Proteins and Their Synthesis - Western Washington University
advertisement

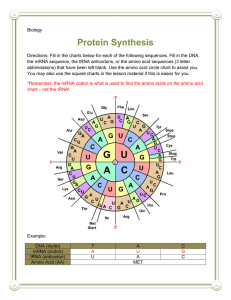
9 Proteins and Their Synthesis WORKING WITH THE FIGURES 1. The primary protein structure is shown in Figure 9-3(a). Where in the mRNA (near the 5′ or 3′ end) would a mutation in R 2 be encoded? Answer: As mRNA is translated 5′ to 3′, polypeptides are assembled from amino end to carboxyl end (the carboxyl end of the growing polypeptide chain is bound to the amino end of the incoming amino acid) Since R 2 is near the amino end of the protein, a mutation for R 2 would be near the 5′ end of the mRNA. 2. In this chapter you were introduced to nonsense suppressor mutations in tRNA genes. However, suppressor mutations also occur in protein-coding genes. Using the tertiary structure of the β subunit of hemoglobin shown in Figure 93(c), explain in structural terms how a mutation could cause the loss of globin protein function. Now explain how a mutation at a second site in the same protein could suppress this mutation and lead to a normal or near-normal protein. Answer: Proper folding is dependent on amino acid sequence and necessary for protein function. An amino acid replacement that disrupted folding of the β subunit would cause a loss of function for the protein because the correct tertiary structure could not form. If a subsequent mutation in another region of β complemented the first mutation by at least partially reestablishing the normal folding pattern, adequate tertiary structure could form and the first mutation would be suppressed. For example, if bonding between points A and B resulted in proper folding, a mutation that changed A would disrupt folding and cause loss of function. A subsequent mutation that changed B so it could bond with mutant A would reestablish the normal folding and normal function. 3. Using the quarternary structure of hemoglobin shown in Figure 9-3(d), explain in structural terms how a mutation in the β subunit protein could be suppressed by a mutation in the α subunit gene. Chapter Nine 283 Answer: A mutation in the β subunit that prevented bonding of α and β subunits would prevent formation of the quaternary structure and block protein function. A complementary mutation in the α subunit that established the capacity to bond with the mutant β subunit would allow formation of a normal quaternary structure and effectively suppress the mutation in β. 4. Transfer RNAs (tRNAs) are examples of RNA molecules that do not encode protein. Based on Figures 9-6 and 9-8, what is the significance of the sequence of tRNA molecules? What do you predict would be the impact on translation of a mutation in one of the bases of one of the stems in the tRNA structure? On the mutant organism? Answer: The sequence of the tRNA molecules determines the three dimensional structure, producing the characteristic L shape. The conservation of the L shape among the different tRNAs implies an important function. If one of the bases in one of the stems were mutant, the formation of the L shape would likely be impaired, reducing or eliminating the capacity of the tRNA to act as an adapter molecule. For example, a mutant tRNA might not be able to bind with the synthetase molecule to become charged, or it might not form a sufficiently stable complex with the ribosome during translation. In the first case, the mutant tRNA would not participate in translation at all; in the second, it could disrupt translation when it was inserted. The overall effect on the mutant organism would probably be minimal because there are several copies of tRNA genes and the normal function would be served by the remaining normal copies of the gene. 5. Ribosomal RNAs (rRNAs) are another example of a functional RNA molecule. Based on Figure 9-11, what do you think is the significance of the secondary structure of rRNA? Answer: The amount of double stranded pairing in the rRNA indicates that a large part of the molecule will have a double-helical structure. The doublehelical regions could potentially interact with ribosomal proteins via their major grooves. rRNA could also interact with other RNAs. The size of the rRNA indicates a complex function. The 16S rRNA contains the ShineDalgarno sequence, which directs the 30S subunit to the start codon, and it could also stabilize bonding between the 30S subunit and mRNA, and between the 30S and 50S subunits. 6. The components of prokaryotic and eukaryotic ribosomes are shown in Figure 9-10. Based on this figure, do you think that the large prokaryotic ribosomal RNA (23S rRNA) would be able to substitute for the eukaryotic 28S rRNA? Justify your answer. 