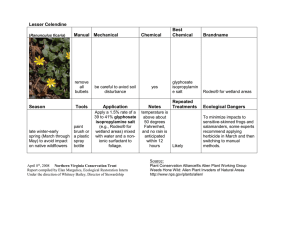
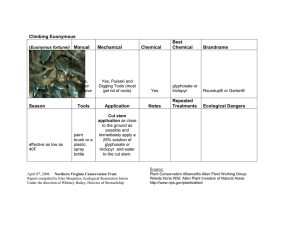
Glyphosate and Spray Water Quality
advertisement

Proceedings of the 2007 CPM Short Course and MCPR Trade Show December 4 – 6, 2007 Minneapolis Convention Center Do not Reproduce or Redistribute Without Written Consent of the Author(s) Improving Glyphosate Performance Calvin G. Messersmith Professor Emeritus Plant Sciences Department North Dakota State University Fargo, North Dakota USA Acknowledgments • Dr. Brad Ramsdale – Nebraska College of Technical Agriculture-Curtis • Dr. John Nalewaja – Professor Emeritus, North Dakota State University • Dr. Zenon Woznica – Visiting Scientist, Poznan (Poland) Agricultural University • Dr. Jingkai Zhou – Former Research Assoc., North Dakota State Univ. Major factors affecting glyphosate efficacy • Chemical and physical characteristics of glyphosate – Water solubility – Antagonistic cations in spray water and environment • Application factors – Spray volume – Sprayer nozzles – Adjuvants • Ammonium sulfate • Surfactants Glyphosate acid O H O - H HO - C - CH2 - N - CH2 - P - OH OH • pKa – 1st phosphonic – • Carboxylate – • 2nd phosphonic • Amine - 0.8 2.3 6.0 11.0 + Glyphosate acid O H O - HO - C - CH2 - N - CH2 - P - OH OH • Sources of ions that affect glyphosate – Glyphosate formulation – Soil – many + and - charges – Spray water – Ca+2, Mg+2, Na+ – Plant factors: surface Ca+2 , stress H+ Water solubility of various salts • Glyphosate salts – Acid – Ammonium – Calcium – Isopropylamine – Potassium – Sodium 160 g/L 300 g/L 30 g/L 500 g/L 900 g/L 500 g/L Water solubility of various salts • Glyphosate salts – Acid – Ammonium – Calcium – Isopropylamine – Potassium – Sodium 160 g/L 300 g/L 30 g/L 500 g/L 900 g/L 500 g/L ~1 lb/gal 3 lb/gal 4.5 lb/gal Reaction of cations with glyphosate K O H O HO - C - CH2- N - CH2- P - O OH CH3 + H3KN - CH + CH3 + Absorption of glyphosate by plants Glyphosate salt Acid Isopropylamine Ammonium Sodium Calcium Potassium LSD (0.05) % 14C absorbed 49 59 44 18 1 ? 5 Nalewaja, J.D., B.L. deVilliers, and R. Matysiak. 1996. Surfactant and salt affect glyphosate retention and absorption. Weed Res. 36:245-247. Solubility and absorption of glyphosate Glyphosate salt Solubility % 14C absorbed Acid 160 g/L 49 Isopropylamine 500 g/L 59 Ammonium 300 g/L 44 Sodium 500 g/L 18 Potassium 900 g/L ? Calcium 30 g/L 1 LSD (0.05) 5 Reaction of cations with glyphosate K O H O HO - C - CH2- N - CH2- P - O OH CH3 + H3KN - CH + CH3 + Glyphosate acid O H O - HO - C - CH2 - N - CH2 - P - OH OH • Sources of ions that affect glyphosate – Glyphosate formulation – Soil – many + and - charges – Spray water – Ca+2, Mg+2, Na+ – Plant factors: surface Ca+2, stress H+ Glyphosate is adsorbed to soil • Glyphosate is immediately inactivated upon contact with soil (or dirty water) • Glyphosate efficacy is reduced by dust on weed leaves (Zhou et al. 2006. Weed Sci. 54:1132-1136) Treatment Plant without dust Plant with dust Control 81% 60% Glyphosate acid O H O - HO - C - CH2 - N - CH2 - P - OH OH • Sources of ions that affect glyphosate – Glyphosate formulation – Soil – many + and - charges – Spray water – Ca+2, Mg+2, Na+ – Plant factors: surface Ca+2, stress H+ Sources of cations in sprayer tanks • Spray carrier water – Ca+2; also other divalents cations - Mg+2, Fe+2 – Na+1; also other monovalent cations • Adjuvants – (NH4)2SO4 fertilizer Glyphosate salts – Isopropylamine+1, K+1, diammonium (2 NH4+) Reaction of cations with glyphosate Ca 2+ O H CH3 O HO - C - CH2- N - CH2- P - O + H32N+ - CH Ca OH CH3 + CH - NH3 CH3 CH3 O H O O - P - CH2- N - CH2- C - OH OH Glyphosate acid O H O - HO - C - CH2 - N - CH2 - P - OH OH • Sources of ions that affect glyphosate – Glyphosate formulation – Soil – many + and - charges – Spray water – Ca+2, Mg+2, Na+ – Plant factors: surface Ca+2, stress H+ Water solubility of various salts • Glyphosate salts – – – – – – Acid Ammonium Calcium Isopropylamine Potassium Sodium 160 g/L 300 g/L 30 g/L 500 g/L 900 g/L 500 g/L • Other salts – – – – Ammonium sulfate Calcium sulfate Potassium sulfate Sodium sulfate 760 g/L 2 g/L 120 g/L 200 g/L Spray deposit characteristics Glyphosate concentration Glyphosate was more phytotoxic when applied in one concentrated drop than nine dilute drops of equal size Cranmer and Linscott Weed Science 1990 and 1991 Spray deposit characteristics Droplet Spread Glyphosate absorption was greater with adjuvants that left a pile deposit Nalewaja and Matysiak 4th International Symposium on Adjuvants for Agrochemicals. 