Packing Density & Coordination: Materials Science Presentation
advertisement
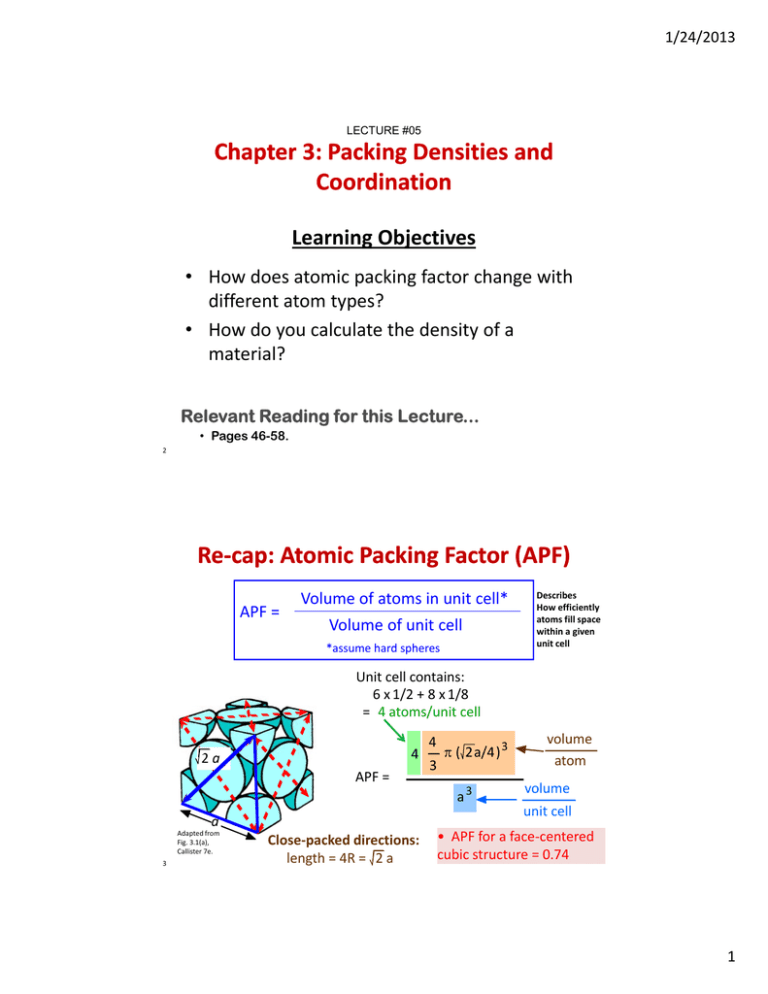
1/24/2013 LECTURE #05 Chapter 3: Packing Densities and Coordination Learning Objecti es Learning Objectives • How does atomic packing factor change with different atom types? • How do you calculate the density of a material? l? Relevant Reading for this Lecture... • Pages 46-58. 2 Re‐‐cap: Atomic Packing Factor (APF) Re Volume of atoms in unit cell* APF = Volume of unit cell * *assume hard spheres h d h Describes How efficiently atoms fill space within a given unit cell unit cell Unit cell contains: 6 x 1/2 + 8 x 1/8 = 4 atoms/unit cell 4 2a 2 a APF = 4 3 ( 2a/4) ( )3 a3 a Adapted from Fig. 3.1(a), Callister 7e. 3 volume atom volume unit cell Close‐packed directions: • APF for a face‐centered cubic structure = 0.74 length = 4R = 2 a 1 1/24/2013 If the unit cell has different atoms – watch out! Ceramic Crystal y Structures! 4 Watch Out! Different Atoms. Must Modify APF Equations. 3a Rblue a a 2a Close‐packed directions: Adapted from Fig. 3.2(a), Callister 7e. a diagonal = 2Rblue + 2Rred = 3 a Must put in correct value for each atom type for each atoms unit cell 2 APF = 5 4 3 ( 3a/4) 3 a3 volume atom volume unit cell 2 1/24/2013 What if ions from the crystal structure? Cs+ Clԟ Same rules as metals w/respect to crystal symmetry. Structure: CsCl type Bravais lattice: simple cubic Ions/unit cell: 1 Cs+ + 1 Clԟ Ionic packing factor (IPF), similar definition at APF. Cesium chloride (CsCl) unit cell showing (a) ion positions and the two ions per lattice point and (b) full‐size ions. Note that the Cs+−Cl− pair associated with a given lattice point is not a molecule because the ionic bonding is non‐directional and because a given Cs+ is equally bonded to eight adjacent Cl−, and vice versa. 6 Calculate the following: (1) The CN of CsCl (2) Ionic Packing Factor of CsCl Recall from the last lecture CN → r/R =r +/r – What are the r’s for Cs+ and Cl-? r Cs+ = 0.170 nm r Cl- = 0.181 nm These numbers came from inside cover of text book! r +/r – = 0.939 7 3 1/24/2013 Now calculate the Ionic Packing Factor of CsCl For these ions, they touch along the body diagonal (make sure you know which direction the ‘hard spheres’ are touching) [# of atoms/unit cell] [volume of a sphere] IPF = volume of unit cell One atom of Cs + One atom of Cl 4/3π(0.170nm)3 + 4/3π(0.181nm)3 VCl VCs 3a = 0.0454 0 0454 nm3 a r Cs+ = 0.170 nm r Cl- = 0.181 nm √3 a = 2rCs+ + 2rCla = 0.405 nm V = a3 = 0.0664nm3 2a IPF = 0.683 8 For kicks, what if the ions touched along the edge length (not the body diagonal)? [# of atoms/unit cell] [volume of a sphere] IPF = volume of unit cell O t t One atom off C Cs + O One atom off Cl 4/3π(0.170nm)3 + 4/3π(0.181nm)3 r Cs+ = 0.170 nm r Cl- = 0.181 nm √3 a = 2rCs+ + 2rCla = 0.405 nm V = a3 = 0.0664nm3 VCl VCs = 0.0454 nm3 which r? average them? a = 2r a = 0.405 nm 0.352nm V = a3 = 0.0436nm3 ravg = 0.176 0 176 IPF = 0.683 1.04 Not possible! >100% packing!!! 