σ σ σ σ σ
advertisement
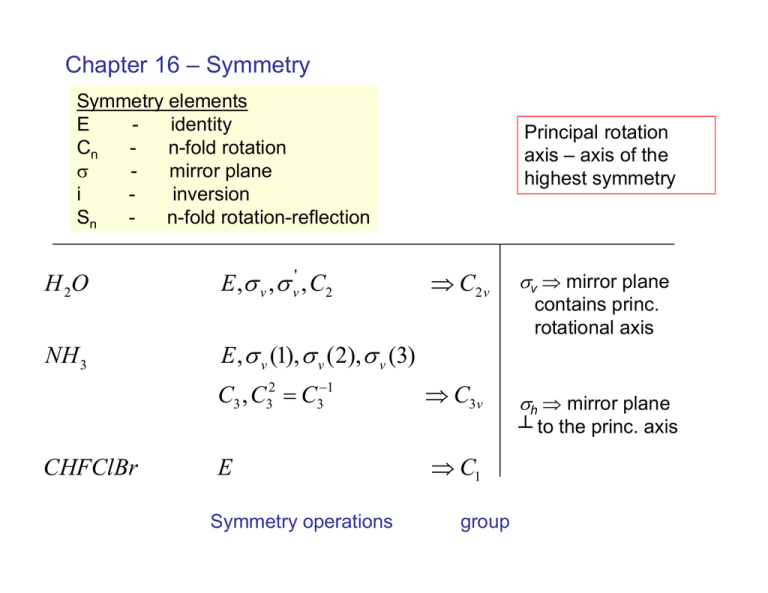
Chapter 16 – Symmetry Symmetry elements E identity Cn n-fold rotation σ mirror plane i inversion Sn n-fold rotation-reflection H 2O E ,σ v , σ v' , C2 NH 3 E , σ v (1), σ v (2), σ v (3) C3 , C32 = C3−1 Principal rotation axis – axis of the highest symmetry ⇒ C2 v σv ⇒ mirror plane ⇒ C3v σh ⇒ mirror plane contains princ. rotational axis ┴ to the princ. axis CHFClBr E Symmetry operations ⇒ C1 group H C=C Br H F H C=C H E,σ H E , C2 , C2' , C2'' H σ , σ ' , σ '' , i ⇒ Cs ⇒ D2 h group Symmetry operators can be represented by matrices Example: H x O H y z , x ⊥ to the plane of the molecule ⎛ 1 0 0⎞ ⎜ ⎟ Eˆ : ⎜ 0 1 0 ⎟ ⎜ 0 0 1⎟ ⎝ ⎠ ⎛ −1 0 0 ⎞ ⎜ ⎟ Cˆ 2 : ⎜ 0 − 1 0 ⎟ ⎜ 0 0 1⎟ ⎝ ⎠ ⎛ 1 0 σˆ v : ⎜⎜ 0 − 1 ⎜ 0 0 ⎝ ⎛ −1 0 σˆ v' : ⎜⎜ 0 1 ⎜ 0 0 ⎝ 0⎞ ⎟ 0⎟ 1 ⎟⎠ 0⎞ ⎟ 0⎟ 1 ⎟⎠ form a representation for the C2v group Cˆ 2 iCˆ 2 = Eˆ Cˆ 2 iσˆ v = σˆ v' etc. In this case, there are simpler representations C2 v Eˆ Cˆ 2 σˆ 2 σˆ v' A1 1 1 1 1 z x2 , y2 , z 2 A2 1 1 −1 −1 Rz xy B1 1 −1 B2 1 −1 −1 1 −1 1 x, Rx xz character table y, Rx yz Ri = rotation about I axis irreducible representations O pz → a1 A1 = totally symmetric representation O px → b1 O p y → b2 for C2V all irreducible representations are one-dimensional ⇒ no degeneracies C3V is an example of a group with a degenerate representation z N x rotate 120°, “mixes” x and y E is a two-fold degenerate representation y The different representations are orthogonal A1 xA2 = 1i1 + 2i1i1 + 3i1i(−1) = 0 A2 xE = 1i2 + 2(1)(−1) + 0 = 0 Symmetry labels of MOs of water Electronic structure C2v group ∫ψ 1 Hˆ ψ 2 dτ = 0 if ψ1, ψ2 not the a22 → A1 same symmetry b1b2 → A2 ∫ψ 1 Aˆψ 2 dτ = 0 if ψ 1 Âψ 2 does not a2b2 → A1 b1a2 → B2 Selection rules contain totally symmetric representation 1 1 a1 → a1 1 2 a1 → b1 ∫ a za dτ ≠ 0 ∫ a zb dτ = 0 ∫ a xb dτ ≠ 0 1 1 etc. allowed transitions C3v e2 → 4 1 0 = c1 (1 1 1) + c2 (1 1 − 1) + c3 (2 − 1 0) c1 = c2 = c3 = 1 ⇒ e 2 → e, a1 , a2 two electrons in an e orbital → E, A1, A2 states Symmetries of vibrational normal modes 1 ⎛ ∂ 2V ⎞ 2 V = ∑ ⎜ 2 ⎟Qi , 2 i ⎝ 2Qi ⎠ Qi are normal coordinates Ψ = ψ 1 ( Q1 )ψ 2 ( Q2 )…ψ N ( QN ) 1⎞ ⎛ E = ∑ ⎜ n j + ⎟hv j 2⎠ j ⎝ Vibrations of the water molecule a1 a1 b2 Procedure (p. 412) for determining how many normal modes there are of each symmetry H 2O = A1 , A1 , B2 ψ 0 ( Q j ) Q jψ m ( Q j ) must belong to the same representation as x, y, or z to be IR active C2V: A1 vibrations are IR active B2 vibration is also IR active Td: example CH4: A1, E, 2T2 vibrations IR forbidden IR active x2, y2, z2, xy, xz, yz belong to representations that are Raman active An example of a more complicated group – D2h (example: ethylene)