Units and SI Prefixes
Matter has both physical and chemical properties. Physical properties include mass and size.
Physical properties are measured. Each measurement contains a number (magnitude) and a
unit indicating size and has a degree of uncertainty (precision). There are seven fundamental
units in science. All other units are derived from these seven. In beginning chemistry courses
only five of the fundamental units are used.
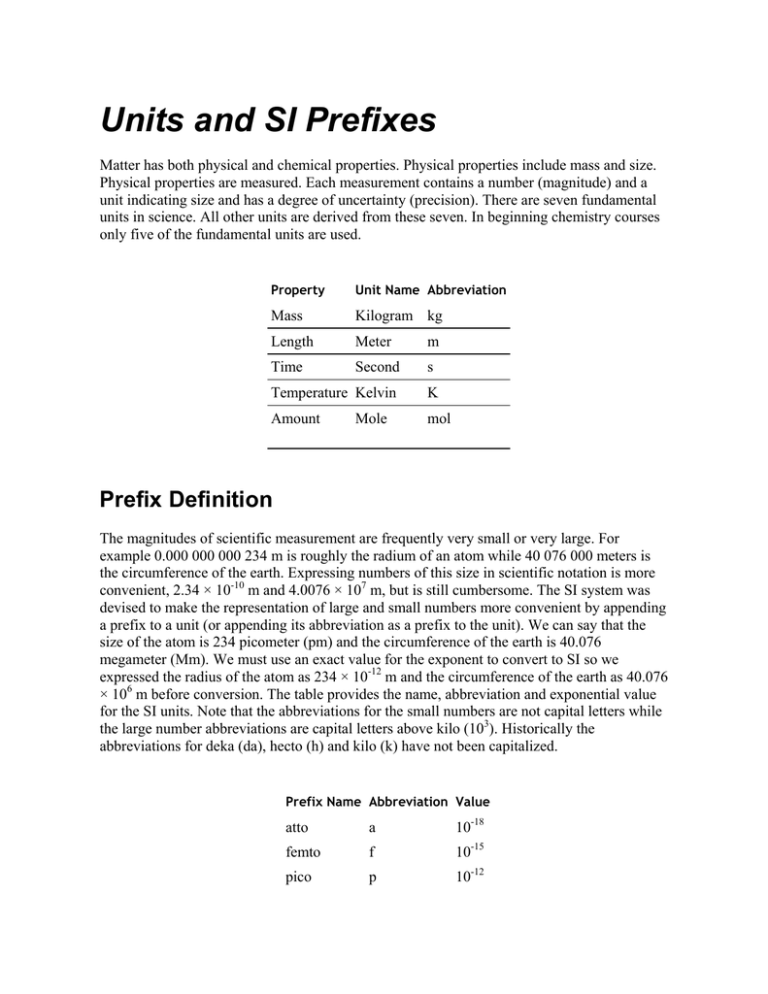
Property
Unit Name Abbreviation
Mass
Kilogram kg
Length
Meter
m
Time
Second
s
Temperature Kelvin
K
Amount
mol
Mole
Prefix Definition
The magnitudes of scientific measurement are frequently very small or very large. For
example 0.000 000 000 234 m is roughly the radium of an atom while 40 076 000 meters is
the circumference of the earth. Expressing numbers of this size in scientific notation is more
convenient, 2.34 × 10-10 m and 4.0076 × 107 m, but is still cumbersome. The SI system was
devised to make the representation of large and small numbers more convenient by appending
a prefix to a unit (or appending its abbreviation as a prefix to the unit). We can say that the
size of the atom is 234 picometer (pm) and the circumference of the earth is 40.076
megameter (Mm). We must use an exact value for the exponent to convert to SI so we
expressed the radius of the atom as 234 × 10-12 m and the circumference of the earth as 40.076
× 106 m before conversion. The table provides the name, abbreviation and exponential value
for the SI units. Note that the abbreviations for the small numbers are not capital letters while
the large number abbreviations are capital letters above kilo (103). Historically the
abbreviations for deka (da), hecto (h) and kilo (k) have not been capitalized.
