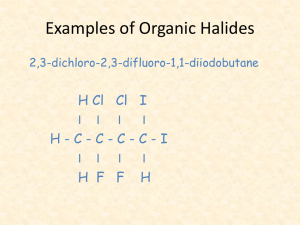
10.2 organic halides and addition and substitution reactions
advertisement

(h) (i) 2. (a) 16 421 (b) Ethane is an important raw material for the production of ethane, which is then used to produce a variety of chemicals. If natural gas containing large amounts of ethane is exported, Alberta and its citizens would not reap the economic benefits associated with the production of these chemicals. (c) As more ethane is burned as a part of natural gas, less will be available for the production of ethene. Assuming that the demand for ethane remains the same, a reduction in the supply of ethane available would increase the price of ethane and all of the chemicals that are derived from it. As the cost of these chemicals spirals upward, the demand for them decreases, which could result in a reduction in the number of petrochemical jobs. 3. 4. Pro Perspectives Ɣ Economic: Saving more fossil fuels for use as petrochemicals will maintain more secondary and tertiary industries, which in turn creates more jobs and more wealth for governments and citizens. Ɣ Ethical: It is not right to deprive future generations of the benefits of petrochemicals and associated products. Con Perspectives Ɣ Technological: Technology of fossil fuel extraction continues to improve and we will have enough fossil fuels for petrochemical use (currently about 5% is used for petrochemicals). Ɣ Economic: The economic benefits of an increased petrochemical industry are mostly lost if we simply export most of the raw materials, feedstocks, and primary petrochemicals. 10.2 ORGANIC HALIDES AND ADDITION AND SUBSTITUTION REACTIONS Practice (Pages 418–419) 1. (a) Copyright © 2007 Thomson Nelson (b) (c) (d) Unit 5 Solutions Manual 349 2. (a) (b) (c) (d) (e) (f) 3. (a) triiodomethane 3-chloro-2-methylpropene (or 3-chloroemethylpropene) dichloromethane 1,2,3-tribromopropane chlorobenzene phenylethene Chloroethene will have a higher boiling point than ethene due to stronger London forces (greater number of electrons) and dipole–dipole forces (polar). Chloroethene has 32 electrons and is polar, due to the chlorine replacing a hydrogen atom, while ethene has only 16 electrons and is nonpolar. (b) Chloroethene will be more soluble in water than ethene will. Chloroethene is polar because of the chlorine, which has replaced a hydrogen atom and would be slightly soluble in the polar water. Ethene is not polar and would essentially be insoluble in water. 4. (a) (b) (c) (d) 5. (a) (b) (c) (d) Investigation 10.1: Substitution and Addition Reactions (Pages 422, 461) Purpose The purpose of this investigation is to test the generalization that addition reactions are faster than substitution reactions. Problem Which compound reacts faster with aqueous bromine: cyclohexane or cyclohexene? Prediction According to empirical generalizations, unsaturated hydrocarbons undergo fast addition reactions and saturated hydrocarbons undergo relatively slow substitution reactions. Cyclohexene, which contains one double bond, will undergo a fast addition reaction, adding two bromine atoms to the double bond. Cyclohexane, which contains only carbon-carbon single bonds, must undergo a substitution reaction, replacing a hydrogen atom with a bromine atom. Since addition to a double bond is faster than substitution to a single bond, cyclohexene will react faster with aqueous bromine. Evidence Substance cyclohexane cyclohexene Al(s) foil covering none covered none covered Initial yellow-brown colour yellow-brown colour colourless colourless Observations After a few minutes slightly lighter yellow-brown colour the same yellow-brown colour colourless colourless Analysis According to the evidence, the reaction of cyclohexene was much faster than that of cyclohexane because the yellow-brown colour of the dissolved bromine disappeared immediately. 350 Unit 5 Solutions Manual Copyright © 2007 Thomson Nelson Evaluation The design is completely adequate to answer the very specific question asked in the Problem. There are no obvious flaws, but the design could be improved by testing other saturated and unsaturated compounds with bromine and other halogens. The materials and procedure were both reasonably adequate. An improvement would be to use some kind of colour meter (colorimeter) to actually measure changes in colour rather than qualitatively estimating. However, this would probably not change the overall results. No special skills were required in this investigation. On the basis of my evaluation of the experiment, I am very certain of the results. There are no significant sources of experimental uncertainty. The prediction is judged to be verified because it clearly agrees with the experimental evidence obtained. Therefore, the generalization about the rate of addition versus substitution reactions appears to be acceptable. I am quite confident about this judgment. The purpose was not really accomplished because it was very general and the problem investigation was restricted to only two hydrocarbons and one halogen. A proper test of the generalization requires many more tests. Practice (Pages 422–423) 6. (a) (b) 7. (a) Substitution: trichloromethane + chlorine o tetrachloromethane + hydrogen chloride (b) Addition: propene + bromine o 1,2-dibromopropane (c) Addition: ethylene + hydrogen iodide o iodoethane (d) Substitution: ethane + chlorine o chloroethane + hydrogen (e) Addition: 1,2-dichloroethyne + fluorine o 1,2-dichloro-1,1,2,2-tetrafluoroethane (f) Addition: but-1-ene + hydrogen chloride o 1-chlorobutane + 2-chlorobutane Copyright © 2007 Thomson Nelson Unit 5 Solutions Manual 351 [Alternatively, two separate chemical equations can be written. This would be a better representation because the relative yields of the two products are not equal.] (g) Substitution: chlorobenzene + chlorine o hydrogen chloride + 1,2-dichlorobenzene + 1,3-dichlorobenzene + 1.4-dichlorobenzene [Alternatively, three separate chemical equations can be written. This would be a better representation because the relative yields of the three isomers are not equal.] 