The reaction albite = jadeite * quartz determined experimentally in
advertisement

American Mineralogkt,
Volume 65, pages 129-134, 1980
The reaction albite = jadeite * quartz determinedexperimentallyin the range600-1200.c
TrMorHy J. B. Hou-eNo
Departmentof the GeophysicalSciences,Universityof Chicago
Chicago,Illinois 60637
Abstract
The reaction
NaAlSirOu+ SiO2 :NaAlSirOs
jadeite
quaftz high albite
has been determinedby direct experimentin the temperaturerange6fi)-1200"C, and the PT line can be describedby the equation
P: 0.35+ 0.0265?("C) + 0.50kbar
The brackets,when comparedwith the gas-apparatusdeterminationsof this reactionby Hays
and Bell (1973)and by Birch and LeComte (1960),show clearly that pressurecorrectionsare
vanishingly small when the NaCl pressurecell is used with the piston-+ylinder device if piston-out methodsare used.The slope(dP/df : 26.5bar oC-t), io conjunction with tabulated
entropiesfor quartz andjadeite, leadsto a value for the entropy ofsynthetic high albite. The
disorderingentropy of albite is (14.2+2.4J K-' mol-'), and the enthalpy is (12950=3320J
mol-t). The disorderingenthalpy is in excellentagreementwith recentcalorimetric measurements on Amelia albite and its heat-treatedmodification. The bestvaluesfrom phase equilibria for the 298 K, I bar, thermochemicaldata for synthetichigh albite are
AJI3I".rt*:
-3922170+ 4300J mol-'
S&r., o- :221.6 + 2.5J K-t mol-'
The lack of curvatureof the reaction in the P-T plane imFlies that high albite doesnot undergo ordering down to 600'C, at least within the duration time of the experiments.This is
also conflrmed by the X-ray patternsof albites from runs at 600"C.
Introduction
The stability of the end-member feldspars is of
fundamental importance to petrology, yet considerable uncertainty exists as to the P-Tlocation of the
albite to jadeite * quartz curve at high temperatures.
In the temperature range 500-600"C the experimental location is precise (Newton and Smith, 1967;
Johannes et al., l97l; Hays and Bell, 1973), but at
higher temperatures the slope and position of the
curve are only loosely constrained by the reversal intervals determined by Birch and LeComte (1960) and
Bell and Roseboom (1969). Boettcher and Wyllie
(1968) published brackets at 600oC and 800"C which
demand a lower dP/d'T slope (19.5 bar deg-') than
expected for the breakdown ofdisordered albite, for
0003-004X/80/0102-0129$02.00
which high_temperature calorimetry (Holm and
Kleppa, 1968) predicts a slope of 27.6 bar deg-', as
shown later.
The importance of this reaction to petrology cannot be overstated, as it forms the basis for a number
of geobarometric inferences on the stability of
plagioclase-bearing assemblagesat depth (Boettcher,
l97l\.
This study defines closely the P-Z stabitty of high
albite relative to jadeite and quartz, using the precise
pressure control aforded by the low-friction NaCl
pressure cell with the piston-cylinder apparatus. The
precision made possible by this technique allows accurate determination of the standard thermodynamic
properties ofhigh albite.
r29
HOLI-AND: REACTION ALBITE :
130
Startingmaterialsand experimentaltechnique
High albite was synthesized hydrothermally at
7 50"C, 2 kbaL from a mixture of natural vein quartz
and NaAlSi,O. glass(preparedby P. A. M. Anderson) in a l: I molar ratio. The X-ray pattern is that of
high albite,with A2d(CuKa) : 1.90ofor (l3l)-(l3l)
(Goldsmith and Laves, 1954).Natural white jadeite
from a vein in serpentinite,donatedto R. C. Newton
by R. G. Coleman,was used;it containsas principal
impurities 0.13 weight percentCaO, 0.12weight percent MgO, and 0.45weight percentFerO, (Coleman,
1961).
