ln ln v v R T T Css dS + =−= T Q dS = kg kJ K kJ T Q dS S / 671.0 298
advertisement
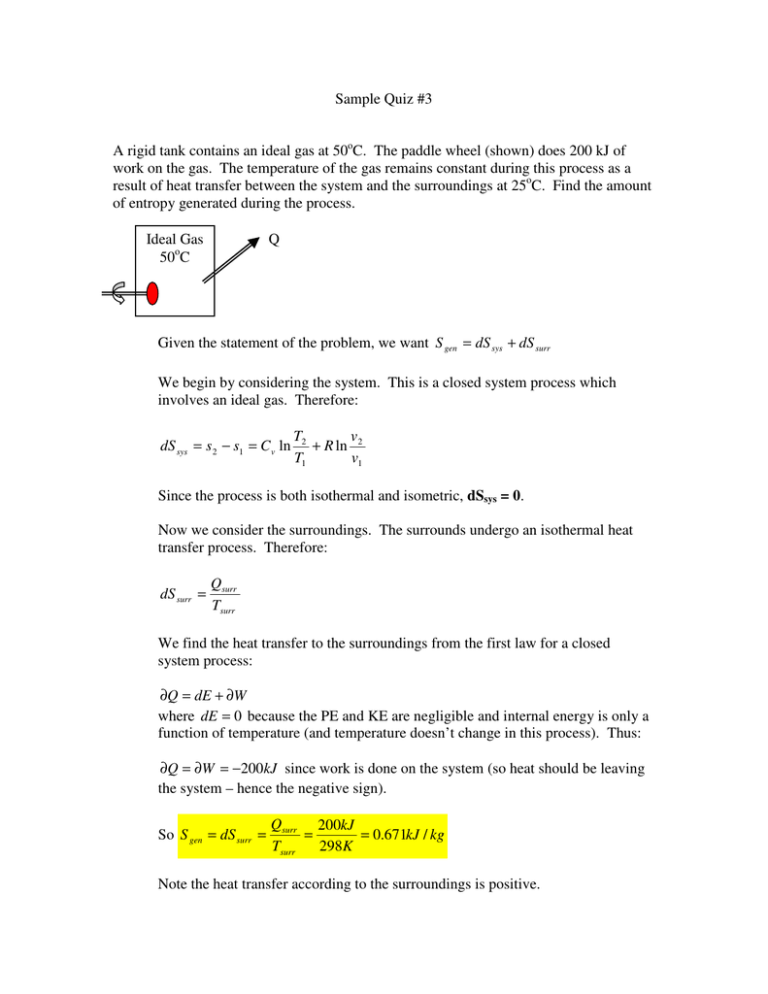
Sample Quiz #3 A rigid tank contains an ideal gas at 50oC. The paddle wheel (shown) does 200 kJ of work on the gas. The temperature of the gas remains constant during this process as a result of heat transfer between the system and the surroundings at 25oC. Find the amount of entropy generated during the process. Ideal Gas 50oC Q Given the statement of the problem, we want S gen = dS sys + dS surr We begin by considering the system. This is a closed system process which involves an ideal gas. Therefore: dS sys = s 2 − s1 = C v ln T2 v + R ln 2 T1 v1 Since the process is both isothermal and isometric, dSsys = 0. Now we consider the surroundings. The surrounds undergo an isothermal heat transfer process. Therefore: dS surr = Qsurr Tsurr We find the heat transfer to the surroundings from the first law for a closed system process: ∂Q = dE + ∂W where dE = 0 because the PE and KE are negligible and internal energy is only a function of temperature (and temperature doesn’t change in this process). Thus: ∂Q = ∂W = −200kJ since work is done on the system (so heat should be leaving the system – hence the negative sign). So S gen = dS surr = Qsurr 200kJ = = 0.671kJ / kg Tsurr 298K Note the heat transfer according to the surroundings is positive.



