Aluminum Technology - The Wiring Harness of the Future
Next Generation Terminals for Aluminum Wire Application
2015-01-0245
Published 04/14/2015
Markus Gaertner
Delphi Deutschland GmbH
CITATION: Gaertner, M., "Aluminum Technology - The Wiring Harness of the Future Next Generation Terminals for Aluminum Wire
Application," SAE Technical Paper 2015-01-0245, 2015, doi:10.4271/2015-01-0245.
Copyright © 2015 SAE International
Abstract
The Wiring Harness of a Vehicle
Historically aluminum was recognized as a valuable material to
achieve weight reduction targets in engines, vehicle chassis and
suspension. Aluminum needs to be also considered in new areas
like vehicle electrification to support the overall weight reduction
targets. The use of aluminum helps to improve fuel economy and
brings down CO2 emissions by reducing weight. This benefit is an
attractive option for the wiring harness to replace heavier copper
conductors. In addition to large cross section wires for power
cable, where aluminum conductors are already in use, the
intermediate aluminum cable cross section of 2.5mm2 to 6.0 mm2
provides a good potential for car implementation to hit weight
saving targets.
Today reducing weight and simultaneously implementing more
functionality is the challenge for the design of wiring harnesses. A
wiring harness for a vehicle from 1960 would have a weight 3 to
5 kg. Today, a wiring harness weight can be in the range of 50 to
70 kg, dependent on the equipped functions.
The major implementation roadblocks for aluminum technology
are the surface oxides Al2O3 which are an insulator and the
potential galvanic corrosion of aluminum in combination with the
always present copper terminal. Galvanic corrosion can occur with
the presence of electrolyte fluids inside the car. Traditional
termination solutions are mostly dependent on the use of additive
materials like paints, grease or similar approaches to protect the
aluminum at the crimp area. To achieve a permanently reliable
electrical contact, the termination technology between a copper
terminal and aluminum wire with single strands, needs to be
specifically optimized.
Delphi now provides a technology named SMC (Selective Metal
Coating). SMC is an innovative technical solution for a wiring
harness equipped with aluminum cable.
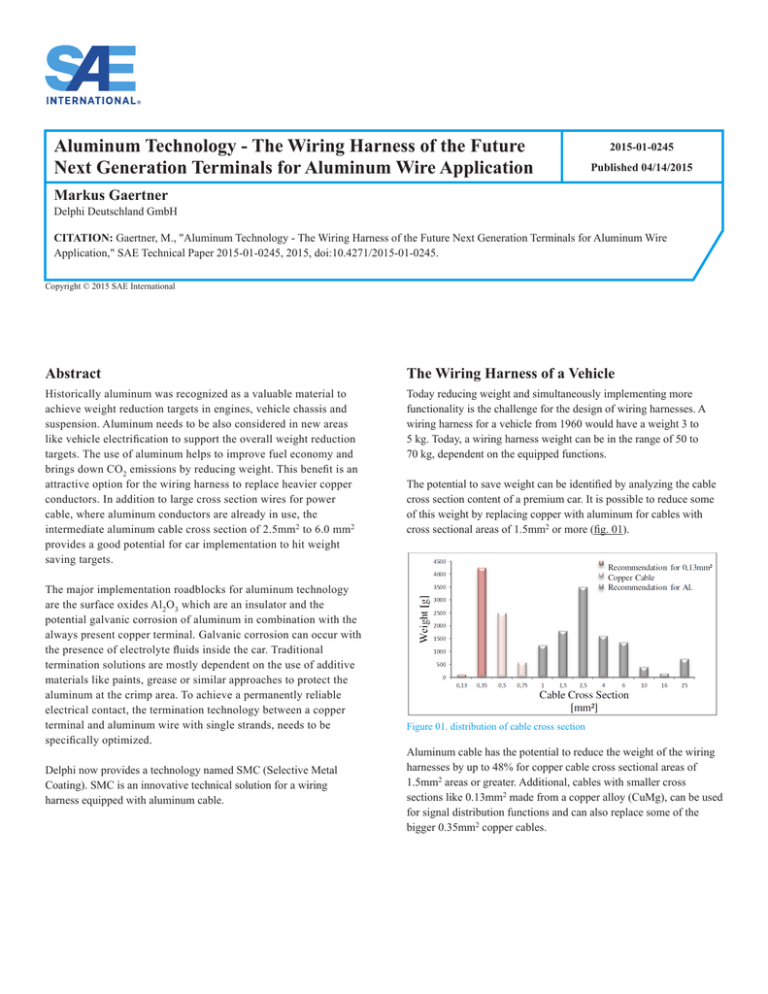
The potential to save weight can be identified by analyzing the cable
cross section content of a premium car. It is possible to reduce some
of this weight by replacing copper with aluminum for cables with
cross sectional areas of 1.5mm2 or more (fig. 01).