284 Chapter Nine Answer: It would be unlikely that the 23S rRNA of prokaryotes would be able to substitute for the 28S rRNA of eukaryotes. Its different size implies a different folding pattern which would affect its affinity for protein components of the 60S subunit. Also, the 60S subunit of eukaryotes contains 49 proteins, whereas the 50S of prokaryotes has 31 proteins. Thus, the 23S rRNA would have to be able to target a related but different set of proteins to function effectively. In both prokaryotes and eukaryotes, rRNA and ribosomal proteins have coevolved as a unit and interact closely during the formation of the ribosome and translation. Although specifics about the differences in the rRNA and proteins cannot be determined from the figure, the differences in rRNA sizes and number of protein components between prokaryotes and eukaryotes indicate evolutionary divergence in this intricate interaction. It would not be expected that a bacterial rRNA would interact functionally with the protein components of a eukaryotic ribosome. 7. In Figure 9-12, is the terminal amino acid emerging from the ribosome encoded by the 5′ or 3′ end of the mRNA? Answer: Figure 9-12(b) shows a growing polypeptide chain attached to the P site. The terminal amino acid shown emerging from the ribosome is at the amino end of the polypeptide and so was the first one added to the polypeptide chain. The amino end of the growing polypeptide chain will be the initial amino acid because the tRNA binds to the carboxyl end, leaving the amino end free. Since translation begins at the 5′ end of the mRNA and terminates at the 3′ end, this initial amino acid would be encoded at the 5′ end. 8. In Figure 9-12(b), what do you think happens to the tRNA that is released from the E site? Answer: Once the tRNA is released from the E site, it returns to the cytoplasm where it will be recharged with another amino acid. In this way, tRNAs are recycled through the translational machinery. 9. In Figure 9-17, what do you think happens next to the ribosomal subunits after they are finished translating that mRNA? Answer: Once translation is terminated, the ribosomal subunits are released from the mRNA and the 30S subunit is free to form a new initiation complex. Both subunits will ultimately participate in the translation of another mRNA, but it will not necessarily be the same mRNA. 10. Based on Figure 9-19, can you predict the position of a mutation that would affect the synthesis of one isoform but not the other? Chapter Nine 285 Answer: Exon 8 (in blue) is present in one isoform only, exon 9 (in green) is present in the other isoform only. A mutation in the 5′ GU in exon 8 would prevent splicing and block synthesis of the blue isoform. This mutation would not affect the green isoform because splicing for the green isoform does not involve the 5′ GU in exon 8. Likewise, a mutation in the 5′ GU in exon 9 would block synthesis of the green isoform only. Additionally, a nonsense mutation in either of these exons would block synthesis of that isoform but not the other. Based on Figure 9-24, can you predict the position of a mutation that would produce an active protein that was not directed to the correct location? 11. Answer: A mutation in the signal sequence could prevent transfer of the protein to the lumen of the ER, thus preventing it from reaching its proper destination. BASIC PROBLEMS Unpacking Problem 12 a. Use the codon dictionary in Figure 9-5 to complete the following table. Assume that reading is from left to right and that the columns represent transcriptional and translational alignments. C DNA double helix T C A G A U G Tr p C Tr p A mRNA transcribed Appropriate tRNA anticodon Amino acids incorporated into protein b. Label the 5′ and 3′ ends of DNA and RNA, as well as the amino and carboxyl ends of the protein. Answer: 3´ CGT ACC ACT GCA 5´ 5´ GCA TGG TGA CGT 3´ 5´ GCA UGG UGA CGU 3´ 3´ CGU ACC ACU GCA 5´ NH 3 – Ala – Trp – (stop) –COOH 13. DNA double helix (transcribed strand) DNA double helix mRNA transcribed appropriate tRNA anticodon amino acids incorporated Consider the following segment of DNA: 5′ GCTTCCCAA 3′ 3′ CGAAGGGTT 5′ Assume that the top strand is the template strand used by RNA polymerase. 