Prefix Name Abbreviation Value
atto
a
10-18
femto
f
10-15
pico
p
10-12
nano
n
10-9
micro
μ
10-6
milli
m
10-3
centi
c
10-2
deci
d
10-1
----
----
100=1
deka
da
101
hecto
h
102
kilo
k
103
Mega
M
106
Giga
G
109
Tera
T
1012
Peta
P
1015
Exa
E
1018
These are the most frequently used names and abbreviations. The names and abbreviations do
extend beyond the extremes (10-18 and 1018) in either direction but these additional SI prefixes
are not listed in this table. An important skill to master is converting between scientific
notation and the SI representation of a number and the reverse.
Converting from a Prefix Unit to an Exponential
Representation
Use the table to establish an identity relationship. Write down the name of the unit for which
your want the identity relationship.
1 picosecond = ? second
Substitute the prefix name with the prefix value to get the identity relationship.
1 picosecond = ? second
Replace the prefix “ pico” with the value “ 10-12” .
1 picosecond = 10-12 second
Keep the unit.
In abbreviated form: 1 ps = ? s
Replace “ p” by “ 10-12” to get 1 ps = 10-12 s
The same process works for the other units.
1 picometer = ? meter
Replace the prefix “ pico” with the value “ 10-12”
1 picometer = 10-12 meter
Keep the unit.
In abbreviated form: 1 pm = ? m
Replace “ p” by “ 10-12” to get 1 pm = 10-12 m
The process is the same whether the value is large or small
1 Gigameter = ? meter
Replace the prefix “ Giga” with the value “ 109”
1 Gigameter = 109 meter
Keep the unit.
In abbreviated form: 1 Gm = ? m
Replace “ G” by “ 109” to get 1 Gm = 109 m
1 Megagram = ? gram
Replace the prefix “ Mega” with the value “ 106”
1 Megagram = 106 gram
Keep the unit.
In abbreviated form: 1 Mg = ? g
Replace “ M” by “ 106” to get1 Mg = 106 g
Conversion ratios are used in solving many common problems in beginning science courses.
Conversion ratios can be derived from either the long form or abbreviated form.
Two conversion ratios can be obtained by replacing the prefix or its abbreviation in the
numerator and denominator of the fraction with the exponential value.
The procedure works for both very small and very large numbers.
For convenience the abbreviated prefix units are normally used during calculations.
It is important to always use the same value for the exponent. Never change the sign of the
exponent when determining conversion ratios. For example the ratio for meter (m) and
centimeter (cm) and liter (L) and milliliter (mL) are as follows.
Do not use 102 cm = 1 m (100 cm = 1m) or 103 mL = 1 L (1000 mL = 1 L). It may seem
easier to use these relationships but unless you are very careful they can lead to confusion and
errors.
Converting From Exponential Representation to a
Prefix Unit
The reverse process can be used to go from an exponential number with fundamental units to
a number without exponents with the corresponding prefix unit. In most cases you will be
dealing with abbreviated prefixes and units as in the examples.
2.34 × 10-6 s Replace × 10-6 with the abbreviated form of micro, μ.
2.34 × 10-6 s = 2.34 μs
The exponents in the number must match one of the prefix values. Adjust the exponent value
so that this is the case.
Consider the number 45.6 × 104 m
Convert first to scientific notation → 4.56 × 105 m
Then convert to a prefix value → 456 × 103 m or 0.456 × 106 m
Then substitute names for exponents → 456 km or 0.456 Mm
Note: the distance is the same, just the name is different.
Prefix Definition
Question: How many megameters are in 1 meter. (1 Mm = 106 m)
Solution: Recognize that “ mega” is 106. There are two conversion ratios.
Question: How many seconds are in 1 kilosecond. (1 ks = 103 s)
Solution: Recognize that “ kilo” is 103. There are two conversion ratios.
Question: How many mol are in one mmol?
Solution: Recognize that “ m” is the abbreviation of “ milli” which is 10-3. There are two
conversion ratios.