An end-loaded piston-cylinder apparatuswith a
0.75" diameter cylindrical pressurechamber and
NaCl pressuremedium was used for all runs. The
chamber contained a smooth,but not polished,steel
liner and was lubricated by dry MoS, powder. Piston-out type runs were made,g5ingpistonswith bevelled tops cappedwith pyrophylli{s ssalinggasketsas
describedin Holland (1979a),by the following procedure. The cold assembly was brought up to a predeterminedpressureof 3.5 kbar or more below the final desiredrun pressureand was then heatedto the
required temp€rature for the run. The large thermal
expansion of the NaCl assembly during heating, a
pre-calibrated function of temperature and final run
Table 1. Experimental results on the reaction jadeite + quartz :
high albite
-o^
Ti-me
(hours)
Resul t*
4
6
24
23
600
600
800
800
16.O
L 6. 5
2l .o
22.O
24.O
8.O
t3.25
23.75
A
JQ
28
25
27
18
22
900
900
900
1000
1000
23.5
24.25
25.O
26.o
27.5
23.5
22.75
2L.50
23.25
A
NR
JQ
A
JQ
l3
L4
to
11
1100
I 100
I 100
I 100
2 8- O
29.O
3 0. 0
3 0 .o
-**
A
A
JQ
JQ
L5
L6
L7
1200
1200
1200
31.O
32.O
33.O
5.4
4.25
A
NR
JQ
NR
**
had
A - albite growth;
no reaction.
JQ -
zz, I)
3.25
5.2
o.2
jadeite
+ quartz
Run was quenched as soon as desired
been reached.
JQ
growthi
P,T conditions
TADEITE + QUARTZ
pressure,ensuresinitiation of the run under conditions of retreating piston. Occasionallyit was necessary to bleed off a little oil pressureduring the final
stagesof heating to prevent pressureovershoot.
Chromel-alumel thermocouples, in AtSiMag
sleeves,with the junctions protected by a layer of
alundum cement, were used to measure temperatures.No pressurecorrectionwas applied to the
thermocouple readings. The reversal mixture was
made up of the crystallinestarting materialshigh albite, jadeite, and quartz in reactingproportions.The
mixture was ground repeatedly under acetone to
thoroughly homogenizethe reactants,and was then
baked at 350oC overnight to burn off any organic
residue from the acetone. Following Boettcher and
Wyllie (1968)in the successfuluse of interstitial melt
as a flux, the chargewas moistenedby breathing on
the powder before sealingin platinum capsules.The
small amount of interstitial water-undersaturated
melt was found sufficient to produce 100 percent reaction in many runs. Direction of reaction was detectedby changesof more than 15 percentin relative
peak intensitieson the diffractometertrace. Runs at
600"C (from Holland,1979a) were conductedunder
HrO-excessconditions.
Results
Kinetics of the albite to jadeite * quartz reaction
in the presenceof small amounts of melt are such
that complete reaction occurred in less than 3 hours
at 1200oand,24hours at 800"C. Runs at and above
ll00"C cannot be consideredreversed,as the albite
breaks down to jadeite + quartz during the approach
to the final P-T conditions (Table l). However,
growth of albite is extremely rapid (Newton, in Johanneset al.,l97l) and the resultscertaidy represent
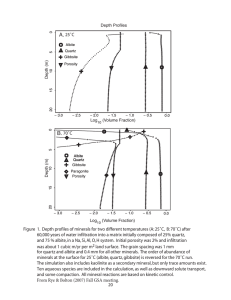
equilibrium. Table 1 and Figure I display the experimental results. The best straight-line fit (by eye)
through the brackets can be described by P (kbar) :
0.0265TC+0.35+0.50.
Discussion
Pressurecalibration of piston cylinder apparatus
The use of the NaCl pressure medium in recent
years has significantly improved the pressuredetermination in piston-cylinder experiments (Johannes
et al., l97l; Mirwald et al., 1975;Johannes,1978;
Holland, 1979a).It
Danckwerth and Newton, 19781,
is instructive to comparethe presentresultsat 600oC
with the gas-apparatusdetermination by Hays and
Bell (1973)and in the range 800-1000'C with the de-
HOLI,AND: REACTION ALBITE :
termination by Birch and Lecomte (1960),also performed with gas pressure medium. At 600'C the
pressuresobtained in this study, 16.0-16.5kbar,
agree well with the Hays and Bell value (16.4+0.5
kbar). The bracketsin the range 800-1000"C all tie
within the reversal interval of Birch and LeComte
and can be regardedas a refinementof their results.
