Recitation 5
advertisement
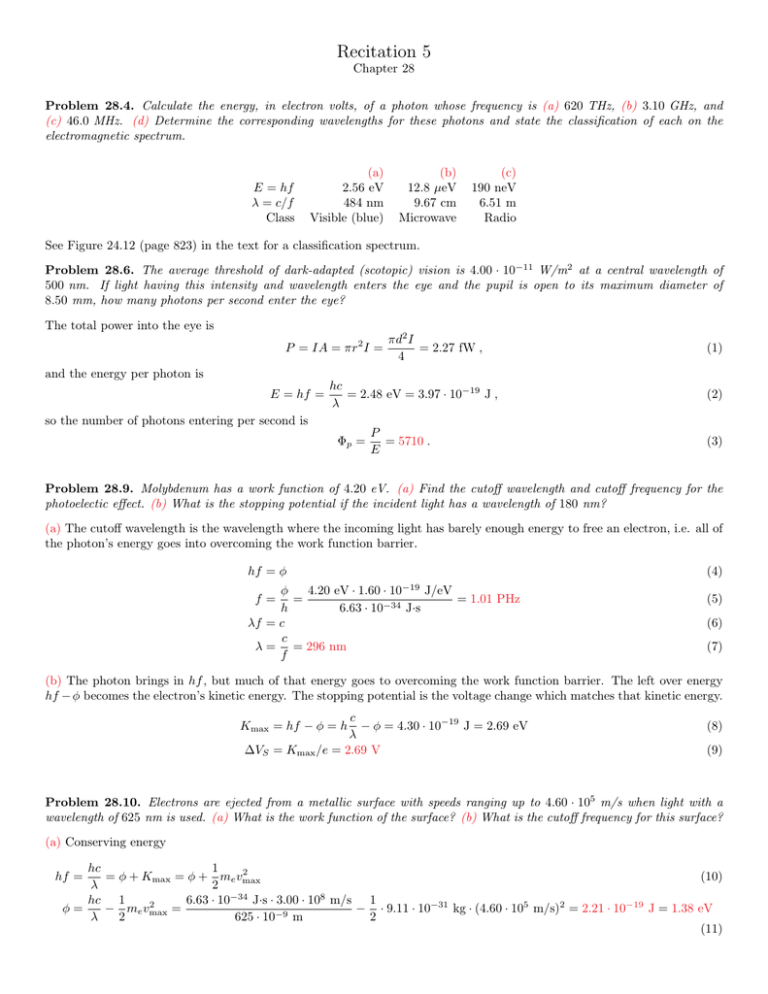
Recitation 5 Chapter 28 Problem 28.4. Calculate the energy, in electron volts, of a photon whose frequency is (a) 620 THz, (b) 3.10 GHz, and (c) 46.0 MHz. (d) Determine the corresponding wavelengths for these photons and state the classification of each on the electromagnetic spectrum. E = hf λ = c/f Class (a) 2.56 eV 484 nm Visible (blue) (b) 12.8 µeV 9.67 cm Microwave (c) 190 neV 6.51 m Radio See Figure 24.12 (page 823) in the text for a classification spectrum. Problem 28.6. The average threshold of dark-adapted (scotopic) vision is 4.00 · 10−11 W/m2 at a central wavelength of 500 nm. If light having this intensity and wavelength enters the eye and the pupil is open to its maximum diameter of 8.50 mm, how many photons per second enter the eye? The total power into the eye is P = IA = πr2 I = πd2 I = 2.27 fW , 4 (1) and the energy per photon is E = hf = hc = 2.48 eV = 3.97 · 10−19 J , λ (2) so the number of photons entering per second is Φp = P = 5710 . E (3) Problem 28.9. Molybdenum has a work function of 4.20 eV. (a) Find the cutoff wavelength and cutoff frequency for the photoelectic effect. (b) What is the stopping potential if the incident light has a wavelength of 180 nm? (a) The cutoff wavelength is the wavelength where the incoming light has barely enough energy to free an electron, i.e. all of the photon’s energy goes into overcoming the work function barrier. hf = φ (4) −19 φ 4.20 eV · 1.60 · 10 J/eV = = 1.01 PHz h 6.63 · 10−34 J·s λf = c c λ = = 296 nm f f= (5) (6) (7) (b) The photon brings in hf , but much of that energy goes to overcoming the work function barrier. The left over energy hf − φ becomes the electron’s kinetic energy. The stopping potential is the voltage change which matches that kinetic energy. c − φ = 4.30 · 10−19 J = 2.69 eV λ ∆VS = Kmax /e = 2.69 V Kmax = hf − φ = h (8) (9) Problem 28.10. Electrons are ejected from a metallic surface with speeds ranging up to 4.60 · 105 m/s when light with a wavelength of 625 nm is used. (a) What is the work function of the surface? (b) What is the cutoff frequency for this surface? (a) Conserving energy hc 1 2 = φ + Kmax = φ + me vmax (10) λ 2 hc 1 6.63 · 10−34 J·s · 3.00 · 108 m/s 1 2 φ= − me vmax = − · 9.11 · 10−31 kg · (4.60 · 105 m/s)2 = 2.21 · 10−19 J = 1.38 eV λ 2 625 · 10−9 m 2 (11) hf = (b) Light at the cutoff frequency only barely supplies enough energy to overcome the work function. hfcut = φ φ fcut = = 334 THz h (12) (13) (14) Problem 28.13. An isolated copper sphere of radius 5.00 cm, initially uncharged, is illuminated by ultraviolet light of wavelength 200 nm. What charge will the photoelectric effect induce on the sphere? The work function for copper is 4.70 eV. As light lands on the sphere, electrons are blasted off into oblivion. As they leave, the sphere accumulates positive charge, so it takes a bit more energy to pull the next electrons away. Eventually the system reaches equilibrium when the initial kinetic energy of electron blasting off is not quite enough for it to escape the electro-static attraction to the positive sphere. In math Kmax = hf − φ = = ke Q= hc − φ = 2.40 · 10−19 J = 1.50 eV λ (15) Qq R (16) Kmax R 2.40 · 10−19 J = 8.34 pC = 52.1 · 106 electrons = 9 ke q 8.99 · 10 N·m2 /C2 · 1.60 · 10−19 C (17) Problem 28.56. Figure P28.56 shows the stopping potential versus the incident photon frequency for the photoelectric effect for sodupm. Use the graph to find (a) the work function, (b) the ratio h/e, and (c) the cutoff wavelength. The data are taken from R.A. Millikan, Physical Review 7:362 (1916). ∆Vs (V) 4 3 2 1 0 400 800 f (THz) 1200 (a) The fit line passes nearby the points (400 THz, 0 V) and (1.20 PHz, 3.3 V). In point-slope form, the fit line is then (3.3 − 0) V (f − 400 THz) (1200 − 400) THz ∆Vs = 4.125mV/THz · (f − 400 THz) ∆Vs − 0 V = (18) (19) The work function is the inverse y-intercept, so φ = −∆Vs (f = 0) = −4.125mV/THz · (−400 THz) = 1.65 eV (20) (b) The theoretical form for the fit line is e∆Vs = hf − φ h ∆Vs = f − φ e (21) (22) so he = 4.12 mV/THz = 4.12 pV/s. (c) The cutoff wavelength is given by the work function and conservation of energy. hc =φ λcut hc = = 751 nm φ hfcut = λcut (23) (24) Problem 28.57. The following table shows data obtained in a photoelectric experiment. (a) Using these data, make a graph similar to Active Figure 28.9 that plots as a straight line. From the graph, determine (b) an experimental value for Planck’s constant (in joule-seconds) and (c) the work function (in electron volts) for the surface. (Two significant figures for each answer are sufficient.) Wavelength (nm) 588 505 445 399 Maximum Kinetic Energy of Photoelectrons (eV) 0.67 0.98 1.35 1.63 (a) We can convert the wavelengths to frequencies and graph them λ (nm) 588 505 445 399 f = c/λ (THz) 510 594 674 751 Kmax (V) 2 1 0 −1 −2 0 200 400 f (THz) 600 800 The least-squares fit yields parameters (c) φ = 1.4 eV and h/e = 4.04 mV/THz. So (b) h = 6.5 · 10−34 J·s.