286 Chapter Nine a. b. c. d. Draw the RNA transcribed. Label its 5′ and 3′ ends. Draw the corresponding amino acid chain. Label its amino and carboxyl ends. Repeat parts a through d, assuming the bottom strand to be the template strand. Answer: a. and b. 5´ UUG GGA AGC 3´ c. and d. Assuming the reading frame starts at the first base: NH3 – Leu – Gly – Ser - COOH For the bottom strand, the mRNA is 5´ GCU UCC CAA 3´ and assuming the reading frame starts at the first base, the corresponding amino acid chain is NH3 - Ala - Ser - Gln - COOH. 14. A mutational event inserts an extra nucleotide pair into DNA. Which of the following outcomes do you expect? (1) No protein at all; (2) a protein in which one amino acid is changed; (3) a protein in which three amino acids are changed; (4) a protein in which two amino acids are changed; (5) a protein in which most amino acids after the site of the insertion are changed. Answer: (5) With an insertion, the reading frame is disrupted. This will result in a drastically altered protein from the insertion to the end of the protein (which may be much shorter or longer than wild type because of the location of stop signals in the altered reading frame). 15. Before the true nature of the genetic coding process was fully understood, it was proposed that the message might be read in overlapping triplets. For example, the sequence GCAUC might be read as GCA CAU AUC: Devise an experimental test of this idea. Answer: A single nucleotide change should result in three adjacent amino acid changes in a protein. One and two adjacent amino acid changes would be expected to be much rarer than the three changes. This is directly the opposite of what is observed in proteins. Also, given any triplet coding for an amino acid, the next triplet could only be one of four. For example, if the first is GGG, Chapter Nine 287 then the next must be GGN (N = any base). This puts severe limits on which amino acids could be adjacent to each other. You could check amino acid sequences of various proteins to show that this is not the case. 16. In protein-synthesizing systems in vitro, the addition of a specific human mRNA to the E. coli translational apparatus (ribosomes, tRNA, and so forth) stimulates the synthesis of a protein very much like that specified by the mRNA. What does this result show? Answer: It suggests very little evolutionary change between E. coli and humans with regard to the translational apparatus. The code is universal, the ribosomes are interchangeable, the tRNAs are interchangeable, and the enzymes involved are interchangeable. (Initiation of translation in prokaryotes in vivo requires specific base-pairing between the 3´ end of the 16s rRNA and a Shine–Dalgarno sequence found in the 5´ untranslated region of the mRNA. A Shine–Dalgarno sequence would not be expected (unless by chance) in a eukaryotic mRNA and therefore initiation of translation might not occur.) 17. Which anticodon would you predict for a tRNA species carrying isoleucine? Is there more than one possible answer? If so, state any alternative answers. Answer: There are three codons for isoleucine: 5´ AUU 3´, 5´ AUC 3´, and 5´ AUA 3´. Possible anticodons are 3´ UAA 5´ (complementary), 3´ UAG 5´ (complementary), and 3´ UAI 5´ (wobble). 5´ UAU 3´, although complementary, would also base-pair with 5´ AUG 3´ (methionine) due to wobble and therefore would not be an acceptable alternative. 18. a. In how many cases in the genetic code would you fail to know the amino acid specified by a codon if you knew only the first two nucleotides of the codon? b. In how many cases would you fail to know the first two nucleotides of the codon if you knew which amino acid is specified by it? Answer: a. By studying the genetic code table provided in the textbook, you will discover that there are 28 codons that do not specify a particular amino acid with the first two positions (32 if you count Tyr and the stop codons starting with UA). b. If you knew the amino acid, you would not know the first two nucleotides in the cases of Arg, Ser, and Leu. 288 Chapter Nine 19. Deduce what the six wild-type codons may have been in the mutants that led Brenner to infer the nature of the amber codon UAG. Answer: The codon for amber is UAG. Listed below are the amino acids that would have been needed to be inserted to continue the wild-type chain and their codons: glutamine lysine glutamic acid tyrosine tryptophan serine CAA, CAG* AAA, AAG* GAA, GAG* UAU*, UAC* UGG* AGU, AGC, UCU, UCC, UCA, UCG* In each case, the codon marked by an asterisk would require a single base change to become UAG. 20. If a polyribonucleotide contains equal amounts of randomly positioned adenine and uracil bases, what proportion of its triplets will encode (a) phenylalanine, (b) isoleucine, (c) leucine, (d) tyrosine? Answer: a. The codons for phenylalanine are UUU and UUC. Only the UUU codon can exist with randomly positioned A and U. Therefore, the chance of UUU is (1/2)(1/2)(1/2) = 1/8. b. The codons for isoleucine are AUU, AUC, and AUA. AUC cannot exist. The probability of AUU is (1/2)(1/2)(1/2) = 1/8, and the probability of AUA is (1/2)(1/2)(1/2) = 1/8. The total probability is thus 1/4. c. The codons for leucine are UUA, UUG, CUU, CUC, CUA, and CUG, of which only UUA can exist. It has a probability of (1/2)(1/2)(1/2) = 1/8. d. The codons for tyrosine are UAU and UAC, of which only UAU can exist. It has a probability of (1/2)(1/2)(1/2) = 1/8. 