Chapter 11 Atomic Mass Spectrometry
advertisement
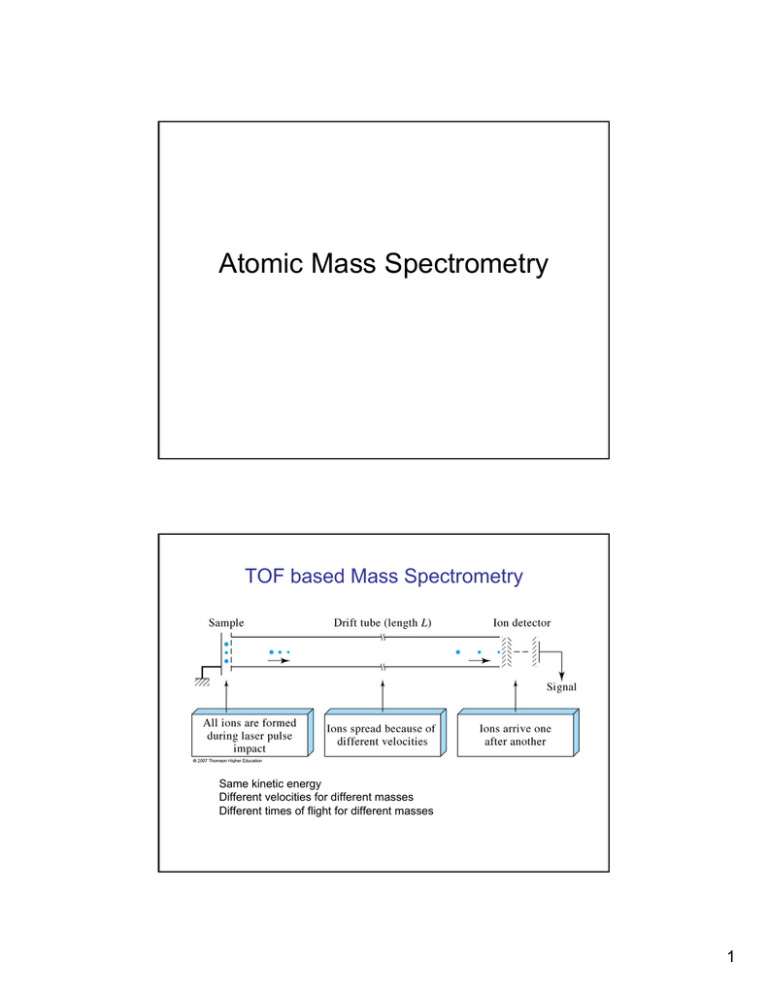
Atomic Mass Spectrometry TOF based Mass Spectrometry Same kinetic energy Different velocities for different masses Different times of flight for different masses 1 Effect of Magnetic Field on Moving Charged Particles Effect of Magnetic Field on Moving Charged Particles • Ions accelerated by a potential V into a magnetic field B • Trajectory in magnetic field is defined by the balance between the force of the magnetic filed on ion and the centrifugal force • The relationship between mass, radius, accelerating potential and magnetic field strength is m eB 2 R 2 = z 2V 2 Relationship between Mass, Radius of Trajectory, Field and Voltage force − of − magnetic − field − on − ion : FB = ezvB mv 2 Centrifugal − force : Fc = R mv 2 KE = = zeV 2 B : magnetic − field − strength R : radius − of − trajectory V : accelerating − voltage (1)ezvB = ( 2) mv 2 R m eRB = z v mv 2 = zeV (3) KE = 2 2 zeV ( 4) v = m m eRB (5) = z 2 zeV m 3 Obtaining a Mass Spectrum • Magnetic scanning: change current in a electromagnet – Keep V constant – Scan B – Keep R constant Quadrupole Analyser • Four parallel rod-like metal electrodes. • Direct (dc) and alternating voltages (ac) are applied to rods • Applied potentials of opposite rods are ¾ (U+Vcos(ωt)) ¾ -(U+Vcos(ωt)) • U is a dc voltage • Vcos(ωt) is an ac voltage. 4 The Quadrupole is a “Mass Filter”. – For given dc and ac voltages, only ions of a certain mass-to-charge ratio pass through the quadrupole filter and all other ions are thrown out of their original path. – A mass spectrum is obtained by monitoring the ions passing through the quadrupole filter as the voltages on the rods are varied. – There are two methods: varying ω and holding U and V constant, or varying U and V (U/V) fixed for a constant ω. The Quadrupole is a “Mass Filter”. • Generally the frequency of the ac field is constant • The radius is constant • Potential of direct current and alternating current are increased to allow all ions through the quadrupole sequentially 5 Quadrupole MS 6