Although Birch and LeComteusednatural low albite
in their starting material, the product albite which
was nucleated and/or grown in their runs was without doubt disorderedhigh albite. On the other hand
the lessstablelow albite in their runs probably broke
down to jadeite + qtrurtz at slightly lower pressures
than thoserequired for the breakdownof high albite.
The equilibrium curve for high albite : jadeite +
qtartz should therefore be expected to lie within or
at marginally higher pressuresthan the Birch and
LeComte reactionbrackets.The fact that the present
resultsall lie within the Birch and LeComte intervals
indicates that the pressuresdetermined with the
NaCl cell, as used here, are not overestimates,and
that subtractive friction correctionsur" yxli5hingly
small. Indeed, any correction due to friction would
be much less at these higher temperatures (8001200'C) than at 600oC, where it was shown to be
negligible (this work; Hays and Bell, 1973;Johannes
et al., l97l). Johannes(1978)reported a pressureof
lessthan 16 kbar for a piston-out reversalat 600oC,
in contrastto the 16.25=0.25kbar reportedhere. Although both studiesusedthe NaCl pressuremedium,
the methodsof arrival at the final pressureand temperaturediffered.It is apparentthat with this method
no pressurecorrection is necessary,and this redetermination is sufficiently preciseto be used as a pressure calibration for alternativepressurecells such as
Pyrex, talc, or soft glass.Results in this laboratory
suggestthat the 7-10 percent subtractive correction
commonly applied to Pyrex-talc assembliesabove
ll00"C with piston-in runs is valid.
JADEITE
+QUARTZ
E.
<.?
ao:<
I
l,!
ALBITE
t/)
t/)
t!
E
o-1
1100
700
900
- DEG
TEMPERATURE
t
500
1300
Fig. l. P,7 brackets on the jadeite + quartz to albite reaction.
Filled squares denote growth of jadeite + qtJaftz, open squares
denote growth of albite, and partially-fiIled squares denote no
apparent reaction.
extrapolatingthe c-quartz heat contentdata in Robie
et al. (1978),the one-barenthalpy for the reactionjadeite + quartz: albite, involving a-qtartz and high
albite, becomes11405+2670I at 964 K. With mean
thermal expansionsand compressibilities from Table
2 and the equilibrium pressureof 18.7kbar, the slope
mav be calculatedfrom:
r^v
dP/dr: ,..+ 1,"Wv- r^@ryd4f
[or,
The result,27.6!1.5 bar "C-', is in good agreement
with the measuredslope,26.5+1.5bar oC-'.
The entropy of synthetichigh albite may be calculated from the experimentalbrackets,using the measured volumes and heat capacities(in Robie et al.,
ttr"-odffifproperties for jadeite,albite
Table2. Standard
s3o*
Thermodynamic consider ations
The slope for the reaction between albite and jadeite + quartz can be readily calculatedfrom hightemperatureoxide melt solution calorimetry.For low
albite, the data of Hlabse and Kleppa (1968) yield
LHn** : -2385+2090 J, for the reaction from jadeite + quartz.Recentmeasurements(R. C. Newton,
personalcommunication) on the enthalpy di-fference
betweenlow albite and albite heat-treatedat 20 kbar,
1200'C yield Aflu,*,o..: 14580+1660
J mol-' at 970
K. Taking account of the a-B quafiz transition by
13r
IADEITE + QUARTZ
.t *-lLJr-t
Jadeite
133,47
+r.25
-3O2427O
IOO 43
{O. 09
221 6
+2.5
-3922170
100 07
J{ 13
2O7.4O
r{ 40
-3935120
4L46
+o 20
- 910700
60 4
I
to
High albiEe
Low albite
Quartz
A,Hloo
.l roitr
22.688
+o o01
dvoxlo5
J b".-l K-l
pvo*ro6
J bar-2
+4180
L5.94
+4300
1 5. 9 4
+3415
5.18
+1000
Thermal exFension data taken from Skinner (1966), conpressibility deEe Eaken frm Birch (1966)
Al1 lhermodynamic daEa are frm
(1978) excepr for high albite and jadeiEe dara which
Robie et al
are from this study,
132
HOLLAND: REACTION ALBITE:
1978), thermal expansion and compressibility data
(Table 2). Solving the relation:
- ZASrr..,:
LH.,n".,
-
JADEITE + QUARTZ
in times of the order of three weeks.Note that the
low-temperature acid calorimetric results (Waldbaum and Robie, l97l; Kracek and Neuvonen',1952)
yield somewhatlow enthalpiesof disorder.