1995 Spray deposit characteristics Spray Water Quality Glyphosate- + IPA+ or K+ Ca++ Na+ Droplet drying Glyphosate – Ca Low solubility & poor absorption Glyphosate- + IPA+ or K+ or Na+ Spray deposit characteristics (NH4)2SO4 Can Overcome Antagonism Glyphosate- + IPA+ or K+ Ca++ Na+ 2 NH4+ + SO4- Droplet drying CaSO4 Precipitates before glyphosate-Ca Glyphosate- + NH4+ or IPA+ or K+ or Na+ Wheat leaf surface with residual of glyphosate (source: Nalewaja et al., 1992) Glyphosate + Tween 20 Wheat leaf surface with residual of glyphosate (source: Nalewaja et al., 1992) Glyphosate + Tween 20 + calcium chloride Wheat leaf surface with residual of glyphosate (source: Nalewaja et. al., 1992) CaSO 4 crystal CaSO 4 Glyphosate + Tween 20 + calcium chloride + ammonium sulfate Plant stress affects glyphosate efficacy: velvetleaf seedlings Zhou et al. 2007. Weed Sci. 55:240-244 Source of stress None Percent control No adjuvant 84 Surf. & AMS 93 Cold 68 80 Drought 46 67 Flooding 50 68 Objective • To evaluate the potential to reduce glyphosate rates by decreasing spray volume • To evaluate relationships among spray volume, adjuvants, formulations, spray water quality, and sprayer nozzles for glyphosate efficacy Reasons for using very low herbicide rates in experiments The herbicide rate selected should provide control on the linear portion of the bioassay curve between about 30% and 70% control 70% Control % • 30% Herbicide rate Herbicide Treatment 2 Herbicide Treatment 1 Diagram of three-species treatments Wheat Oat Barley Herbicide Efficacy Research - Reduced herbicide rates - Solid seeded assay species - wheat, barley, oat, proso millet - All-terrain-vehicle (ATV) sprayer Experimental Design - Randomized complete block - 4 replicates - Multiple years or locations - Control evaluated visually Spray water quality of two sources CaCO3 Ca++ Mg++ Na+ K+ Soft Hard ------ mg/L -----110 1550 30 230 8 238 59 146 6 51 Glyphosate with hard water Roundup Custom® + 0.5% oxysorbic surfactant 100 % control 90 80 70 LSD (0.05) = 9 60 50 40 30 20 10 0 0.5 oz/A 1.0 oz/A 2.0 oz/A Glyphosate Rate No AMS 0.5% w/v 1% w/v Combined over 2 years and 3 grass species 2% w/v Conclusions from previous slide • Ammonium sulfate at 0.5% w/v was sufficient to overcome the antagonism from Ca and other ions • Efficacy of glyphosate at low rates in hard water is enhanced by adding ammonium sulfate – Ca+2 is precipitated as CaSO4 – Glyphosate-NH4 is an effective herbicide • Glyphosate at high rates in hard water includes enough glyphosate to allow some (glyphosate)2Ca to form and still have extra glyphosate to provide adequate control Glyphosate with soft water Roundup Custom® + 0.5% oxysorbic surfactant 100 90 80 LSD (0.05) = 7 % control 70 60 50 40 30 20 10 0 0.5 oz/A 1.0 oz/A 2.0 oz/A Glyphosate Rate No AMS 0.5% w/v 1% w/v Combined over 2 years and 3 grass species 2% w/v Conclusions from previous slide • Efficacy of glyphosate at very low rates in soft water is enhanced slightly by adding ammonium sulfate at 0.5% w/v, but the commercial formulation contains adequate glyphosate and surfactant to optimize glyphosate efficacy at medium to high glyphosate rates • Ammonium sulfate at 2% w/v may be slightly antagonistic at low spray volumes in soft water Spray volume - Grass species % control Roundup Ultra® + 0.5% v/v surfactant 100 90 80 70 60 50 40 30 20 10 0 LSD (0.05) = 11 2.0 oz/A 2.5 gal/A 1.0 oz/A 5 gal/A 10 gal/A 0.5 oz/A 20 gal/A Combined over oat and wheat, 1 location Conclusions from the previous slide • Efficacy of glyphosate at all rates that