21. You have synthesized three different messenger RNAs with bases incorporated in random sequence in the following ratios: (a) 1 U:5 C’s, (b) 1 A:1 C:4 U’s, (c) 1 A:1 C:1 G:1 U. In a protein-synthesizing system in vitro, indicate the identities and proportions of amino acids that will be incorporated into proteins when each of these mRNAs is tested. (Refer to Figure 9-5.) Answer: a. 1 U : 5 C — The probability of a U is 1/6, and the probability of a C is 5/6. Chapter Nine 289 Codon UUU UUC CCC CCU UCC UCU CUC CUU Amino acid Phe Phe Pro Pro Ser Ser Leu Leu Probability (1/6)(1/6)(1/6) = 0.005 (1/6)(1/6)(5/6) = 0.023 (5/6)(5/6)(5/6) = 0.578 (5/6)(5/6)(1/6) = 0.116 (1/6)(5/6)(5/6) = 0.116 (1/6)(5/6)(1/6) = 0.023 (5/6)(1/6)(5/6) = 0.116 (5/6)(1/6)(1/6) = 0.023 Sum Phe = 0.028 Pro 0.694 Ser = 0.139 Leu = 0.139 1 Phe : 25 Pro : 5 Ser : 5 Leu b. Using the same method as above, the final answer is 4 stop : 80 Phe : 40 Leu : 24 Ile : 24 Ser : 20 Tyr : 6 Pro : 6 Thr : 5 Asn : 5 His : 1 Lys : 1 Gln. c. All amino acids are found in the proportions seen in the code table. 22. In the fungus Neurospora, some mutants were obtained that lacked activity for a certain enzyme. The mutations were found, by mapping, to be in either of two unlinked genes. Provide a possible explanation in reference to quaternary protein structure. Answer: Quaternary structure is due to the interactions of subunits of a protein. In this example, the enzyme activity being studied may be from a protein consisting of two different subunits. Both subunits are required for activity. The polypeptides of the subunits are encoded by separate and unlinked genes. 23. A mutant is found that lacks all detectable function for one specific enzyme. If you had a labeled antibody that detects this protein in a Western blot (see Chapter 1), would you expect there to be any protein detectable by the antibody in the mutant? Explain. Answer: There are a number of mutational changes that can lead to the absence of enzymatic function in the product of a gene. Some of these changes would result in the complete absence of protein product and therefore also the absence of a detectable band on a Western blot. Mutations such as deletions of the gene, for example, would result in the lack of detectable protein. Other mutations that destroy function (missense, for example) may not alter the production of the protein and would be detected on a Western blot. Still other mutations (nonsense, frameshift) could alter the size of the protein yet would still lead to detectable protein. 24. In a Western blot (see Chapter 1), the enzyme tryptophan synthetase usually shows two bands of different mobility on the gel. Some mutants with no 290 Chapter Nine enzyme activity showed exactly the same bands as the wild type. Other mutants with no activity showed just the slow band; still others, just the fast band. a. Explain the different types of mutants at the level of protein structure. b. Why do you think there were no mutants that showed no bands? Answer: a. Tryptophan synthetase is a heterotetramer of two copies each of two different polypeptides, each encoded by a separate gene. Mutations that prevent the synthesis of one subunit would lead to the loss of one of the bands on a Western blot. Mutants that still make both subunits (those with exactly the same bands as wild type) might have mutations that prevent the subunits from interacting or disrupt the active site of the enzyme. b. Because the two subunits are encoded by separate genes, the absence of both bands simultaneously would require two independent and rare mutagenic events. 25. In the Crick–Brenner experiments described in this chapter, three “insertions” or three “deletions” restored the normal reading frame and the deduction was that the code was read in groups of three. Is this deduction really proved by the experiments? Could a codon have been composed of six bases, for example? Answer: Yes. It was not known at the time what number of bases the “plus” and “minus” mutations actually were. If each mutation was two bases, then a codon would have been six bases. Since the mutations were actually adding or subtracting single bases, the codon is indeed three bases. 