3.1 Introduction to the First Law of Thermodynamics 3.2 Heat Transfer
advertisement
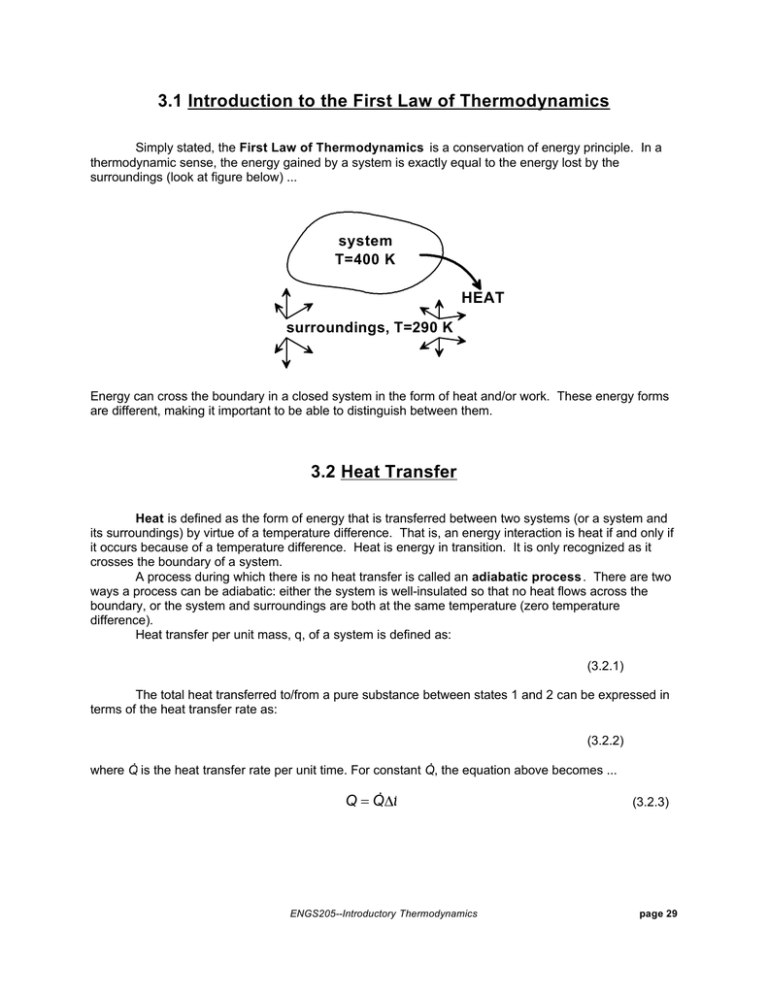
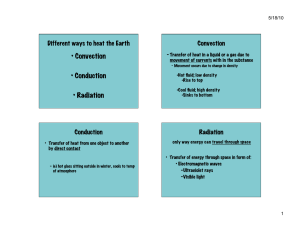
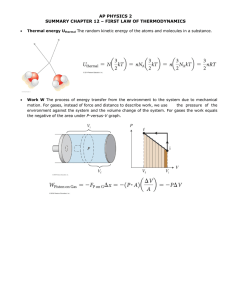
3.1 Introduction to the First Law of Thermodynamics Simply stated, the First Law of Thermodynamics is a conservation of energy principle. In a thermodynamic sense, the energy gained by a system is exactly equal to the energy lost by the surroundings (look at figure below) ... system T=400 K HEAT surroundings, T=290 K Energy can cross the boundary in a closed system in the form of heat and/or work. These energy forms are different, making it important to be able to distinguish between them. 3.2 Heat Transfer Heat is defined as the form of energy that is transferred between two systems (or a system and its surroundings) by virtue of a temperature difference. That is, an energy interaction is heat if and only if it occurs because of a temperature difference. Heat is energy in transition. It is only recognized as it crosses the boundary of a system. A process during which there is no heat transfer is called an adiabatic process. There are two ways a process can be adiabatic: either the system is well-insulated so that no heat flows across the boundary, or the system and surroundings are both at the same temperature (zero temperature difference). Heat transfer per unit mass, q, of a system is defined as: (3.2.1) The total heat transferred to/from a pure substance between states 1 and 2 can be expressed in terms of the heat transfer rate as: (3.2.2) . . where Q is the heat transfer rate per unit time. For constant Q, the equation above becomes ... . Q=Q t ENGS205--Introductory Thermodynamics (3.2.3) page 29 Very Important Sign Convention: Since heat is a directional quantity (it can be lost or gained), it is very important to establish a sign convention! Heat transfer to a system is positive and heat transfer from a system is negative! Heat In Q=5 kJ SYSTEM Heat out Q=-5 kJ Heat can be transferred in three different ways: conduction, convection, and radiation. Below is a brief description to familiarize you with the basic mechanisms of heat transfer. ‚ Conduction Heat Transfer: The transfer of energy from the more energetic particles of a substance to the adjacent less energetic ones as a result of interactions between the. particles. Conduction takes place in solids, liquids, and gases. The rate of heat conduction, Q cond , is given by Fourier's Law of heat conduction: (3.2.4) where k is a constant of proportionality called the thermal conductivity and A is the area normal to the direction of heat transfer. Fourier's law indicates that the rate of heat conduction in a direction is proportional to the temperature gradient in that direction. Note! Have you ever wondered why sitting in a cold steel chair feels colder than sitting in a wooden chair at the same temperature? The cold sensation you feel when sitting in the chair is due to heat being conducted from your body to the chair. The thermal conductivities of carbon steel and wood are, respectively, 60.5 and 0.17 W/(m K). This means you lose 350 times more heat from your body by sitting in the steel chair as opposed to the wooden chair, initially !! ‚ Convection Heat Transfer: The mode of energy transfer between a solid surface and an adjacent liquid or gas which is in motion, and it involves the combined effects of conduction and fluid motion. The faster the fluid motion, the greater the convection. In the absence of bulk fluid motion, the heat transfer between the solid and fluid occur via conduction. There are two specific kinds of convection: v Forced convection: occurs when the fluid is forced to flow over a surface by external means such as a fan, blower, or the wind. v Free (or natural) convection: occurs if fluid motion is caused by