provide less than complete control is greatest at very low spray volumes (2.5 to 5 gal/A) • Glyphosate efficacy is reduced at spray volumes above about 5 gal/A • Glyphosate rates may be reduced by about 1/3 at low spray volumes • Probably glyphosate efficacy in higher spray volumes is reduced because the glyphosate and surfactant concentration in the spray droplet is too diluted for optimum efficacy Glyphosate and surfactant concentrations Spray volume Glyphosate rate 2.5 gal/A 5 gal/A 10 gal/A 20 gal/A (relative Glyp and surf. concentration) 2 oz/A 8x 4x 2x 1x 1 oz/A 4x 2x 1x 0.5x 0.5 oz/A 2x 1x 0.5x 0.25x Efficacy vs. relative Glyp and surf. concentration: r = 0.83 Thus, glyphosate efficacy is greatest when the glyphosate and surfactant concentration in the spray droplet is high Spray Volume – Quackgrass (Elytrigia repens) % quackgrass control Roundup UltraMAX® 100 90 80 70 60 50 40 30 20 10 0 LSD (0.05) = 10 6 oz/A 5 gal/A 3 oz/A 10 gal/A 1.5 oz/A 20 gal/A Combined over 2 years and treatments +/- NIS Conclusions from the previous slide • Efficacy of glyphosate at all rates that provide less than complete control is greatest at the lowest spray volume of 5 gal/A and was reduced by higher spray volumes • Probably glyphosate efficacy in higher spray volumes is reduced because the glyphosate and surfactant concentration in the spray droplet is too diluted for optimum efficacy Glyphosate and surfactant concentrations on quackgrass Spray volume Glyphosate rate 5 gal/A 10 gal/A 20 gal/A (relative Glyp and surf. concentration) 6 oz/A 24x 12x 6x 3 oz/A 12x 6x 3x 1.5 oz/A 6x 3x 1.5x Efficacy vs. relative Glyp and surf. concentration: r = 0.76 Commercial formulations Glyphosate applied at 1 oz/A 100 LSD (0.05) = 8 90 80 Control (%) 70 60 50 40 30 20 10 0 None AMS (1% w/v) 2.5 gal/A None AMS (1% w/v) 10 gal/A Roundup Custom + NIS Roundup Ultra Glyphomax Plus Glyfos X-tra Touchdown Roundup UltraDry Combined over grass species and locations Pre-orifice Drift Guard Spraying Systems Inc. Recommended spray pressures: 30 to 60 psi Turbo TeeJet Spraying Systems Inc. Pre-orifice Recommended spray pressures: 15 to 90 psi Air-induction (venturi) nozzles Venturi Air-induction Large exit tip Sprayer Nozzles % control Glyphosate (Roundup Ultra®) at 1 oz/A 100 90 80 70 60 50 40 30 20 10 0 LSD (0.05) = 8 2.5 gal/A Standard TurboDrop 10 gal/A Turbo TeeJet Ultra-Lo-Drift Combined over grass species and environments Summary • AMS at 0.5% w/v was sufficient for hard water sources at low spray volumes – AMS at 2% w/v may be slightly antagonistic at low spray volumes and without antagonistic salts • Glyphosate efficacy was greatest at spray volumes of 2.5 or 5 gal/A. – Rates may be reduced by 1/3 at low spray volumes – Advantages include high glyphosate concentration that increased absorption, overcoming antagonistic salts, and higher concentrations of surfactant in commercial formulations Summary • Commercial formulations of glyphosate that contain surfactant provided similar grass control • Glyphosate efficacy was similar for several driftreducing nozzles and a standard flat-fan nozzle The bottom line • Use clean spray water and an adequate rate of glyphosate • Use as low a rate of application (gal/A) as is practical • Add ammonium sulfate at 0.5% w/v in most cases • Add nonionic surfactant when glyphosate rates are very low or spray volumes are high • Spray non-stressed plants, when possible Application of these concepts to other herbicides, especially weak acid herbicides • Other weak acid herbicides, e.g., 2,4-D, dicamba, MCPA,…are antagonized by cations like Ca+2 and Na+1 • Ammonium sulfate is an adjuvant that can overcome antagonism of cations with other weak acid herbicides • Most postemergence herbicides are most effective at very low application volumes • Most postemergence herbicides are equally effective when applied with conventional and drift-reducing nozzles, even at very low application volumes Research supported by USDA-CSREES grants under agreements 97-34361-3960, 98-34361-6831, and 99-34361-8432.