26. A mutant has no activity for the enzyme isocitrate lyase. Does this result prove that the mutation is in the gene encoding isocitrate lyase? Answer: No. The enzyme may require post-translational modification to be active. Mutations in the enzymes required for these modifications would not map to the isocitrate lyase gene. 27. A certain nonsense suppressor corrects a nongrowing mutant to a state that is near, but not exactly, wild type (it has abnormal growth). Suggest a possible reason why the reversion is not a full correction. Answer: A nonsense suppressor is a mutation in a tRNA such that its anticodon can base-pair with a stop codon. In this way, a mutant stop codon (nonsense mutation) can be read through and the polypeptide can be fully synthesized. However, the mutant tRNA may be for an amino acid that was not encoded in that position in the original gene. For example, the codon UCG (serine) is Chapter Nine 291 instead UAG in the nonsense mutant. The suppressor mutation could be in tRNA for tryptophan such that its anticodon now recognizes UAG instead of UGG. During translation in the double mutant, the machinery puts tryptophan into the location of the mutant stop codon. This allows translation to continue but does place tryptophan into a position that was serine in the wild-type gene. This may create a protein that is not as active and a cell that is “not exactly wild type.” Another explanation is that translation of the mutant gene is not as efficient and that premature termination still occurs some of the time. This would lead to less product and, again, a state that is “not exactly wild type.” 28. In bacterial genes, as soon as any partial mRNA transcript is produced by the RNA polymerase system, the ribosome jumps on it and starts translating. Draw a diagram of this process, identifying 5′ and 3′ ends of mRNA, the COOH and NH 2 ends of the protein, the RNA polymerase, and at least one ribosome. (Why couldn’t this system work in eukaryotes?) Answer: C T A GG C T GC A RNA polymerase CU DNA A GC U G C G A T TC C G A C G AG NH 2 polypeptide ribosome 5´ RNA In eukaryotes, transcription occurs within the nucleus while translation occurs in the cytoplasm. Thus, the two processes cannot occur together. 29. In a haploid, a nonsense suppressor su1 acts on mutation 1 but not on mutation 2 or 3 of gene P. An unlinked nonsense suppressor su2 works on P mutation 2 but not on 1 or 3. Explain this pattern of suppression in regard to the nature of the mutations and the suppressors. Answer: Assuming that the three mutations of gene P are all nonsense mutations, there are three different possible stop codons that might be the cause (amber, ochre, or opal). A suppressor mutation would be specific to one type of nonsense codon. For example, amber suppressors would suppress amber mutants but not opal or ochre. 30. In vitro translation systems have been developed in which specific RNA molecules can be added to a test tube containing a bacterial cell extract that includes all the components needed for translation (ribosomes, tRNAs, amino acids). If a radioactively labeled amino acid is included, any protein translated 292 Chapter Nine from that RNA can be detected and displayed on a gel. If a eukaryotic mRNA is added to the test tube, would radioactive protein be produced? Explain. Answer: Initiation of translation in prokaryotes requires specific base-pairing between the 3´ end of the 16s rRNA and a Shine–Dalgarno sequence found in the 5´ untranslated region of the mRNA. A Shine–Dalgarno sequence would not be expected (unless by chance) in a eukaryotic mRNA and therefore initiation of translation would not occur. 31. In a eukaryotic translation system (containing a cell extract from a eukaryotic cell) comparable with that in Problem 30, would a protein be produced by a bacterial RNA? If not, why not? Answer: Initiaton of translation in eukaryotes requires initiation factors (eIF4a, b, and G) that associate with the 5´ cap of the mRNA. Because prokaryotic mRNAs are not capped, translation would not initiate. 32. Would a chimeric translation system containing the large ribosomal subunit from E. coli and the small ribosomal subunit from yeast (a unicellular eukaryote) be able to function in protein synthesis? Explain why or why not. Answer: Not likely. Although the steps of translation and the components of ribosomes are similar in both eukaryotes and prokaryotes, the ribosomes are not identical. The sizes of both subunits are larger in eukaryotes and the many specific and intricate interactions that must take place between the small and large subunits would not be possible in a chimeric system. 