buoyancy forces induced by density differences due to the variation of temperature in the fluid. This is how a baseboard heater works. Heat transfer processes involving change of phase of a fluid are considered convection processes (including boiling and condensation). ENGS205--Introductory Thermodynamics page 29 . The rate of heat transfer by convection, Q conv , is determined from Newton's Law of Cooling , which is expressed as: . Q conv = hA(T s − T f ) (3.2.5) where h is the convection heat transfer coefficient, A is the surface area through which heat transfer takes place, Ts is the surface temperature, and Tf is the bulk fluid temperature away from the surface. ‚ Radiation Heat Transfer: The energy emitted by matter in the form of electromagnetic waves (or photons) as a result of the changes in the electronic configurations of the atoms or molecules. Unlike conduction and convection, the transfer of energy by radiation requires no intervening medium. In heat transfer, we're interested in thermal radiation, or radiation emitted by bodies because of their temperature. Thermal radiation is a volumetric phenomenon, and all solids, liquids, and gases, emit, absorb, and transmit thermal radiation to varying degrees. For opaque solids, thermal radiation is a surface phenomenon and their maximum emission of radiant energy is given by the Stefan-Boltzmann law: . Q emit,max = AT 4s (3.2.6) where A is the surface area and σ = 5.67 x 10-8 W/(m2 K4 ) is the Stefan-Boltzmann constant. The idealized surface which emits radiation at this maximum rate is called a blackbody. The radiation of real surfaces is less than radiation emitted by a blackbody at the same temperature and is expressed as: . Q emit = AT 4s (3.2.7) 1 is where ε is the emissivity of the surface. The property emissivity, whose value is in the range 0 a measure of how closely a surface approximates a blackbody for which ε = 1. Another important property of a surface is called the absorptivity, α, which is the fraction of radiant energy incident on a surface which is absorbed by the surface. A blackbody absorbs all incident radiation (α=1). In general, both ε and α depend on temperature and wavelength of radiation. Kirchoff's law of radiation states that the emissivity and absorptivity of a surface are equal at the same temperature and wavelength. In most practical applications, the temperature and wavelength dependencies of surface emissivity and absorptivity is ignored and the average emissivity is taken as the average absorptivity. In general, the determination of the net rate of heat transfer by radiation is a very complicated matter since it depends on the properties of the surfaces, the orientation relative to each other, and the intervening medium. However, the following simple equation is used to get a ballpark figure as to the net radiative transfer between a system and its surroundings: . Q rad,net = A T 4s − T 4surr (3.2.8) where Ts is the surface temperature and Tsurr is the temperature of the surroundings. All temperatures used in radiation calculations must be absolute! Note! In the winter, people in a room with the curtains open may experience a faint chilly feeling. The window glass separating the people in the room and the cold surroundings outside is semi-transparent. That is, the windows can partially transmit thermal radiation. This causes people in the room to radiate to the surroundings, producing that uncomfortable feeling! (Drawing the curtains should alleviate this problem). ENGS205--Introductory Thermodynamics page 29 3.3 Work Work, like heat is an energy interaction between a system and its surroundings. More specifically, work is the energy transfer associated with a force acting through a distance( e.g. rotating shaft, rising piston, etc ...). The work done per unit mass, w, of a system is defined as: (3.3.1) . The