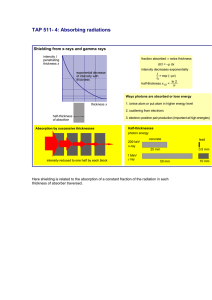
Linear Absorption Coefficient Experiment
advertisement

Experiment 3 Linear Absorption Coefficient Objective: 1- Verification of the absorption law of Gamma radiation. 2- Determination: a. The linear absorption coefficient (µ). b. The mass absorption coefficient. c. The half value thickness of the absorbing material (X 1/2). 3- Verification of the relation between the atomic number (Z) and linear absorption coefficient (µ) for the absorbing materials. Theory: When Gamma radiation passes through matter, it undergoes absorption primarily by Compton, photoelectric and pair production interactions. The intensity of the radiation is thus decreased as a function of thickness of the absorbing material. The mathematical expression for intensity ( I ) is given by the following expression: (1) where, I0 is the original intensity of the beam. I is the intensity transmission through an absorber to thickness X. µ is the linear absorption coefficient for the absorbing material. If we rearrange eq.(1) and take the logarithm of both sides, the expression becomes, X (2) The half value layer (HVL) of the absorbing material is defined as that thickness X1/2 which will cut the initial intensity in half. That is, I=I0/2. 1 Prepared By Najat AL-Twarqi If we substitute this into eq.(2), (3) Putting in numerical values and rearranging eq.(3) becomes, (4) Radiation strongly depends on the material density, this dependence is revealed by dividing the linear absorption coefficient by the material density ρ, this is called the mass absorption coefficient. Mass absorption coefficient = µ / ρ (5) Apparatus: Source of radiation . Sheets of different absorbing materials (Aluminum and Lead). Geiger detector. HV power supply. Procedure: 12345678- Connect the plugs of the electric mains. Set the timer to 60s and the operating voltage to 380 Volt. Record the count rate per one minute for the back ground (IB.G). Put the source in front of the GM tube. Record the count rate ( I0 ). Place Al sheet midway between the source and the GM tube. Record the count rate (I1 and I2) and then find Iavg.. Repeat steps 6 and 7 with increasing the thickness of the absorbing material. 9- Place Pb sheet and repeat steps 6,7 and 8. 2 Prepared By Najat AL-Twarqi 10- Plot a graph between ln( I0 / I ) and thickness X, if the relation is a straight line, then the absorption law is verified. 11- Find the slope from the graph, this is equal to the linear absorption coefficient. 12- Calculate the mass absorption coefficient. 13- Plot a graph between ( I ) and thickness (X), then find the value of the half thickness graphically. 14- Calculate the half thickness theoretically by using eq.(4). 15- Plot a graph between the linear absorption coefficient (µ) and atomic number (Z). 3 Prepared By Najat AL-Twarqi Results: Source description Activity (A0) ( µCi ) Element For Al: Z = 13 and For Pb: Z = 82 and Half life (t1/2) ( year ) ρ = 2.7 g/cm3. ρ = 11.4 g/cm3. IB.G = ……….. min-1. I' 0 = ………… min-1. I0 = I' 0 - IB.G = ……… min-1 sheet Strip no. X (cm) I1 (min-1) and I2 (min-1) I0 /2 = ……… min-1. Iavg. (min-1) I =Iavg.-IB.G (min-1) ln( I0 / I) Al Pb Thickness(X) = , where ρ is the density of absorbing material. 4 Prepared By Najat AL-Twarqi µ= slope = ……….. cm-1. ( From Fig.1) Mass absorption coefficient = ………… cm2/g. (X1/2 )Thoertically = ln (2)/ µ= ………. cm. (X1/2 )graphically = ………..cm. ( From Fig.2) I (min-1) ln(I0/I) I0 I0/2 X (cm) X1/2 Fig.1 X (cm) Fig.2 µ (cm-1) Z Fig.3 5 Prepared By Najat AL-Twarqi