33. Mutations that change a single amino acid in the active site of an enzyme can result in the synthesis of wild-type amounts of an inactive enzyme. Can you think of other regions in a protein where a single amino acid change might have the same result? Answer: Single amino acid changes can result in changes in protein folding, protein targeting, or post-translational modifications. Any of these changes could give the results indicated. 34. What evidence supports the view that ribosomal RNAs are a more important component of the ribosome than the ribosomal proteins? Answer: The first indication of rRNAs importance was the discovery of ribozymes. Recently, structural studies have shown that both the decoding center in the 30S subunit and the peptidyl transferase center in the 50S subunit Chapter Nine 293 are composed entirely of rRNA and that the important contacts in these centers are all tRNA/rRNA contacts. 35. Explain why antibiotics, such as erythromycin and Zithromax, that bind the large ribosomal subunit do not harm us. Answer: Antibiotics need to selectively target bacterial structures and functions that are essential for life but unique or sufficiently different from the equivalent structure and functions of their animal hosts. The large bacterial ribosomal subunit fits these criteria as its function is obviously essential yet its structure is sufficiently different from the large eukaryotic ribosomal subunit. While the steps of protein synthesis are similar overall, eukaryotic ribosomes have larger and more numerous components. These differences make it possible to develop drugs that specifically bind bacterial ribosomes but have little or no affinity for eukaryotic ribosomes. 36. Why do multicellular eukaryotes need to have hundreds of kinase-encoding genes? Answer: Recent studies indicate that most proteins function by interacting with other proteins. (The complete set of such interactions is called the interactome.) Most of these essential protein-protein interactions are regulated by phosphorylation/dephosphorylation modifications. Kinases are the enzymes that catalyze phosphorylations. Therefore, the complexity of the interactome necessary for the complexity of multicellularity requires the very large number of kinase-encoding genes. 37. Our immune system makes many different proteins that protect us from viral and bacterial infection. Biotechnology companies must produce large quantities of these immune proteins for human testing and eventual sale to the public. To this end, their scientists engineer bacterial or human cell cultures to express these immune proteins. Explain why proteins isolated from bacterial cultures are often inactive, whereas the same proteins isolated from human cell cultures are active (functional). Answer: Bacterial and human cell cultures are both capable of producing the same polypeptide from the same mRNA, but that does not mean that the resulting protein will be active. Many proteins require posttranslational processing to become functional, and the enzymes and control necessary for such processing is not universal. The proteins produced and isolated from a human cell culture system will contain the necessary posttranslational modifications necessary for human protein function. 294 Chapter Nine CHALLENGING PROBLEMS 38. A single nucleotide addition and a single nucleotide deletion approximately 15 sites apart in the DNA cause a protein change in sequence from Lys–Ser–Pro–Ser–Leu–Asn–Ala–Ala–Lys to Lys–Val–His–His–Leu–Met–Ala–Ala–Lys a. What are the old and new mRNA nucleotide sequences? (Use the codon dictionary in Figure 9-5.) b. Which nucleotide has been added and which has been deleted? (Problem 38 is from W. D. Stansfield, Theory and Problems of Genetics. McGraw-Hill, 1969.) Answer: a. and b. The goal of this type of problem is to align the two sequences. You are told that there is a single nucleotide addition and single nucleotide deletion, so look for single base differences that effect this alignment. These should be located where the protein sequence changes (i.e., between Lys-Ser and Asn-Ala). Remember also that the genetic code is redundant. (N = any base) Lys Ser Pro Ser Leu Asn Ala Ala Lys A – AGU AG U C UU G C A U AAA G UCN CCN UCN CUN AA C GCN GCN AA G U U AAA GCN AA A G GUN CA C CA C CUNA AUG GCN G + UU G Lys Val His His Leu Met Ala Ala Lys Base deleted Old: A