work done per unit time is called power and is denoted by W. Very Important Sign Convention: The sign convention used by your text asserts work done by a system as being positive and work done on a system as being negative. (+) Work done by system SYSTEM (-) Work done on system Heat transfer and work are interactions between a system and its surroundings, and there are many similarities between the two: ‚ Both are recognized at the boundaries of the system as they cross them. That is, both heat transfer and work are boundary phenomena. ‚ Systems possess energy, but not heat transfer or work. That is, heat transfer and work are transient phenomena. ‚ Both are associated with a process, not a state. Unlike properties, heat transfer or work has no meaning at a state. ‚ Both are path functions (i.e. their magnitudes depend on the path followed during a process as well as the end states). Path functions have inexact differentials designated by the symbol δ . Therefore, a differential amount of heat or work is represented by δQ or δW , respectively, instead of dQ or dW. Properties (like temperature, pressure, specific volume, etc ...), however, are point functions (i.e., they depend on the state only, and not on how a system reaches that state), and they have exact differentials designated by the symbol d. The work done on or by a system between two states is represented as W 12 , not ∆ W! The same goes for heat. ENGS205--Introductory Thermodynamics page 29 Electrical Work: The flow of electrons, usually through a wire, across a system boundary forms the basis for electrical work. When N coulombs of electrons move through a potential difference V, the electrical work done is: W e = VN (3.3.2) which can also be expressed in the rate form as: . W e = VI (3.3.3) . where W e is the electrical power and I is the electrical current (usually measured in amperes, A). . We = VI I 2 =I R R 2 = V /R V In general, both V and I vary with time, and the electrical work done during a time interval ∆t is: 2 W e = VIdt ... and for constant V and I during ∆t ... W e = VI t (3.3.4) 1 3.4 Mechanical Forms of Work There are several ways of doing work, each in some way related to a force F acting through a distance s ... 2 W = Fds ... or, for a constant force F ... W = Fs (3.4.1) 1 There are two requirements for a work interaction between a system and its surroundings to exist: ‚ There must be a force acting on the boundary. ‚ The boundary must move. Some important forms of mechanical work are listed below ... ENGS205--Introductory Thermodynamics page 29 Moving Boundary Work: One form of work frequently encountered in practice is associated with the expansion or compression of a gas in a piston-cylinder device. During this process, part of the boundary (the inner face of the piston) moves back and forth (as in an automobile engine). Therefore, the expansion and compression work is often called the moving boundary work , or boundary work. F ds P The boundary work between two states is the area under the P-V diagram, or ... 2 W b = PdV (3.4.2) 1 ... or, for an isobaric process in a piston-cylinder device ... Wb = P V (3.4.3) Polytropic Process: During expansion and compression processes of real gases, pressure and volume are often related by PVn = C, where n and C are constants. A process of this kind is a polytropic process. From Eq. (3.4.2), the boundary work is ... (3.4.4) ... and for ideal gases (PV=mRT), this equation can also be written as: (3.4.5) Question to think about! In the boundary work expressions given above (Eqs. (3.4.4) and (3.4.5)), what happens to the boundary work when the constant n is equal to 1? ENGS205--Introductory Thermodynamics page 29 Gravitational Work: Gravitational work can be defined as the work done by or against a gravitational force field. The work required to raise a body from level