9.2 Electromagnetic Waves in Vacuum
advertisement

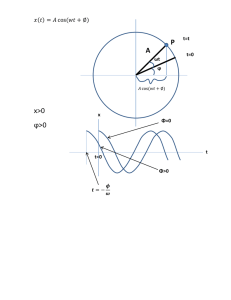
9.2 Electromagnetic Waves in Vacuum 9.2.1 The Wave Equation for E and B Source Free Wave Equations: In vacuum, Maxwell’s equations reduce to: In Vacuum: r r r r r r ∂B ∂E , ∇ ⋅ B = 0 , ∇ × B = µ0ε 0 ∇⋅ E = 0, ∇× E = − ∂t ∂t r r r r r r ∂ ∂2E 1 ∂2E 2 ∇ × ∇ × E = − ∇ × B = − µ0ε 0 2 Æ − ∇ E + ∇ ∇ ⋅ E = − 2 2 ∂t ∂t c ∂t r r r ∂ ∂2B ∇ × ∇ × B = µ0ε 0 ∇ × E = − µ0ε 0 2 ∂t ∂t ( ) ( ) ( ) ( ( ) ) ⎛ 2 1 ∂2 ⎞ r ⎛ 2 1 ∂2 ⎞ r Æ ⎜⎜ ∇ − 2 2 ⎟⎟ E = 0 and ⎜⎜ ∇ − 2 2 ⎟⎟ B = 0 (homogeneous vector wave c ∂t ⎠ c ∂t ⎠ ⎝ ⎝ equations) In linear, isotropic, and homogeneous nonconducting media, Maxwell’s equations reduce to: In Linear Media: r r r r r r r ∂B ∂H ∂E = −µ ∇⋅ E = 0, ∇× E = − , ∇× H = ε , ∇⋅H = 0 ∂t ∂t ∂t r r r r r r ∂ ∂2E 1 ∂2E 2 ∇ × ∇ × E = − µ ∇ × H = − µε 2 Æ − ∇ E + ∇ ∇ ⋅ E = − 2 2 ∂t ∂t v ∂t ( ) ( ) ( ) ⎛ ⎛ 1 ∂2 ⎞ r 1 ∂2 ⎞ r Æ ⎜⎜ ∇ 2 − 2 2 ⎟⎟ E = 0 and ⎜⎜ ∇ 2 − 2 2 ⎟⎟ H = 0 (homogeneous vector wave v ∂t ⎠ v ∂t ⎠ ⎝ ⎝ equations) r r E and B satisfy the three-dimensional wave equation, ∇2 f = 1 ∂2 f . v 2 ∂t 2 The Solution of The Nonhomogeneous Wave Equations: r ⎛ 2 1 ∂2 ⎞ 1 ∂2 ⎞ r ρ ⎛ ⎜⎜ ∇ − 2 2 ⎟⎟V = − , ⎜⎜ ∇ 2 − 2 2 ⎟⎟ A = − µ0 J ? c ∂t ⎠ c ∂t ⎠ ε0 ⎝ ⎝ r The solutions are V (r , t ) = 1 4πε 0 ∫ r ρ (r ' , tr ) r r µ0 r r dτ ' and A(r , t ) = 4π r − r' r r J (r ' , tr ) ∫ rr − rr' dτ ' . 9.2.2 Monochromatic Plane Waves: The Electromagnetic Spectrum: The Electromagnetic Spectrum Frequency Wavelength 23 Photon Energy Type 9 10 10 eV 1022 1021 γ rays 10-13m 20 6 10 10 eV 1019 1018 1Å 17 10 1 nm x rays 3 10 eV 16 10 Ultraviolet 15 10 1 µm 1014 1 eV 13 10 Infrared 12 10 1011 10 10 1 mm EHF (mm wave) 1 cm SHF (micro wave) 9 10 UHF (mobile phone) 8 10 1m VHF (TV, FM) 7 10 HF 106 MF (AM) 5 10 1 km LF (Navigation) 4 10 VLF (RF, Navigation, Sonar) 103 ULF 2 6 10 10 m SLF 1 10 ELF The Visible Range Frequency (Hz) Color Wavelength (nm) 1.0 X 1015 Near ultraviolet 300 14 Shortest visible blue 400 14 6.5 X 10 Blue 460 5.6 X 1014 Green 540 7.5 X 10 5.1 X 1014 Yellow 590 14 Orange 610 14 Longest visible red 760 14 Near infrared 1000 4.9 X 10 3.9 X 10 3.0 X 10 I. ORIGINAL THERMAL 2.45 GHz MICROWAVE EFFECT Frequencies ranging from 3 MHz to 30 GHz i.e. from radio-frequencies to the infrared are being used to process food. Depending on the chosen frequency and the particular design of the applicator, treatment by electromagnetic energy at different wavelengths has distinct features. For example, in microwave ovens electromagnetic waves with centimeters wavelength freely propagate and are absorbed by solid or liquid phase food products. The principle of microwave heating is that the changing electrical field interacts with the molecular dipoles and charged ions. The heat generated by the molecular rotation is due to friction of this motion. The influence of microwave energy on chemical or biochemical reactions is strictly thermal. The microwave energy quantum is given by the usual equation W = h ν. Within the frequency domain of microwaves and hyper-frequencies (300 MHz - 300 GHz), the corresponding energies are respectively 1.24 10-6 eV - 1.24 10-3 eV. These energies are much lower than the usual ionisation energies of biological compounds (13.6 eV), of covalent bond energies like OH (5 eV), hydrogen bonds (2 eV), Van der Waals intermolecular interactions (lower than 2 eV) and even lower than the energy associated to Brownian motion