Elektrik ve Manyetizma
advertisement
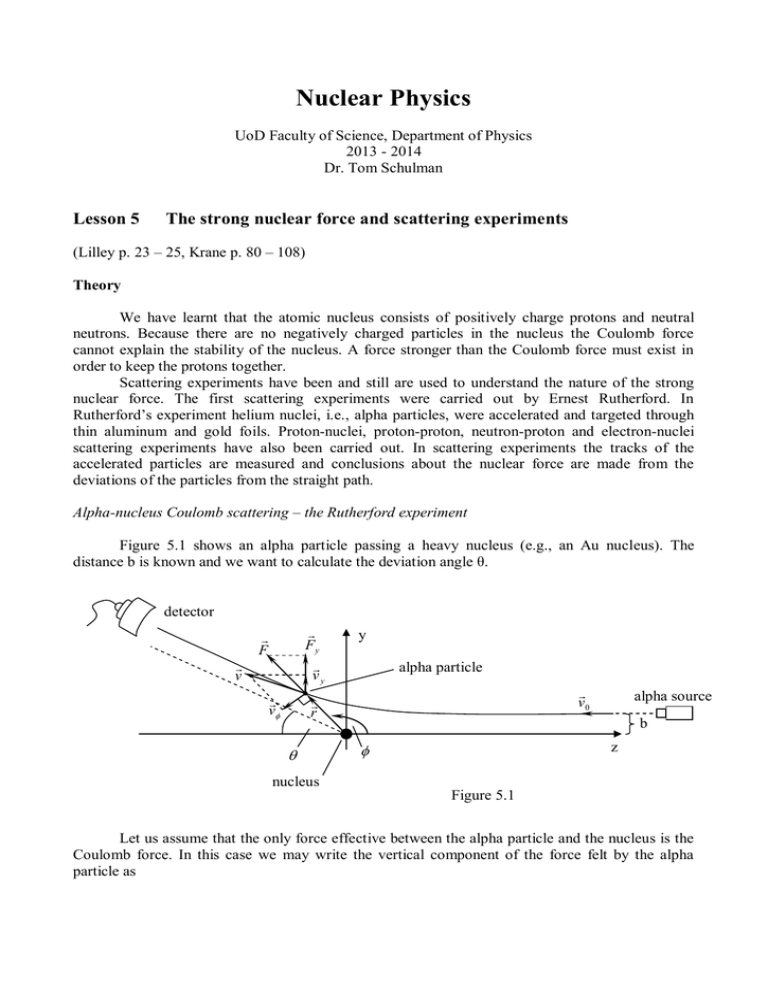
Nuclear Physics UoD Faculty of Science, Department of Physics 2013 - 2014 Dr. Tom Schulman Lesson 5 The strong nuclear force and scattering experiments (Lilley p. 23 – 25, Krane p. 80 – 108) Theory We have learnt that the atomic nucleus consists of positively charge protons and neutral neutrons. Because there are no negatively charged particles in the nucleus the Coulomb force cannot explain the stability of the nucleus. A force stronger than the Coulomb force must exist in order to keep the protons together. Scattering experiments have been and still are used to understand the nature of the strong nuclear force. The first scattering experiments were carried out by Ernest Rutherford. In Rutherford’s experiment helium nuclei, i.e., alpha particles, were accelerated and targeted through thin aluminum and gold foils. Proton-nuclei, proton-proton, neutron-proton and electron-nuclei scattering experiments have also been carried out. In scattering experiments the tracks of the accelerated particles are measured and conclusions about the nuclear force are made from the deviations of the particles from the straight path. Alpha-nucleus Coulomb scattering – the Rutherford experiment Figure 5.1 shows an alpha particle passing a heavy nucleus (e.g., an Au nucleus). The distance b is known and we want to calculate the deviation angle θ. detector Fy vy F v v y alpha particle v0 r alpha source b z nucleus Figure 5.1 Let us assume that the only force effective between the alpha particle and the nucleus is the Coulomb force. In this case we may write the vertical component of the force felt by the alpha particle as Fy F sin Qq 2Ze 2 sin sin 4 0 r 2 4 0 r 2 (5.1) In equation 5.1 Q = Ze is the charge of the atomic nucleus and q = 2e is the charge of the alpha particle. Then the equation of motion is Fy ma m dv y dt dv y dt 2Ze 2 sin 4 0 r 2 m (5.2) The angular momentum of the alpha particle must remain equal to the initial angular momentum: mv0 b mv r m d 2 1 1 d r 2 dt v0 b dt r (5.3) Applying equation 5.3 to equation 5.2 gives dv y dt 2Ze 2 d 2Ze 2 sin dv y sin d 4 0 mv0 b dt 4 0 mv0 b (5.4) Integrating both sides (with vy = 0 and = 0 at the beginning and vy = v0sin and = - at the end) gives v0 sin 2Ze 2 dv 0 y 4 0 mv0b Because tan 2 sin d v0 sin 0 2Ze 2 (1 cos ) 4 0 mv0 b (5.5) sin tan we can write 1 cos 2 2Ze 2 4 0 mv02 b (5.6) The scattering angle is plotted as a function of b in figure 5.2 when v0 1.0 10 6 m s . 180,00 150,00 120,00 90,00 60,00 30,00 0,00 0,0E+00 2,0E-12 4,0E-12 6,0E-12 Mesafe b/m 8,0E-12 1,0E-11 Figure 5.2 If the only force effective between the alpha particle and the nucleus is the Coulomb force then the calculated curve plotted in figure 5.2 should match experimental observations. But this is not the case in reality. If b is very small and v0 is high enough the measured values of are different from the ones predicted by the calculation above. This means that there must be a force present in the nucleus that is effective at very small distances. This force is called the strong nuclear force. In actual real scattering experiments the target is a thin sheet of metal. A beam of alpha particles is directed towards and through the metal sheet. The distance b for the many alpha particles differs slightly for different alphas and the alphas thus scatter as shown in figure 5.3. We therefore define a scattering cross section σ as The number of scattered particles per unit solid angle and per unit time The number of particles hitting the metal sheet per unit time ( ) (5.7) metal sheet d alpha beam nucleus b Figure 5.3 db If N is the density of alphas in the beam hitting the metal sheet then the number of alphas passing the sheet per unit time in a circular section of radius b and width db is n v0 N (2bdb) . When these alphas pass through the metal sheet they scatter into the solid angle d. Then the scattering cross section is ( ) v0 N (2bdb) db 2b v0 Nd d (5.8) The distance b and db can be solved from equation 5.6 and expressed as a function of the scattering angle and d. The solid angle d can be written as d 2 sin d . We then get for the scattering cross section (after some trigonometry) coulomb( ) Z 2e4 (4 0 ) 2 m 2 v04 sin 4 ( 2) (5.9) coulomb( ) - /rad Figure 5.4 The scattering cross section is plotted in figure 5.4 as a function of the scattering angle . The calculations above were