z1 to level z2 is: (3.4.6) This expression is the change in potential energy! Gravitational work is positive if it is done by the system (as the system falls) and negative if done on the system (as the system is raised). Accelerational Work: The work associated with the change in velocity of a system is called accelerational work. By combining Newton's second law (F=ma) and Eq. (3.4.1), the following accelerational work expression is obtained: (3.4.7) This expression is recognized as being the change in kinetic energy of mass m! As always, this form of work is positive if done by the system and negative if done on the system. Shaft Work: Energy transmission via a rotating shaft is known as shaft work. The shaft work generated by applying a constant torque τ for n revolutions of the shaft is given by: W sh = 2 n (3.4.8) ... and the power transmitted through the shaft is: . . W sh = 2 n (3.4.9) . where n is the number of revolution per unit time. Spring Work: The work performed when displacing a spring from its equilibrium position is known as spring work. The spring work is obtained by combining Hooke's Law (F=kx) and Eq. (3.4.1) giving the following expression for a linear (k is constant) elastic spring ... W spring = 12 k x 22 − x 21 (3.4.10) where x1 and x2 are initial and final displacements of the spring measured relative to its equilibrium position. ENGS205--Introductory Thermodynamics page 29 3.5 The First Law of Thermodynamics The first law of thermodynamics , a simple re-statement of the conservation of energy principle for a closed system or control mass, may be expressed as follows: Q − = W Net energy transfer to (or from) the system as heat and work E where: Q = net heat transfer across system boundaries Q = W = net work done in all forms (W = (3.5.1) Net increase (or decrease in the total energy of the system Q in − Q out W out − W in ) ∆E = net change in total energy of system ( E = E 2 − E 1 ) As discussed previously, the total energy of a system is considered to be comprised of three parts: internal energy U, kinetic energy KE, and potential energy PE. The change in total energy ∆E is: E = U + KE + PE (3.5.2) U = m(u 2 − u 1 ) where: For stationary closed systems , the changes in kinetic and potential energies are negligible, and the first-law relation reduces to: Q−W= U (3.5.3) Other Forms of the First-Law Relation: ... on a unit-mass basis: q−w = e (3.5.4) ... on a time rate basis: ... in differential forms: (3.5.5) Q − W = dE (3.5.6) q − w = de (3.5.7) For a cyclic process, the initial and final states are identical (∆E=0). Therefore, Q−W=0 Q=W ENGS205--Introductory Thermodynamics (3.5.8) page 29 3.7 Specific Heats The specific heat is defined as the energy required to raise the temperature of a unit mass of a substance by one degree. In general, this energy depends on how the process is executed. In thermodynamics, we are interested in two kinds of specific heats: ‚ specific heat at constant volume Cv : the energy required to raise the temperature of a unit mass of a substance by one degree as the volume is maintained constant. 3.13 kJ V = constant m = 1 kg ∆T = 1 K C = 3.13 kJ/(kg K) v ‚ specific heat at constant pressure Cp : the energy required to raise the temperature of a unit mass of a substance by one degree as the pressure is maintained constant. P = constant m = 1 kg ∆T = 1 K Cp = 5.2 kJ/(kg k) 5.2 kJ Important Point! The value of Cp will always be greater than Cv for a pure substance because the constant pressure system is allowed to expand. Extra energy must be supplied to the system to account for the expansion (boundary) work. Consider the differential form of the first-law (on a per-mass basis) ... q − w other − w b = de ENGS205--Introductory Thermodynamics (3.7.1) page 29 For a constant volume process (δwb =0) with no work interactions, the following must