Photoelectric effect - University of Colorado Boulder
advertisement

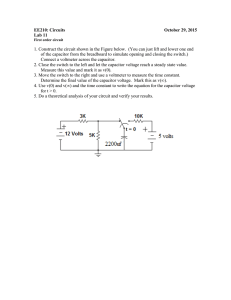
Experiment 5 Photoelectric Effect Physics 2150 Experiment No. 5 University of Colorado 1 Introduction Photons of frequency 𝑓 falling on a surface whose work function is Φ cause photoelectrons to be ejected with maximum kinetic energies given by the Einstein equation ℎ𝑓 = 𝑇!"# + Φ (1) with ℎ𝜐 the energy of the incident photon, ℎ being Planck’s constant. In this experiment, Einstein’s equation is tested by allowing the ejected electrons to advance against a potential difference which just suffices to bring them to rest and which is thus called a “stopping potential.” This stopping potential, Vs, is a measure of the maximum kinetic energy, Tmax, with which electrons leave the surface: 𝑒𝑉! = 𝑇!"# . (2) The stopping potential is not dependent on light intensity, but on photon energy. Monochromatic light obtained by isolating lines of the mercury spectrum is allowed to fall upon the cathode of a photoelectric cell, ejecting electrons. Across this photocell is connected a storage capacitor; the complete circuit diagram is given in Fig. 1. Each photoelectron emitted creates in effect a positive charge, +e, on the cathode and thus on one Figure 1: Circuit Diagram for Photoelectric Effect Experiment Experiment 5 2 plate of the capacitor. Electrons that fly to the anode end up effectively on the other plate of the capacitor. Current will flow thus charging up the capacitor until the charge imbalance is great enough that the resulting potential difference across the capacitor prevents the buildup of additional charge. The apparatus thus generates its own stopping potential. Since the anode may have a different work function than the cathode, and in general the wires and other circuit elements may have different work functions and different contact potentials, it is not easy to measure the actual value of the stopping potential. However, such effects will always contribute the same constant (but unknown) amount to the measured stopping potential and can be eliminated by varying the frequency of light used and hence the value of 𝑉! . Let 𝑉! be the measured stopping potential, and 𝑉! be the unknown contributions to 𝑉! from differences in work function, etc. Then 𝑉! = 𝑉! + 𝑉! = !!"# ! (3) Substituting into Eq. (1), ℎ𝑓 = 𝑒 𝑉! − 𝑉! + Φ and solving for 𝑉! , ! Φ 𝑉! = ! 𝑓 + 𝑉! − ! . + 𝑉! . (4) (5) This equation shows that 𝑉! plotted against frequency should yield a straight line of slope ℎ/𝑒. If 𝑒 has already been found from an experiment such as Millikan’s Oil-­‐Drop experiment, ℎ can be found from observations of this slope. In this experiment, the potential 𝑉! is measured by discharging the capacitor through a ballistic galvanometer in which the observed deflection, 𝐷, is proportional to the charge 𝑄 passing through the galvanometer coil 𝐷 = 𝑘𝑄 (6) where 𝑘 is the galvanometer constant. If the potential 𝑉! corresponds to a charge 𝑄 built up on the capacitance 𝐶 , then 𝑄 = 𝐶𝑉! (7) and hence ! 𝑉! = !" 𝐷. (8) Experiment 5 3 The galvanometer deflection is thus a direct measure of the potential 𝑉! . The constant 𝑘𝐶 is measured by using a standard voltage source and measuring the deflection 𝐷 as a function of known voltage. From these data, a calibration curve is made which is later used to determine 𝑉! for various frequencies of light incident on the photocell. Procedure Five major spectral lines of mercury are isolated by means of interference filters mounted on a wheel. Each filter transmits only one wavelength and is selected by means of a knob at one end of the light-­‐tight box enclosing the lamp, filters, and photocell. Remove the lid of the box and identify the components. Replace the lid and be sure it is latched in place. Note the thin strip of aluminum placed through the slot in the side of the box. This strip, which moves horizontally, is used as a shutter to block light from the mercury lamp while making a galvanometer reading. (The galvanometer in this experiment looks like a crossbow with a telescope mounted on the pillar). Part I: Calibration A. Preliminary Steps and Notes 1. Be sure that the AC power switch, located on one end of the box, is turned off. 2. Look through the telescope and focus it on the eye piece index line and the galvanometer scale. 3. Zero the galvanometer scale (cm) by adjusting either the scale or the telescope’s position. Do not touch the galvanometer suspension. 4. Once set into motion, the galvanometer suspension will keep swinging. Stop it by pressing and holding the short switch. 5. All component references are to Fig. 1. B. Calibration 1. To provide a well-­‐defined linear calibration curve over the range 0-­‐2V, take between 10 and 20 readings of galvanometer deflection (i.e. charge the capacitor in 0.1 or 0.2 V steps). Place the Calibrate-­‐Operate switch S2 in the calibrate position. Press switch S1 on the front of the Voltage Calibration Source box and adjust the voltage with R1 to the desired value. Hold the switch S1 down for at least 2 seconds while the capacitor charges, and while holding it down, flip the Calibrate-­‐Operate switch to Operate. Note: if S1 is not held down, the capacitor will discharge back through the low-­‐resistance voltmeter and the variable resistor R1. While looking through the galvanometer telescope, depress the Discharge switch and note and record the maximum deflection seen. A graph of voltage versus deflection obtained from such readings then determines Experiment 5 4 the constant (1/kC) in Eq. (8). Use a least-­‐squares fitting program to determine the best value of 1/kC and the uncertainty in this value. Part II: Stopping Potential as a Function of Frequency A. The Calibrate-­‐Operate switch is placed in the Operate position for the remainder of the experiment. Turn on the AC power to the mercury lamp and allow several minutes for it to warm up. Select one wavelength indicated on the filter selector knob at the end of the box. B. Check the shutter to see that it is closed. Depress and then release the discharge switch to discharge the capacitor completely. You are now ready to take data. Open the shutter for a time interval sufficient to charge the capacitor. Typically, 5 minutes is required for each wavelength. In principle, the time necessary to charge the capacitor depends on the intensity of the light of each wavelength incident on the photocell. The required time interval could be found by charging for longer and longer intervals, until the maximum deflection of the galvanometer upon discharge does not continue to increase. C. Close the shutter before discharging the capacitor to take a reading. Record the highest reading obtained (i.e. the peak deflection of the galvanometer) with its corresponding wavelength. D. Follow the above procedure for all five wavelengths indicated in Table I. Plot voltage versus frequency and determine 𝒉/𝒆. Use a least-­‐squares fitting program to determine the best value of 𝒉/𝒆 and the uncertainty in this value. Compare it with the accepted value of 4.136 x 10-­‐15 J-­‐sec/C. What factors might explain the discrepancy you found? Φ E. By extending the line of your graph to 𝑣 = 0, determine the quantity (𝑉! − ! ) in Eq. 5. Ultraviolet 3650 Å Violet 4050 Å Blue 4360 Å Green 5460 Å Yellow 5780 Å Table 1: High Spectral Lines Isolated by Filters