss of capacity at low temperature No freezing at temperatures below 32°F (0°C) Robust against deep discharges Long shelf life No passivation in case of prolonged storage in partial discharge conditions • Robust against abusive environment & optimized for harsh operating conditions • No electrolyte stratification Main disadvantages of NiCd • Contains cadmium • Cost • More cells needed to reach operating voltage • Possible memory effect • High self discharge Summary • NiCd batteries have unique operating characteristics • NiCd batteries have distinct advantages and disadvantages • Choose the right battery for the application