Figure 01. distribution of cable cross section
Aluminum cable has the potential to reduce the weight of the wiring
harnesses by up to 48% for copper cable cross sectional areas of
1.5mm2 areas or greater. Additional, cables with smaller cross
sections like 0.13mm2 made from a copper alloy (CuMg), can be used
for signal distribution functions and can also replace some of the
bigger 0.35mm2 copper cables.
Although replacing the copper with aluminum will result in a larger
harness cross section due to the physical characteristics of aluminum
compared to copper.
Figure 02. shows the relation between copper and aluminum cable in terms of
cross section for the same current carrying capacity
Aluminum Cable Intermediate Size
Aluminum cables with cross sections greater than ≥ 2,5mm2 are
designed out of nearly pure aluminum. A typical alloy used is Al
99,7. For lower cross sections, due to cable mechanical
requirements several alloys are available that have a higher tensile
strength characteristic.
Aluminum Cable, Current Load Capacity
The theoretical approach of getting the current equivalent cross
section between aluminum and copper is the equation (1) based on
the same electrical current. The cross section is reversely
proportional to the electrical conductivity. The cross section for
aluminum needs to be about 1.6 times larger than for copper for
carrying the same current.
Figure 04. conductivity Cu-ETP & Al99,7 according LV 112-1/2 [2] The
corresponding cross section between aluminum and copper are compared to
each other. The test shows that the current carrying capacity of aluminum is
mostly higher Imax,Al >= Imax,Cu. than is the current equivalent of copper.
Connection Technology, Cable to Terminal
Crimp Technology
Crimp technology is used to connect a terminal to a wire in automotive
industry today. Crimping is a process where the core crimp wings of
the terminal are bent around the core of the wire. The core crimp
provides the mechanical and electrical connection of the cable to the
terminal. The primary function and design of the core crimp is to
optimize the electrical performance of the cable to terminal attachment
for the service life of the terminal. The wire is compressed by the core
crimper and provides a good electrical and mechanical connection. The
insolation of the wire is in contact with the insolation crimp of the
terminal. It provides a strain relief support function for the core crimp.
For the use of aluminum cable, the connection technology crimping
and terminal design need a few modifications.
(1)
To get the equivalent cross section based on equal current capacity in
addition to the power loss due to resistance, the cooling on the cable
surface is important to consider. On the basis of the larger aluminum
cross section and the better thermal radiation the cross section
relation decreases to 1.5 times.
(2)
Figure 05. crimp terminal
Aluminum Background and Technical Challenge
Aluminum contains surface oxides Al2O3. The oxides are an electrical
insulator. The technology of connecting a terminal to an aluminum
wire has therefore two challenges to overcome.
Figure 03. conductivity Cu-ETP & Al99,7 according LV 112-1/2. [2] The
maximal current load capacity with FLRY-B copper cable in comparison to
FLALRY aluminum cable, both with the same cross section.
1.
The terminal to strand conductivity, because of the
nonconductive aluminum surface oxides, is the main challenge.
2.
The strand to strand conductivity, because of the non-conductive
aluminum surface oxides, defines an additional challenge for the
intermediate wires sizes.
Terminal to Strand Conductivity
For the terminal to strand conductivity, the terminal has a knurl
geometry inside the core crimp that assures that the hard oxide
surface is going to break.
In addition to optimized strand to strand conductivity, the nugget
gives a more robust mechanical characteristic to the design due to the
prevention of relative strand to strand movement.
Figure 09. cross section view through an aluminum core crimp after Crimping
Figure 06. Delphi terminal knurl design
Aluminum Field Test
Delphi has conducted a field test on high-mileage daily use vehicles
that have been equipped with aluminum wire (fig.10). The objective
was to gain durability and design verification experience with
aluminum cable and termination. In order to gain real world
experience with aluminum cable and aluminum specific crimp
technologies Delphi performed data monitoring on these vehicles.
The result was real-world operating conditions for our products under
constant monitoring.
Figure 07. the geometry of the terminal knurls has been adapted by the
aluminum cable
Due to the flowing characteristic of the aluminum, the oxides are
broken and a good electrical connection can be achieved.
The Strand to Strand Conductivity
Before crimping the aluminum wire to a terminal, the single strands
of an aluminum wire welded to each other by an ultra-sonic welding
process. This provides a cable that has a welding geometry
afterwards, a so called “nugget” with an electrical strand to strand
connection. This nugget is going to be crimped to the terminal.