AAA G AGU CCA UCA CUU AAU GCN GCN AA G New: A AAA G GUC CAU CAC UUA AUG GCN GCN AA G Base added 39. You are studying an E. coli gene that specifies a protein. A part of its sequence is Chapter Nine 295 –Ala–Pro–Trp–Ser–Glu–Lys–Cys–His– You recover a series of mutants for this gene that show no enzymatic activity. By isolating the mutant enzyme products, you find the following sequences: Mutant 1: –Ala–Pro–Trp–Arg–Glu–Lys–Cys–His– Mutant 2: –Ala–Pro– Mutant 3: –Ala–Pro–Gly–Val–Lys–Asn–Cys–His– Mutant 4: –Ala–Pro–Trp–Phe–Phe–Thr–Cys–His– What is the molecular basis for each mutation? What is the DNA sequence that specifies this part of the protein? Answer: Mutant 1: A simple substitution of Arg for Ser exists, suggesting a nucleotide change. Two codons for Arg are AGA and AGG, and one codon for Ser is AGU. The U of the Ser codon could have been replaced by either an A or a G. Mutant 2: The Trp codon (UGG) changed to a stop codon (UGA or UAG). Mutant 3: Two frameshift mutations occurred: 5´–GCN CCN (–U)GGA GUG AAA AA(+U or C) UGU(or C) CAU(or C)–3´. Mutant 4: An inversion occurred after Trp and before Cys. The DNA original sequence (with both strands shown for the area of inversion) was 3´–CGN GGN ACC TCA CTT TTT ACA(or G) GTA(or G) –5´ 5´– –AGT GAA AAA– –3´ Therefore, the complementary RNA sequence was 5´–GCN CCN UGG AGU GAA AAA UGU/C CAU/C–3´ The DNA inverted sequence became 3´–CGN GGN ACC AAA AAG TGA ACA/G GTA/G–5´ ˆ ˆ Therefore, the complementary RNA sequence was 5´–GCN CCN UGG UUU UUC ACU UGU/C CAU/C–3´ ˆ ˆ 296 Chapter Nine 40. Suppressors of frameshift mutations are now known. Propose a mechanism for their action. Answer: If the anticodon on a tRNA molecule also was altered by mutation to be four bases long, with the fourth base on the 5´ side of the anticodon, it would suppress the insertion. Alterations in the ribosome can also induce frameshifting. 41. Consider the gene that specifies the structure of hemoglobin. Arrange the following events in the most likely sequence in which they would take place. a. b. c. d. e. f. g. h. i. j. Anemia is observed. The shape of the oxygen-binding site is altered. An incorrect codon is transcribed into hemoglobin mRNA. The ovum (female gamete) receives a high radiation dose. An incorrect codon is generated in the DNA of the hemoglobin gene. A mother (an X-ray technician) accidentally steps in front of an operating X-ray generator. A child dies. The oxygen-transport capacity of the body is severely impaired. The tRNA anticodon that lines up is one of a type that brings an unsuitable amino acid. Nucleotide-pair substitution occurs in the DNA of the gene for hemoglobin. Answer: f, d, j, e, c, i, b, h, a, g 42. An induced cell mutant is isolated from a hamster tissue culture because of its resistance to -amanitin (a poison derived from a fungus). Electrophoresis shows that the mutant has an altered RNA polymerase; just one electrophoretic band is in a position different from that of the wild-type polymerase. The cells are presumed to be diploid. What do the results of this experiment tell you about ways in which to detect recessive mutants in such cells? Answer: Cells in long-established culture lines usually are not fully diploid. For reasons that are currently unknown, adaptation to culture frequently results in both karyotypic and gene dosage changes. This can result in hemizygosity for some genes, which allows for the expression of previously hidden recessive alleles. 43. A double-stranded DNA molecule with the sequence shown here produces, in vivo, a polypeptide that is five amino acids long. Chapter Nine 297 TACATGATCATTTCACGGAATTTCTAGCATGTA ATGTACTAGTAAAGTGCCTTAAAGATCGTACAT a. Which strand of DNA is transcribed and in which direction? b. Label the 5′ and the 3′ ends of each strand. c. If an inversion occurs between the second and the third triplets from the left and right ends, respectively, and the same strand of DNA is transcribed, how long will the resultant polypeptide be? d. Assume that the original molecule is intact and that the bottom strand is transcribed from left to right. Give the base sequence, and label the 5′ and 3′ ends of the anticodon that inserts the fourth amino acid into the nascent polypeptide. What is this amino acid? Answer: a. and b. The sequence of double-stranded DNA is as follows: 5´—TAC ATG ATC ATT TCA CGG AAT TTC TAG CAT GTA—3´ 3´—ATG TAC TAG TAA AGT GCC TTA AAG ATC GTA CAT—5´ First look for stop codons. Next, look for the initiating codon, AUG (3´— TAC—5´ in DNA). Only the upper strand contains the necessary codons. DNA RNA protein 3´ TAC GAT CTT TAA GGC ACT 5´ 5´ AUG CUA GAA AUU CCG UGA 3´ Met Leu Glu Ile Pro stop The DNA strand is read from right to left as written in your text and is written above in reverse order from your text. c. Remember that polarity must be taken into account. The inversion is DNA 5´ TAC ATG CTA GAA ATT CCG TGA AAT GAT CAT GTA 3´ RNA 3´ –GAU CUU UAA GGC ACU UUA CUA GUA– 5´ amino acids HOOC 7 6 5 4 3 2 1 –NH3 d. DNA 3´ATG TAC TAG TAA AGT GCC TTA AAG ATC GTA CAT 5´ mRNA 5´UAC AUG AUC AUU UCA CGG AAU UUC UAG 3´ 1 2 3 4 5 6 7 stop Codon 4 is 5´–UCA–3´, which codes for Ser. Anticodon 4 would be 3´–AGU– 5´ (or 3´–AGI–5´ given wobble). 