Applications
- r+
tv
arl
ar
dr
1,"
I:"^+
The improved precision in the location of the al[/-o.o
by plotting the right side against absolute temperature yields ASrnr,,as slope and LH2s8'as intercept.
The derived entropy of albite is 221.6+2.5 J K-l
mol-'. Comparing this value with that for low albite
gives the disordering entropy as 14.2+2.4J K-' per
mole of albite, which is somewhat lower than the
maximum configurationalterm -4R (0.25 ln 0.25 +
0.75 ln 0.75) : 18.7 J K-' mol-'. X-ray crystallographic site-occupancystudiesshow that natural low
albite is not fully ordered and that high albite may
not be completely disordered (Holm and Kleppa,
1968).From the observeddistribution of Al and Si
on the tetrahedralsitesin low and heat-treatedalbite,
the configurational disordering entropy is calculated
as 16.0J K-' mol-' (Hotm and Kleppa, 1968).The
agreementwith the value, 14.2!2.4 J K-t, derived
from the slopeof the experinental study is encouraging.
By comparing the derived LHr"".,for the reaction
(12800+2580J) obtained from the experimental
bracketswith that for the reaction involving low albite (-150+2090J) calculated from the data of
Hlabse and Kleppa (1968)with the help of the hightemperature heat contents (Robie et al., 1978),we
can estimatethe disorderingenthalpy of albite. This
estimate, 12950t3320 J, can be compared with the
measuredenthalpiesof disorder ll0l5+840 J mol-'
(Waldbaumand Robie, l97l),9640 J mol-'(Kracek
and Neuvonen, 1952), and 14220=1660J mol-'
(R.C.Newton and T. V. Charlu,unpublisheddata).
This last value was determinedfrom the differencein
heatsof solution at 970 K of Amelia albite and high
albite crystallized from Amelia albite glass at 20
kbar, l200oC, and should representmaximum disordering enthalpy for albite. All enthalpies, unless
otherwise stated, are given corrected to 298 K for
easeof comparison.The high-temperaturecalorimetric measurementsof Holm and Kleppa (1968)on albites heat-treatedfor times of the order of one month
at 1050"C yielded a disordering enthalpy of
J mol-' at97l K, which is equivalentto
14230+1050
J mol-' at298K.It may be (R. C. New13870+1050
ton, personal communication) that albite does not
disorder fully at 1050"C under atmosphericpressure
bite breakdowncurveto high temperaturesallows for
more reliable geobarometryof sodic pyroxenesthan
was possible before. An important application is in
the estimation of the stability of jadeitic pyroxenes
coexisting with quartz in natural rocks. As an example we consider the metamorphic rocks of the
Sezia-Lanzo zone of the Western Alps, and in particular the assemblagepyroxene I quartz, describedby
Reinsch (19-17).Reinsch estimated680'C as an appropriate temperaturefor the equilibration of the pyroxene, which contained 8l percent of the jadeite
end-member.Direct applicationof the presentresults
yield a pressureof 18.4 kbar below which pure jadeite + qrartz would break down. However, the effects of 19 percent diluent in the natural pyroxene
can be calculatedand will result in a destabilization
of the quartz * pyroxene assemblageby an amount
AP = -(RT/LV")lno,a
where R is the gas constant, T the absolutetemperature,LV" the volume changefor the reactionjadeite
* quartz: high albite, and crothe activity ofjadeite
in the pyroxene.The experimentalwork of Holland
(1979b)on the activity-compositionrelationshipsfor
the join jadeite-diopside at 600"C showed that the
activity ofjadeite can be expressedas
o.,d: Ka"lta
where Xro and yrdare the mol fraction and the activity coefficient of jadeite respectively.no was determined (Holland, 1979b)as
1,ra: €xPI(W/RZ) (l X,")1
where the interaction energy, W, was found to be
24kJ. For Reinsch'spyroxenewe find 1',a: l.l2 ar.d
: 0.73at 680oC,which leadsto a
aro: (0.81)'z(1.12)
1.4kbar, and an estimateof a
of
AP:
destabilization
17
kbar for the assemblage.