9 It is critical that you identify the correct ‘hard sphere’ touching directions! 4 1/24/2013 Let’s do another example: Consider NaCl What is the CN? What is the IPF? How do we determine CN? Correct, CN → r +/r – What are the r’s for Na+ and Cl-? r Na+ = 0.102 nm r Cl- = 0.181 nm Again, got these numbers from inside cover of text book! r +/r – = 0.564 10 Not a very clear representation – let’s expand it! Motif 2 ions per lattice point Do you see how the green spheres form a FCC structure?! The blue spheres do the same thing, form a FCC structure We have two interpenetrating FCC lattices Commonly called a Rock Salt structure – structure seen in other materials including TiC, TaC, MgO, CaO, etc. 11 5 1/24/2013 Calculate the IPF of NaCl IPF = [# of atoms/unit cell] [volume of a sphere] volume of unit cell What type of unit cell do we have? Two FCC lattices – one for Na and one for Cl 4 atoms/Na FCC + 4 atoms/Cl FCC = 8 atoms/unit cell total Four atoms of Na + Four atoms of Cl 4 x 4/3π(0.102 nm)3 + 4 x 4/3π(0.181 nm)3 = 0.117nm3 12 What is the volume of the unit cell? a = 2r Na + 2rCl a = 0.566nm V = a3 = 0.181nm3 IPF = 0.117nm3 (previous slide) 0.181nm3 = 0.65 W t h O t! Diff Watch Out! Different Ions. tI 2 a a CORRECTION! Though it looks like FCC symmetry, the face diagonal atoms don’t touch; but the edge atoms do touch! Why? Cations and anions do not have the same size! Before we considered atoms of the same size 13 6 1/24/2013 Atoms can occupy ‘interstitial’ sites Unoccupied space in center can accommodate small atoms, e.g. He in UO2 fuel rods Fl it (C F2) Fluorite (CaF ) unit cell showing (a) ion positions (b) full‐size ions. FCC interstitial sites FCC interstitial sites Note: useful info on atom placement Structure: fluorite (CaF2) type Bravais lattice: FCC Ions/unit cell: 4Ca2+ + 8FTypical ceramics: UO2, ThO2, TeO2 14 CLASS ROOM EXAMPLE: Calculate the ionic packing factor for UO2, which has the CaF2 structure U and O ions touch along a portion of the body diagonal 15 7 1/24/2013 CLASS ROOM EXAMPLE: SOLUTION Calculate the ionic packing factor for UO2, which has the CaF2 structure This problem is tricky! The face diagonal has a length of 2a. The body diagonal has a length of 3a. Along with the cell edge, they form a right triangle within the unit cell. U2+ a 3a O2a a a The Ca2+ and F- ions touch a short distance along the body diagonal. By the principle of similitude, this smaller triangle, measures as ¼ the size of the large triangle one. Because of this, the length of the bond becomes: 1 3a RU 4 RO 2 0.105 0.132 nm 0.548 nm 4 Now you can calculate a and the corresponding unit cell volume. 