8. (a) Substitution: (b) Addition: (c) Substitution: (d) Addition: (e) Substitution: 9. (a) Ethyne (acetylene) is reacted with an equal chemical amount of chlorine. The product of this reaction is reacted with hydrogen chloride gas. (b) Chemists and chemical engineers invent chemical processes to produce chemicals that are beneficial to industries or individuals within society. Chemical processes are also invented to accomplish a task as part of the process of creating a new product or improving an existing product. New processes may produce new chemicals, which can lead to new technologies and discoveries, or a new process may make the production of an already known chemical safer or more efficient. (c) Perspectives that can be considered while inventing a new technology include: Ɣ Economic: will the process make money? Ɣ Ecological: will the process or chemical produced damage the environment? Ɣ Political: will the new technology be hindered or helped by government legislation? Ɣ Militaristic: can this process produce something that can be used as a weapon? 352 Unit 5 Solutions Manual Copyright © 2007 Thomson Nelson 10. According to the intermolecular force theories of London dispersion and dipole–dipole, the boiling points of these two isomers should be the same. Both 1-bromopropane and 2bromopropane are isoelectronic (having the same number of electrons), and both now, are polar substances. There is nothing in the intermolecular theories studied until that can account for the significant difference in boiling points. The explanatory power of the intermolecular force theory is nonexistent in this case. [The shape of the molecules is a major factor.] 11. (a) ethical (or social) (b) technological (c) scientific (d) economic (e) political (f) political (or legal) (g) ecological (or ethical) (h) ecological Lab Exercise 10.A: Synthesis of an Organic Halide (Page 423) Purpose The purpose of the Lab Exercise is to create a reaction series to produce a product. Problem What are two reaction series to produce 1,2-dichloroethane from hydrocarbons? Hypothesis One possibility would be an addition reaction of ethane with chlorine, as shown below. A second possibility would be a series of two substitution reactions, starting with ethane and chlorine. The desired product would have to be separated from the final mixture. Copyright © 2007 Thomson Nelson Unit 5 Solutions Manual 353 Section 10.2 Questions (Page 424) 1. Cyclohexane is reacted with bromine and timed for a reaction (colour change), in both the presence and the absence of light. Ɣ Manipulated variable: quantity of light during the reaction Ɣ Responding variable: time of reaction, as evidenced by a colour change of the solution Ɣ Controlled variables: volume of cyclohexane, volume of bromine, temperature 2. (a) (b) (c) (d) 3. (a) The order of the boiling points depends on the order of the strengths of the intermolecular forces among molecules in a substance. All substances will have London forces with strengths depending on the number of electrons per molecule. In this case, the number of electrons per molecule increases as the halogen in these compounds increases in size. Since iodoethane contains the highest number of electrons, it has the strongest London forces and hence the highest boiling point. (b) These compounds will be more soluble in ethanol because they are more similar in structure to ethanol than they are to water. 4. (a) (b) (c) 354 Unit 5 Solutions Manual Copyright © 2007 Thomson Nelson Extension 5. (a) Hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) are alternatives to chlorofluorocarbons (CFCs) that are still in use. They appear to be less damaging to the ozone layer than CFCs (although the “hole” in the ozone layer is still growing). However, HCFCs and HFCs may act as greenhouse gases (GHGs), possibly leading to global warming. (b) An aerosol can is designed with a pump-action top so that the can will be pressurized with air when necessary. The contents to be sprayed can be pre-packaged into the can or added by the consumer. The marketing strategy will focus on environmental safety (no propellant added to the atmosphere to decrease the ozone layer or increase global warming) and economy (re-usable for a variety of ingredients). 6. Pro Perspectives Ɣ Technological: Many organic halides are important chemicals for use in various industries or serve a useful purpose such as pest control. Con Perspectives Ɣ Ecological: Many organic halides are toxic and many are also carcinogenic. Some organic halides have caused and continue to cause the depletion of the ozone layer. 7. 8. Toxicity of organic halides depends on the specific compound and whether it interferes with necessary biological processes. Toxicity also depends on the concentration of the compound. For small organic halides that are somewhat polar, their water solubility will mean that the organic halide has a good chance of being excreted from the body of an organism. However, larger organic halides whose composition is mostly hydrocarbon tend to have a very low water solubility and a very high fat solubility. Ingesting fat-soluble organic halides likely means that they will be stored in the fatty deposits of an organism and not excreted. The concentration slowly increases above the local environmental level in a process called bioaccumulation. As small organisms like algae are consumed by larger organisms like water fleas, bioaccumlation is magnified. Going up the food chain from water fleas to minnows to larger fish to eagles or humans, the concentration of fat-soluble organic halides is magnified at each step, resulting in final concentrations that may be lethal or cause serious effects (e.g., reproductive). The classic case of this biomagnification is dichloro diphenyl trichloroethane (DDT). Another common organic halide example is polychlorinated biphenyls (PCBs). 10.3 ALCOHOLS AND ELIMINATION REACTIONS Practice (Page 426) 1. The introduction of alcohols into gasoline has a number of roles. Alcohols contain oxygen, and when mixed with gasoline in the combustion chamber of the engine they burn more completely and produce less carbon monoxide. Alcohols increase the octane rating of the fuel, allowing the engine to run more efficiently. Alcohols are also hydrophilic, which means that they can absorb and dissolve water into the fuel. This keeps any condensation of water in the gas tank from freezing the gas lines. Copyright © 2007 Thomson Nelson Unit 5 Solutions Manual 355