pressure
of
minimum
(Ganguly, 1973)of
interpretation
quantitative
The
(1969)
on the join diopKushiro
of
the experiments
lack
of
data for the alby
hampered
was
side-albite
inhigh
temperatures
the
at
curve
bite breakdown
discussed
relations
mixing
of
the
Application
volved.
aboveto Kushiro's data showsthat predictionsbased
HOLLAND: REACTION ALBITE:
upon the location of the albite breakdowncurve together with the activity-composition relations determined at 600oC are in good agreementwith the experiments of Kushiro. The agreementis particularly
gratifying becauseKushiro's work was performed at
temperatures(1050-1250"C)far removedfrom those
at which the mixing relations were determined.
The results on the albite breakdown curve can be
used together with the recent determination of the
anorthite breakdown curve to grossular+ kyanite *
qtrafiz (Goldsmith, 1979) to predict the maximum
stability pressuresfor plagioclase feldspars in the
lower crust. Reliable estfunatesfor these limits await
accurate determination of the mixing relations in
plagioclasefeldspar.
JADEITE + QUARTZ
133
supported by NSF grant EAR 74-22851 (R. C. Newton), with
additional support from the Minerals ResearchLaboratory, NSF.
References
Bell, P. M. and E. H. Roseboom(1969) Melting relations of jadeite and albite to 45 kilobars with commentson mghing diagrams of binary systemsat high pressures.Minerat. Soc.Am
Spec.Pap., 2, 15l-161.
Birch, F. (1966)Compressibility;Elastic constants.In S. P. Clark,
Ed., Handbook of PhysicalConstants,p.97-173. Geol. Soc.Am.
Mem.97.
and P. LeComte(1960)Temperaturepressureplane for albite composition.Am. J. Sci.,258, 209-217.
Boettcher,A. L. (1971)The nature of the crust of the earth, with
specialemphasison the role of plagioclase.In J. G. Heacock,
Ed., The Structure and PhysicalPropertiesof the Earth's Crust, p.
Conclusions
261-277. Geophys. Monogr. Ser. 14, Am. Geophys. Union,
The experimental determination of the jadeite *
Washington,D.C.
and P. J. Wyllie (1968)Jadeitestability measuredin the
qaartz: albite reactionrepresentsa considerableim- presenceof silicateliquids in the systemNaAlSiO.-SiO2-H2O.
provement in location of the curve as well as an exGeochim.Cosmochim.Acta, 32, 999-1012.
tension of the measurementsto higher temperatures. Coleman, R. G. (1961)Jadeitedepositsof the Clear Creek area,
This study demonstratesthat the P-T lne showsno
New Idria district, San Benito County, California. J. Petrol., 2,
209-247.
curvature, at least in the range 600-1200oC, and
hencethat albite doesnot undergoAl-Si ordering in Danckwerth, P. and R. C. Newton (1978)Experimentaldetermination of the spinel peridotite to garnet peridotite reaction in
the experinental runs.
the systemMgO-Al2O3-SiOr in the range 900oC-ll00oC and
The location of the curve agreesprecisely with
Al2O3 isopleths of enstatite in the spinel field. Contrib. Mineral.
both pre-existing gas-apparatus determinations
Petrol.,66,189-201.
(Birch and LeComte, 1960;Hays and Bell, 1973)and Ganguly, J. (1973)Activity compositionrelation of jadeite ln omphacite pyroxene.Earlh Planet. Sci.Lett., 19,145-153.
demonstratesthe reliability of the NaCl pressureme(1979)Melting and breakdownreactionsof anordium in piston-cylinder experiments.No pressure Goldsmith, J. R.pressures
and temperatures.EOS, 60,420.
thite at high
correction need be applied for such determinations and F. Laves(1954)The microcline-sanidinestability relawith the experinental techniqueused in this study.
tions. Geochim.Cosmochim.Acta, 5, l-19.