16 CLASS ROOM EXAMPLE: SOLUTION ‐ continued 3a RU 4 RO2 0.105 0.132 nm 4 IPF Vions in unit cell Vunit cell Solving for a we get: a 0.548 nm Vunit cell a 3 0.548 nm 0.164 nm3 3 4 Vsingle ion R 3 3 4 4 16 32 3 3 Vions 4 RU3 4 8 RO3 2 0.105 0.132 0.0965 nm3 3 3 3 3 IPF Vions 0.0965 nm3 0.588 Vunit cell 0.164 nm3 17 8 1/24/2013 THEORETICAL DENSITY, Density = mass/volume mass = number of atoms per unit cell mass of each atom mass = number of atoms per unit cell mass of each atom mass of each atom = atomic weight / Avogadro’s number # atoms/unit cell nA VcNA Volume/unit cell (cm3/unit cell) Atomic weight (g/mol) Avogadro's number (6.023 x 10 23 atoms/mol) 18 THEORETICAL DENSITY, # atoms/unit cell nA VcNA Volume/unit cell (cm3/unit cell) Atomic weight (g/mol) Avogadro's number (6.023 x 10 23 atoms/mol) Example: Copper • crystal structure = FCC: 4 atoms/unit cell • atomic weight = 63.55 g/mol (1 amu = 1 g/mol) • atomic radius R 0 128 nm (1 nm 10‐7 cm) • atomic radius R = 0.128 nm (1 nm = 10 Vc = a3 ; For FCC, a = 4R/ 2 ; Vc = 4.75 x 10-23cm3 Result: theoretical Cu = 8.89 g/cm3 Compare to actual: Cu = 8.94 g/cm3 Why the difference? 19 9 1/24/2013 Theoretical Density, • Ex: Cr (BCC) A = 52.00 g/mol R = 0.125 nm n = 2 R a a = 4R/ 3 = 0.2887 nm atoms g mol 2 52.00 unit cell = a 3 6.023 x 1023 volume theoretical = 7.18 g/cm3 actual = 7.19 g/cm3 atoms mol unit cell 20 20 Densities of Material Classes In general metals > ceramics > polymers Why? Metals/ Alloys 30 Metals have... have Ceramics have... • less dense packing • often lighter elements Polymers y have... • low packing density (often amorphous) • lighter elements (C,H,O) Composites have... • intermediate values 3 (g/cm ) • close‐packing (metallic bonding) • often large atomic masses 20 Platinum Gold, W Tantalum 10 Silver, Mo Cu,Ni Steels Tin, Zinc 5 4 3 2 Titanium Aluminum Magnesium Graphite/ Ceramics/ Semicond Polymers Composites/ fibers B ased on data in Table B1, Callister *GFRE, CFRE, & AFRE are Glass, Carbon & Aramid Fiber‐Reinforced Carbon, & Aramid Fiber Reinforced Epoxy composites (values based on 60% volume fraction of aligned fibers in an epoxy matrix). Zirconia Al oxide Diamond Si nitride Glass ‐soda Concrete Silicon G raphite 1 0.5 0.4 0.3 PTFE Silicone PVC PET PC H DPE, PS PP, LDPE Glass fibers GFRE* Carbon fibers CFRE * A ramid fibers AFRE * Wood Data from Table B1, Callister 7e. 21 10 1/24/2013 Summary • If there are different ions in the unit cell, APF/IPF equations must be modified! Find out APF/IPF equations must be modified! Find out which direction the ‘hard spheres’ touch • The ionic packing factor can be calculated by IPF • Vions in unit cell Vunit cell Theoretical Density can be calculated by Theoretical Density can be calculated by # atoms/unit cell nA VcNA Volume/unit cell (cm3/unit cell) Atomic weight (g/mol) Avogadro's number (6.023 x 10 23 atoms/mol) 22 11