be true ... q = C v dT = du ... or ... C v = u T v (3.7.2) For a constant pressure process (δwb =Pdv) with no work interactions, we have ... q = C p dT = du + Pdv = dh C p = ... or ... h T p (3.7.3) Note that Cv is the change in specific internal energy u per unit change in temperature at constant volume and Cp is the change in specific enthalpy h per unit change in temperature at constant pressure. Generally, specific heats vary with temperature. That is to say, the amount of energy it takes to raise the temperature of a unit mass of a pure substance by degree (for constant volume & pressure processes) varies at different body temperatures. However, this difference is usually not very large. Specific heat are sometimes given on a molar basis, and are denoted by C v and C p . Specific heats are properties of pure substances and their values can be found in engineering tables. 3.8 Internal Energy, Enthalpy, and Specific Heats of Ideal Gases It has been demonstrated experimentally (and proven theoretically) that the internal energy and enthalpy of an ideal gas is a function of its temperature only. Thus for ideal gases, the partial derivatives in Eqs. (3.7.2) and (3.7.3) can be replaced with ordinary derivatives and we get: 2 du = C v (T )dT u = u 2 − u 1 = C v (T )dT (3.7.4) 1 2 dh = C p (T )dT h = h 2 − h 1 = C p (T )dT (3.7.5) 1 To carry out these integrations, we need to have relations for Cv and Cp as a function of temperature. At low pressures, all real gases approach ideal-gas behavior, and therefore their specific heats depend on temperature only. The specific heats of real gases at low pressures are called ideal-gas specific heats , or zero-pressure specific heats , and are often denoted Cv0 and Cp0 . These zero-pressure specific heat data are available in the Appendix of your text as a function of temperature and can be used for real gases as long as they don't deviate from ideal-gas behavior significantly (check the compressibility factor! ). Another way of evaluating the change in specific internal energy/enthalpy is to refer to tabulated data. Tables A17-A20 in the Appendix contain molar internal energy/enthalpy data for air, O2 , N2 , CO2 , H2 , and H2O. And yet another way involves finding an average zero-pressure specific heat value (look at Table A-2b in the Appendix) for an average temperature of (T1 + T2)/2. Specific internal energies/enthalpies are then computed using the following formulas: u 2 − u 1 = C v0,av (T 2 − T 1 ) (3.7.6) h 2 − h 1 = C p0,av (T 2 − T 1 ) (3.7.7) ENGS205--Introductory Thermodynamics page 29 Specific-Heat Relations of Ideal Gases: For ideal gases, the following relationships hold: C p = C v + R ... and ... C p = C v + R u (3.7.8) where R and Ru are the gas constant and universal gas constant, respectively. The specific heat ratio k is defined as ... (3.7.9) 3.9 Internal Energy, Enthalpy, and Specific Heats of Solids and Liquids A substance whose specific volume (or density) is constant is called an incompressible substance. The specific volumes of solids and liquids essentially remain constant during a process and are usually regarded as being incompressible (e.g. hydraulics). It can be mathematically shown that the constant-volume and constant-pressure specific heats for incompressible substances are identical. Therefore, Cp = Cv = C (3.9.1) and the change in specific internal energy u of an incompressible substance is: 2 du = C(T )dT u = u 2 − u 1 = C(T )dT C av (T 2 − T 1 ) (3.9.2) 1 The enthalpy change of incompressible substances (solids or liquids) during a process can be determined from the definition of enthalpy (h=u+Pv) to be: h 2 − h 1 = (u 2 − u 1 ) + v(P 2 − P 1 ) u2 − u1 (3.9.3) The last term of Eq. (3.9.3) is usually very small and can be neglected. ENGS205--Introductory Thermodynamics page 29 ENGS205--Introductory Thermodynamics page 29