carried out according to the classical theory of mechanics but the result is essentially the same if quantum theory had been used. From the difference between the measured cross section and the coulomb cross section coulomb of equation 5.9 it is possible to make conclusions about the strong nuclear force. Neutron-proton scattering In neutron-proton scattering experiments neutrons from a nuclear reactor are guided through a hydrogen target and the scattered neutrons are measured with a neutron detector. Since the neutrons are neutral the scattering is caused only by the strong nuclear force. Using the neutron wave functions from quantum theory it is possible to calculate the scattering cross section of neutron-hydrogen (i.e., neutron-proton) scattering. Again, by comparing the theoretical results to the experimental one can obtain an understanding of the nuclear strong force. Electron-nucleus scattering High energy electrons may pass through a nucleus. Then the scattering is effected not only by the protons but also by the quarks of the nucleons. Electron-nucleus scattering experiments have provided important knowledge on the inner structure of the nucleons (quarks). Based on the various scattering experiments the following conclusions about the nuclear force have been made: 1. The range of the nuclear force is very short. Two particles interact through the nuclear force only if the distance between them is < 10-15 m. 2. The nuclear force is not dependent on the electrical charge. 3. The nuclear force is not completely central. This result has been concluded from the nonconstant angular momentum of the two nucleons of the deuteron ( 12 H ) nucleus. 4. The nuclear force becomes repulsive at very short distances (<<10 -15 m). 5. The nuclear force is affected by the spin of the nucleons. Despite all this the exact form of the potential energy of the nuclear force is unknown. The Japanese physicist Hideki Yukawa has suggested an exponential function to describe the nuclear potential: E p (r ) E0 r0 e r r0 r (5.10) The Yukawa potential falls rapidly to zero when r increases but it does not explain the repulsive nature of the nuclear force at very short distances. The estimate of the nuclear potential based on experiments is shown below in figure 5.4. Ep r Figure 5.4 Examples 5.1 Proton-nucleus collision Figure 5.5 shows the potential energy of a gold nucleus (Z = 79), i.e., the combination of the energy from the nuclear and from the Coulomb force. Let us calculate the minimum kinetic energy required for a proton to reach the effective range of the nuclear strong force. Ep Emin proton r0 = 10-15 m r Figure 5.5 The kinetic energy of the proton is E k Ep 1 2 mv and the electric potential energy of the nucleus is 2 Ze 2 . The kinetic energy of the proton must exceed the Coulomb barrier: 4 0 r Ek E p (r0 ) 79e 2 1.823 10 11 J 114 MeV vmin 4 0 r0 2Ek 148 10 6 m s . m proton 5.2 Rutherford’s estimation of the radius of the Au nucleus In Rutherford’s first experiments alpha particles were accelerated against gold foils. Some of the alphas returned back at 180. Because the speed of these alphas were equal to the initial speed one may estimate the the radius of the nucleus by writing V Electric potential 1 2 79e 2 79e 2 malphav 2 rAu 2 4 0 rAu 0 malphav 2 r0 Rutherford’s result was r rAu 3.2 10 14 m . This corresponded to the maximum speed v 18 10 6 m s of the alphas returning at 180 (alphas faster than this did not return, why?). The nuclear radius estimated by Rutherford is not exactly in agreement with equation 1.1 but was still a very important result for the scientific community 100 years ago. Problems 5.1 Calculate the scattering angle for b 1 10 10 m and b 1 10 11 m (figure 5.1). If v0 1 10 7 m s how do the values of change? 5.2 Find the limit for b after which > 90 when v0 1 10 7 m s in figure 5.1. 5.3 Find the limit for b after which < 1 when v0 1 10 7 m s in figure 5.1. Compare the result to the radius of the gold atom which is 1.2 10 10 m . 5.4 Find the radii of the silver and the aluminum nuclei based on Rutherford’s experiment in example 5.2 and compare the results with the results given by equation 1.1 (Ag: Z = 47, Al: Z = 13 and v 18 10 6 m s is the maximum speed of the alphas returning at 180 degree). 5.5 a) Draw a graph of the Yukawa potential. b) Sketch a graph of the total nuclear potential (including the Coulomb effect) according to experimental results.