The slope of the reaction as experimentallydeter- Hays, J. F. and P. M. Bell (1973)Albite-jadeite-quartz equilibrium: a hydrostatic determination. Carnegie Inst. lil/'ash. Year
mined is consistentwith recenthigh-temperaturecaBook, 72,706-708.
lorimetry and yields an entropy of disordering which
Hlabse, T. and O. J. Kleppa (1968)The thermochemistryof jais in accord with predictions based upon site occudeite.Am. Mineral., 53, 128l-1292.
pancy studies on natural and heat-treated albites. Holland, T. J. B. (1979a)Experimentaldeterminationof the reaction paragonite : jadeite + kyanite + wat€r, and lnternally conThe enthalpy of disordering in albite can be similarly
sistent thermodynamic data for part of the systemNa2O-Al2O3determined from the experimental brackets and is
SiO2-H2O,with applicationsto eclogitesand blueschists.Cozalso in agreement with thermochemical measuretrib. Mineral. Petrol., 68, 293-301.
ments. Thermodynamic data derived for hydro- ( I 979b) Reversedhydrothermal determinationof jadeitethermally crystallized synthetic high albite are tied to
diopsideactivities.EOS, 60,405.
the entropy and enthalpy of low albite given in Hokn, J. L. and O. J. Kleppa (1968)Thermodynamicsof the disRobie et al. (1978)and form part of a self-consistent ordering processin albite (NaAlSirO). Am. Mineral., 53, 123133.
set which describesaccuratelyall the measuredreac- Johannes,W. (1978)Pressurecomparingexperimentswith NaCl,
tions among albite, paragonite,kyanite, andalusite, AgCl, talc, and pyrophyllite assembliesin a piston cylinder apjadeite, q\artz, corundum, and water (Holland,
paratus. N euesJahrb. Mineral. Monat sh., 84-92.
P. M. Bell, A. L. Boettcher,D. W. Chipman, J. F. Hays,
1979a).
H. K. Mao, R. C. Newton and F. Seifert (1971) An interlaboratory comparison of piston--cylinder pressure calibration
Acknowledgments
using the albite-breakdown reaction. Contrib. Mineral. Petrol.,
R. C. Newton and J. R. Goldsmith have contributed greatly in
32,2+38.
discussionsduring the course of this work. The project was Kracek, F. C. and K. J. Neuvoncn (1952) Thermochemistryof
134
plagioclase
HOLLAND: REACTION ALBITE:
and alkali
feldspars. Am J. Sci., Bowen Volume,
29t-3r8.
Kushiro,I. (1969)Clinopyroxene
solidsolutionsformedby reactions between diopside and plagioclase at high pressures.Mrzeral Soc.Am. Spec.Pap.,2,179-191.
Mirwald, P. W., I. C. Getting and G. C. Kennedy (1975)Low-friction cell for piston-cylinder high-pressure apparatus. "L
Geophys.Res.,80, 1519-1525.
Newton, R. C. and J. V. Smith (1967)Investigationsconcerning
the breakdown of albite at depth in the earth. J. Geol., 75, 268286.
Reinsch,D. (19'fD High pressurerocksfrom Val Chiusella(SeziaLanzo zone,Italian Alps). NeuesJahrb. Mineral. Abh., 130,89to2.
TADEITE + QUARTZ
Robie, R. A., B. S. Hemingwayand J. R. Fisher (1978)Thermodyat 298'15K
namic propertiesofminerals and relatedsubstances
and I bar (105 Pascals)pressureand at higher temperatures.
U. S. Geol. Sun. Bull. 1452.
Skinner, B. J. (1966) Thermal expansion.In S. P. Clark, Ed.,
Handbook of PhysicalConstants,p.75-96. Geol.Soc.Am. Mem.
97.
Waldbaum, D. R. and R. A. Robie (1971)Calorimetric investigation of Na-K mixing and polymorphismin the alkali feldspars.
Z. Kristallogr., 184, 381420.
Manuscript received, December 13, 1978;
acceptedfor publication, May 24, 1979.