at 370C (2.7 X 10-3eV). From this scientific point of view, direct molecular activation of microwaves should be excluded. Some kind of step by step accumulation of the energy, giving rise to a high-activated state should be totally excluded due to fast relaxation. Like Peterson wrote in many of his articles: "The question and the debate of the non thermal effect of microwave give a lot of damage for the reputation of this technology and its application in industry". Microwaves are only absorbed by dipoles, transforming their energy into heat. Ref: http://www.mdpi.net/ecsoc-5/e0017/e0017.htm ~ ~ ~ ~ E( z , t ) = E0ei (kz −ωt ) , B( z , t ) = B 0ei (kz −ωt ) ( ) ( ) ( ) ~ ~ ~ ~ E0 = E0 x xˆ + E0 y yˆ + E0 z zˆ r 1. ∇ ⋅ E = 0 ∂ ∂ ∂ Ex + E y + Ez = 0 ∂x ∂y ∂z include direction, amplitude, phase; vector phasor [( ) ] [( ) ] ]= 0 ~ Æ E0 z = 0 [( ) ] ∂ ~ ∂ ~ ∂ ~ E0 x ei (kz −ωt ) + E0 y ei (kz −ωt ) + E0 z ei (kz −ωt ) = 0 ∂x ∂y ∂z (E~ ) ∂∂z [e ( i kz − ωt ) 0 z ( ) ( ) ~ The same as B 0 z =0 r r ∂B 2. ∇ × E = − ∂t xˆ yˆ zˆ ∂ ∂ ∂ ~ ~ = − xˆik E0 y ei (kz −ωt ) + yˆ ik E0 x ei (kz −ωt ) ∂x ∂y ∂z ~ ~ i ( kz − ωt ) i ( kz −ωt ) E0 x e E0 y e 0 ~ ~ = iω xˆ B 0 x ei (kz −ωt ) + yˆ B 0 y ei (kz −ωt ) ( ) ( ) ( ) [( ) ( ) ( ) ~ k E0 y ( ) ~ = −ω B 0 ( ) Æ x ] ( ) ~ and k E 0 ( ) ( ) x ( ) ~ = ω B0 y ( ) ( ) (( ) ( ) ) k ~ k ~ k ~ ~ ~ ~ ~ B 0 = B 0 x xˆ + B 0 y yˆ = − E0 y xˆ + E0 x yˆ = zˆ × E0 x xˆ + E0 y yˆ = k ω ω ω ω ~ zˆ × E0 r r E and B are in phase and mutually perpendicular. r 1 r B0 = E0 c r r If E points in the x direction, then B points in the y direction: 1~ ~ ~ ~ E( z , t ) = E0ei (kz −ωt ) xˆ , B( z , t ) = E0ei (kz −ωt ) yˆ c 1 real part: E( z , t ) = E0 cos(kz − ωt + δ )xˆ , B( z , t ) = E0 cos(kz − ωt + δ ) yˆ c r let n̂ be the polarization vector and k be the propagation vector r transverse wave Æ nˆ ⋅ k = 0 1~ rr 1 ~ ~ rr ~ ~ E(r, t ) = E0ei (k ⋅r −ωt )nˆ , B(r, t ) = E0ei (k ⋅r −ωt ) kˆ × nˆ = kˆ × E c c r r r r 1 real part: E(r, t ) = E0 cos k ⋅ r − ωt + δ nˆ , B(r, t ) = E0 cos k ⋅ r − ωt + δ kˆ × nˆ c ( ) ( ) ( )( ) 9.2.3 Energy and Momentum in EM Waves 1⎛ B2 ⎞ B2 E ⎟⎟ ( ε 0 E 2 = Æ B = ε 0 µ0 E = ) u = ⎜⎜ ε 0 E 2 + 2⎝ µ0 ⎠ µ0 c As the wave travels, it carries the energy u = ε 0 E 2 = ε 0 E02 cos 2 (kz − ωt + δ ) along with it. The energy flux transported by the fields is given by the Poynting vector. Poynting vector: the energy flux density (energy per unit area, per unit time) ( r 1 r r S= E×B µ0 ) Taking electromagnetic fields as E( z, t ) = E0 cos(kz − ωt + δ )xˆ and 1 B(z , t ) = E0 cos(kz − ωt + δ ) yˆ , the energy flux density is c r 1 2 S= E0 cos 2 (kx − ωt + δ )zˆ = cε 0 E02 cos 2 (kx − ωt + δ )zˆ = cuzˆ . cµ0 r r S The momentum density stored in the field is P = 2 . c For monochromatic plane waves, we have the momentum density of r u P = zˆ . c Any macroscopic measurements will encompass many cycles of the wave oscillations, so we want to know the average value. Average over a period: 1 u = ε 0 E02 cos 2 (kz − ωt + δ ) = ε 0 E02 2 S = ⎛ ε E2 ⎞ E cε 1 1 1 1 E0 B0 = E0 0 = 0 E02 = c × ⎜⎜ 0 0 ⎟⎟ c 2 µ0 2 µ0 2 ⎝ 2 ⎠ P = 1 ε 0 E02 2c ⎛ ε E2 ⎞ I ≡ S = c × ⎜⎜ 0 ⎟⎟ velocity * energy density per unit volume ⎝ 2 ⎠ When light falls on a perfect absorber, it delivers its momentum to the surface. In a r r time ∆t the momentum transfer is ∆p = PAc∆t , so the radiation pressure is P= r r 1 1 ∆p I = cP = ε 0 E02 = . 2 c A ∆t What about the light pressure on a perfect reflector? Exercise: 9.10