44. One of the techniques used to decipher the genetic code was to synthesize polypeptides in vitro, with the use of synthetic mRNA with various repeating base sequences—for example, (AGA) n , which can be written out as AGAAGAAGAAGAAGA. . . . Sometimes the synthesized polypeptide 298 Chapter Nine contained just one amino acid (a homopolymer), and sometimes it contained more than one (a heteropolymer), depending on the repeating sequence used. Furthermore, sometimes different polypeptides were made from the same synthetic mRNA, suggesting that the initiation of protein synthesis in the system in vitro does not always start on the end nucleotide of the messenger. For example, from (AGA) n , three polypeptides may have been made: aa 1 homopolymer (abbreviated aa 1 –aa 1 ), aa 2 homopolymer (aa 2 –aa 2 ), and aa 3 homopolymer (aa 3 –aa 3 ). These polypeptides probably correspond to the following readings derived by starting at different places in the sequence: AGA AGA AGA AGA . . . GAA GAA GAA GAA . . . AAG AAG AAG AAG . . . The following table shows the actual results obtained from the experiment done by Khorana. Synthetic mRNA (UC) n (UG) n (AC) n (AG) n (UUC) n (UUG) n (AAG) n (CAA) n (UAC) n (AUC) n (GUA) n (GAU) n (UAUC) n (UUAC) n (GAUA) n (GUAA) n Polypeptide(s) synthesized (Ser–Leu) (Val–Cys) (Thr–His) (Arg–Glu) (Ser–Ser) and (Leu–Leu) and (Phe–Phe) (Leu–Leu) and (Val–Val) and (Cys–Cys) (Arg–Arg) and (Lys–Lys) and (Glu–Glu) (Thr–Thr) and (Asn–Asn) and (Gln–Gln) (Thr–Thr) and (Leu–Leu) and (Tyr–Tyr) (Ile–Ile) and (Ser–Ser) and (His–His) (Ser–Ser) and (Val–Val) (Asp–Asp) and (Met–Met) (Tyr–Leu–Ser–Ile) (Leu–Leu–Thr–Tyr) None None Note: The order in which the polypeptides or amino acids are listed in the table is not significant except for (UAUC) n and (UUAC) n . a. Why do (GUA) n and (GAU) n each encode only two homopolypeptides? b. Why do (GAUA) n and (GUAA) n fail to stimulate synthesis? c. Assign an amino acid to each triplet in the following list. Bear in mind that there are often several codons for a single amino acid and that the first two letters in a codon are usually the important ones (but that the third letter is occasionally significant). Also, remember that some very different-looking codons sometimes encode the same amino acid. Try to carry out this task without consulting Figure 9-5. Chapter Nine 299 AUG GUG GUU GUA UGU CAC ACA GAU UUC CUC CUU CUA UCU AGU UUG UUA AUC UAU UAC ACU AAG AAC CAA AGA GAG GAA UAG UGA To solve this problem requires both logic and trial and error. Don’t be disheartened: Khorana received a Nobel Prize for doing it. Good luck! (Problem 44 is from J. Kuspira and G. W. Walker, Genetics: Questions and Problems. McGraw-Hill, 1973.) Answer: a. (GAU) n codes for Asp n (GAU) n , Met n (AUG) n , and stop n (UGA) n . (GUA) n codes for Val n (GUA) n , Ser n (AGU) n , and stop n (UAG) n . One reading frame in each contains a stop codon. b. Each of the three reading frames contains a stop codon. c. The way to approach this problem is to focus initially on one amino acid at a time. For instance, line 4 indicates that the codon for Arg might be AGA or GAG. Line 7 indicates it might be AAG, AGA, or GAA. Therefore, Arg is at least AGA. That also means that Glu is GAG (line 4). Lys and Glu can be AAG or GAA (line 7). Because no other combinations except the ones already mentioned result in either Lys or Glu, no further decision can be made with respect to them. However, taking wobble into consideration, Glu may also be GAA, which leaves Lys as AAG. Next, focus on lines 1 and 5. Ser and Leu can be UCU and CUC. Ser, Leu, and Phe can be UUC, UCU, and CUU. Phe is not UCU, which is seen in both lines. From line 14, CUU is Leu. Therefore, UUC is Phe, and UCU is Ser. The footnote states that lines 13 and 14 are in the correct order. In line 13, if UCU is Ser (see above), then Ile is AUC, Tyr is UAU, and Leu is CUA. Continued application of this approach will allow the assignment of an amino acid to each codon.