Question: How many nanonewtons are in one N?
Solution: Recognize that “ nano” is 10-9. There are two conversion ratios.
Question: One _________________ equals 10-6 volt.
Solution: The prefix for 10-6 is micro with the abbreviation “ μ” . The answer is
“ microvolt” or “ μV” .
Metric Conversion
Question: How many seconds are in 1.20 × 10-2 microsecond? Express your answer in
standard scientific notation.
Solution: Micro is 10-6. One microsecond is 10-6 second (1 μs = 10-6 s). There are two
conversion ratios.
Note: The answer has the proper units of second as the microsecond units cancel.
Question: How many V are in 0.466 nanovolt? Express your answer in standard scientific
notation.
Solution: Nano is 10-9. One nanovolt is equal to 10-9 volts (1nV = 10-9 V). There are two
conversion ratios.
Metric Volume, Area and Density
Question: How many dm3 are present in 135 liters? Express your answer in standard
scientific notation.
Solution: Recognize that 1 cm3 = 1 mL and 1 mL = 10-3 L. Also recognize that 1 cm = 10-2 m
while a decimeter is one tenth of a meter (1 dm = 10-1 m). Use these conversion ratios.
Note: The answer is exactly the same as the starting value since the definition of a L is a
decimeter cubed (1 L = 1 dm3). The problem is more efficiently solved using the conversion
ratios of the definition.
Question: The density of a gaseous substance was determined to be 1.66 g/L. What is the
density in microgram/cubic millimeter? Express your answer in standard scientific notation.
Solution: Recognize that 1 cm3 = 1 mL and 1 mL = 10-3 L so that 1 cm3 = 10-3 L. Also
conversion factors must be cubed in order to attain the proper units. Use the following
conversion ratios.
Note the magnitude of the number is exactly the same as for the starting units.
English/Metric Conversion
Question: Convert 8.05 yd into centimeters. Express your answer in scientific notation. (1 in
= 2.54 cm)
Solution: In every yard there are three feet (1yd = 3 ft). In every foot there are 12 inches (12
in = 1ft). In every inch there are 2.54 centimeters (1 in = 2.54 cm). Express these relationships
as conversion ratios.
Use the conversion ratios to convert from yd → ft → in → cm.
Note: The units of feet, yard and inch cancel to leave the answer in the desired unit of
centimeter:
Question: Convert 24.2 bar into μPa. Express your answer in standard scientific notation. (1
bar = 105 Pa)
Solution: For every bar there are 105 Pa (1 bar = 105 Pa). Micro (μ) is 10-6. One μPa is equal
to 10-6 Pa (1 μPa = 10-6 Pa). There are two conversion ratios. Convert from bar → Pa → μPa.
Question: Convert 0.564 kJ into British thermal units. Express your answer in standard
scientific notation. (1 Btu = 1055.06 J)
Solution: For every Btu there are 1055.06 J (1 Btu = 1055.06 J). Kilo is the standard prefix
for 103 (1 kJ = 103 J). Convert kJ → J → Btu. There are two conversion ratios.
Note: The original number had three significant figures so that is the number of significant
figures the answer should have.
Volume, Area and Density Conversion
Question: Convert 913 ft3 into kL. Express your answer in standard scientific notation.
Solution: Convert cubic ft into cubic inches, cubic inches into cubic centimeters, cubic
centimeters into liters and liters into kiloliters (ft3→ in3 → cm3 → L → kL). There are 2.54
cm to an inch. Recall that 1 cm3 = 1 mL and the prefix k is kilo and it has a value of 103. Use
these conversion ratios.
Note: Remember to cube the numerical values when the units are cubed.
Question: Convert 234 km3 into in3. Express your answer in scientific notation.
Solution: Convert cubic kilometers into cubic meters and cubic meters into cubic centimeters
and cubic centimeters into cubic inches ( km3 → m3 → cm3 → in3). There are exactly 2.54 cm
in every inch.
Copyright © 2007 John Wiley & Sons, Inc. All rights reserved.