Figure 10. test set up of the aluminum wire installed inside the engine
compartment with constant data collection of contact resistance
Different technologies were evaluated through the field test. The table
(fig. 11) shows the test result after a duration of 300,000 hours of
vehicle operation and with a total over 1,300,000 miles.
Figure 08. Delphi ultra-sonic welding nugget design
Figure 11. [%] percentage of passed terminals
Technology 01 as an example is designed without any additional
corrosion protection. It passed the test even though being located at
the exterior of the engine compartment.
Selective Metal Coating (SMC)
The connection of an aluminum cable to a copper terminal requires
protection against galvanic corrosion in some vehicle compartments.
Figure15 shows that copper is freely accessible at the punched edges
of the terminal at the core crimp and spatially located right next to the
aluminum wire. At electrical connection location, galvanic corrosion
is particularly likely occur under the influence of an electrolyte.
Figure 12. the picture shows the terminal after one year. It has low resistance
and no visible corrosion.
Aluminum Technologies
Corrosion protection should be used depending on the vehicle
installation location. As the field test indicates, corrosion protection is
not always needed everywhere. Delphi offers the OEM a modular
approach where corrosion protection for the terminal is available as
an additional option that can be used in the compartment areas where
it is recommended.
Figure 15. schematic view of a core crimp
The terminal is formed mainly out of a copper based lead frame
material. The Delphi SMC-protective layer is applied on the hot-dip
tin of the copper base material (Fig.16) as well as on the bare copper
of the punched edges of the terminal. The selectively applied SMC
layer consists of an electroplated layer of brass (CuZn) and an
overlying deposited layer of tin.
Figure 13. recommendation for aluminum terminals
The experience of testing and evaluation in field vehicles results in an
allocation of three different zones for the vehicle as shown in fig. 13.
Zone 1 is without any need for further corrosion protection for “high
and dry” locations, for example the instrument panel. Corrosion
protection is recommended for zone 2 and zone 3. Zone 2 is for
interior compartments that can accidentally be exposed to a corrosive
atmosphere. Here corrosion protection is realized by a selective metal
coating treatment of the terminal. Zone 3 is for exterior, wet
application areas and corrosion protection realized by a connector
housing with a single seal design.
Figure 16. Delphi SMC protection layer
During the corrosion test, a sodium chloride solution is used as an
electrolyte. Chloride is the reactive element which promotes the
corrosion. Therefore, its influence is considered in more detail.
Chlorides are compounds of the chemical element chlorine. Metal
chlorides such as sodium and cobalt chloride are salts.
Sodium chloride dissociates in water into Na + and Cl-ions. The
resulting solution is conductive, a prerequisite for galvanic corrosion.
Chloride is able to react with copper, tin, zinc and aluminum and to
dissolve these metals. It preferentially reacts with zinc and aluminum,
and only then with the nobler metals, tin and copper.
Figure 14. Delphi aluminum technologies
The outer tin layer of the terminal is connected to the aluminum
cable. This connection has an electrochemical potential between tin
and aluminum. This electrochemical potential is available to initiate
galvanic corrosion. ΔE (mV), is the electromotive force (EMF) of the
galvanic corrosion.
Copper has a tendency to form intermetallic phases with tin (Cu3Sn
and Cu6Sn5), and in doing so diffuse into an adjacent layer of tin.
Chloride penetrates from outside into the tin and dissolves it.
Galvanic Corrosion Potential
The basis of galvanic corrosion is the corrosion potential. If metals
with a sufficiently high potential difference are in electrical
connection to each other, a conductive solution (e.g. sodium chloride
in water), dissolves the metal with the more negative potential under
the formation of hydrogen.
Figure 19. REM picture, chloride is in copper by SEM/EDX barely detectable
Figure 17. potentiostat measurement with three electrode set up
The current-density-potential curves of the corrosion potential have
shown (fig.17) that the used aluminum has its potential at −901 mV.
Before the measurement has been done, the oxides of aluminum have
been removed with 25% sodium hydroxide solution by pickling and
rinsing. Measurements with the slightly nobler aluminum oxide layer
result in a potential of −733 mV. At the contacts between the
aluminum wire and the terminal the aluminum oxide layer is the
actual galvanic partner. The measurement for copper has its potential
at −298 mV. Thus, the ΔE between copper and aluminum is 435 mV.
The SMC protective layer has a reduced corrosion potential of
306 mV. In comparison to bare copper the ΔE can be reduced by
30%.In order to avoid galvanic corrosion direct contact between
aluminum, copper and chloride should be prevented (fig.15).
If copper and aluminum are placed in a sodium chloride solution while
in electrical contact with each other, the less noble metal aluminum
behaves as the anode and the more noble metal as a cathode. This
battery effect speeds up the dissolution of the aluminum.
