Pie-Pan Convection
advertisement

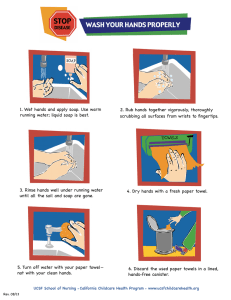
Welcome, Guest Login Teacher Tools Home Help Search Advanced Search Standards Assessment Curriculum Framework Instruction Materials & Resources Safe & Supportive Schools Activity Send to a Friend Printer Friendly Version Pie-Pan Convection Grade Levels 6th Grade , 7th Grade , 8th Grade Related Academic Standards Commonwealth of Pennsylvania Standards and Anchors 3.2.3.A3 Demonstrate how heating and cooling may cause changes in the properties of materials including phase changes. 3.2.3.B2 Explore energy’s ability to cause motion or create change. Explore how energy can be found in moving objects, light, sound, and heat. 3.2.3.B3 Explore temperature changes that result from the addition or removal of heat. 3.2.4.B1 Explain how an object’s change in motion can be observed and measured. 3.2.6.B3 Give examples of how heat moves in predictable ways, normally flowing from warmer objects to cooler ones until they reach the same temperature. Explain the effect of heat on particle motion by describing what happens to particles during a phase change. View All Content Provider © Exploratorium To Do and Notice Let your soap solution settle for a minute (so that there is very little fluid motion). Plug in the hot plate and place it on a low setting. Your hot plate should be hot enough within a few seconds. Place the pie-pan on top of the heating element. Watch what happens, but don't let the pan and liquid get too hot. The stearate molecules will break down and the pearly/metallic luster will vanish from the solution. After awhile remove the pan from the heating unit and place it on a flat cool surface. Wait and watch what happens now. Description By heating a solution of soap, water and food coloring, fluid motion can be visualized. Materials • Aluminum pie-pan • Hot plate • Dye --- any color • Tap water • Bottle of liquid hand soap or shampoo that has a pearly or metallic appearance. Look for the following ingredient on the label: glycol stearate, glycol distearate or glycerol stearate. Good brands to try are Softsoap® and Walgreen's Liquid Soap. Duration 20 - 25 minutes Assembly Fill the pie-pan between 1/2 to 3/4 full with tap water and squeeze in about 2 tablespoons of hand soap. Gently stir the soap and water; try not to create bubbles. Stir until the soap is mixed throughout the solution. To see convection currents and fluid flow more easily, darken the soapy solution by adding a few drops of dye to the mixture. Websites Exploratorium - Science Activities What's Going On? The soap solution at the bottom of the pan heats-up and becomes less dense. This lighter liquid rises in localized columns. When the warm fluid reaches the surface, it cools, becomes heavier and sinks. The region where the fluid rises and sinks is called a convection cell. When on the heater, convection cells rise directly above the heating element. This allows you to see the shape of the hot plate coil below. When on the flat cool surface, the convection process slows. This allows convection cells to widen and become extremely well defined. Etcetera Try doing this without a hot plate. Place the soapy solution on some one's warm hand and watch what happens. Back Copyright © 2013 Commonwealth of Pennsylvania About SAS Contact Us Terms of Use FAQ