The aluminum dissolves with the formation of hydrogen gas (H2). Thus,
the pH value in the sodium chloride solution increases which in turn
accelerates the aluminum dissolution. If conductive contact between
copper and aluminum can be avoided corrosion is slowed down.
Tin Layer as a Corrosion Protection
Corrosion tests have indicated that a re-tinning of terminals e.g. at the
copper punched edges, slows down the galvanic corrosion effect and
offer a significant protection.
Due to the difference in electronegativity between the potential of
CuSn and copper of the base material, the intermetallic CuSn-phase
protects the copper base material. Using a tin plated copper base
material without a CuZn intermediate layer, the contact between the
aluminum, copper and chloride, cannot be prevented sufficiently over
the entire time of the required corrosion tests. CuSn-phases are
formed. But these are not enough to prevent contact corrosion.
CuZn as an Additional Corrosion Protection
Copper and brass (CuZn) have a different diffusion behavior with
tin layers.
If equally 5 μm thick tin layers are deposited directly on brass and on
a copper interlayer (fig.20), after a storage temperature of 170 ° C,
the layer on the copper interlayer is dissolved after 25 days, directly
on the brass, however, it is dissolved in only about 60 days. [1]
This study shows clearly that copper out of the brass layer is
significantly slower diffusing into the tin in comparison to pure
copper. The concentrated copper has a high priority to alloy with tin.
Zinc and tin do not form intermetallic phases. The endeavor of the
copper from the brass layer to mix with tin is significantly lower.
The brass phase dissolves very slowly. The diffusion of copper in the
overlying tin is significantly slowed down by the CuZn-interlayer.
Inspecting the SMC-layer system at the end of the corrosion test, the
chloride is distributed in the outer Sn layer (fig. 21) and diffuses into
the CuZn-intermediate layer, but not beyond that.
Chloride reacts with the zinc in the CuZn-intermediate layer and
dissolves it slowly.
Summary/Conclusions
The SMC Terminal integrates solutions to break the aluminum oxides
through knurls and nugget, and metal plating in the crimp area to
reduce the galvanic corrosion to stay inside customer requirements.
For the SMC layer system, the following protective effects can
be determined.
Figure 20. diffusion of copper into a tin layer
1.
Reduction of electrochemical potential between the terminal and
the aluminum wire.
2.
The chloride is distributed homogeneously in the outer tin layer,
thereby it will be slowed down in the diffusion behavior towards
the copper.
3.
The chloride remains in the brass phase (CuZn) and is prevented
from penetrating directly into the copper of the base material.
Preferably chloride reacts with the zinc, it engages the zinc in
the CuZn layer and dissolves it. As long as zinc is available for
the reaction, the chloride does not penetrate into the copper or
bronze layer.
4.
As the investigation [1] shows, copper from the brass
intermediate layer has a slow diffusion behavior into the
overlying Sn layer. Thus copper as a galvanic element, is lately
available. The diffusion of copper from the base material to the
upper layer of tin, through the brass phase, can be neglected.
5.
By the slowed down aluminum corrosion, the pH value
increases slowly in the electrolyte, sodium chloride. Thus, the
dissolution of the aluminum is delayed.
SMC technology is based on a material optimization for the crimp
design of the terminal. The corrosion protection is inside the terminal
integrated. SMC technology does not need any additional materials like
conformal coating, grease, powder, etc. Delphi aluminum terminals do
not entail any fundamental change for cable harness manufacturers.
References
1.
Source: Manfred Jordan, Die galvanische Abscheidung von
Zinn und Zinnlegierungen, Saulgau 1993, page 206. (Scott, B.
C.: Inst. Metal Finish, Ausgabe 65 (1987), page 90).
2.
LV 112-1/2, Delivery Specification for automotive cable of German
OEMs, LV112-1 for copper cable, LV112-2 for aluminum cable.
Contact Information
Markus.Gaertner@delphi.com
Figure 21. REM picture, SMC protection layer after corrosion test
Acknowledgments
The preparation of this paper was supported by the Delphi aluminum
team: George Drew, Kurt Seifert, Thomas Reinders, Petra Bauer,
Thomas Plinta
The Engineering Meetings Board has approved this paper for publication. It has successfully completed SAE’s peer review process under the supervision of the session organizer. The process
requires a minimum of three (3) reviews by industry experts.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or
otherwise, without the prior written permission of SAE International.
Positions and opinions advanced in this paper are those of the author(s) and not necessarily those of SAE International. The author is solely responsible for the content of the paper.
ISSN 0148-7191
http://papers.sae.org/2015-01-0245



