Double-Helix Coil for Occlusion of Large Patent Ductus Arteriosus
advertisement

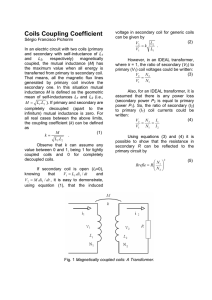
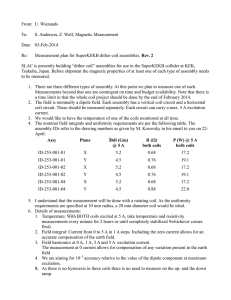
677 JACC Vol. 31, No. 3 March 1, 1998:677– 83 EXPERIMENTAL STUDIES Double-Helix Coil for Occlusion of Large Patent Ductus Arteriosus: Evaluation in a Chronic Lamb Model RALPH G. GRABITZ, MD, FRANZ FREUDENTHAL, MD, MATTHIAS SIGLER, MD, TRONG-PHI LE, MD,* CHRISTOPH BOOSFELD, PHD,† STEFAN HANDT, MD,‡ GÖTZ VON BERNUTH, MD Aachen, Bonn and Cologne, Germany Objectives. We sought to evaluate the efficacy and tissue reaction of a new miniature interventional device for occlusion of large patent ductus arteriosus (PDA) in a neonatal lamb model. Background. A variety of devices are used to close PDAs by interventional measures. Spring coils found to have a high cumulative occlusion rate have thus far been limited to smaller PDAs because of the physical limitation of grip forces. Methods. Memory-shaped double-cone stainless steel coils with enhanced stiffness of the outer rings by a double-helix configuration were mounted on a titanium/nickel core wire. A snap-in mechanism attaches the coil to the delivery wire, allowing intravascular coil retrieval and repositioning. The system was placed through a 4F or 5F Teflon catheter. A chronic lamb model (n 5 8) of PDA (>5 mm) was used in which ductus patency was secured by a protocol of repetitive angioplasty procedures. The animals were killed after 1 to 181 days, and the ductal region was examined by inspection as well as by light and electron microscopy. Results. Placement of the coils within the PDA was possible in all lambs. Before final detachment, the coils were retrieved or repositioned, or both, up to 12 times. In all but one animal the ductus was closed within 6 days after the procedure. The coils caused no infections or aortic and pulmonary artery obstruction. Histologic and electron microscopic studies revealed endothelial coverage of the implants but no foreign body reaction or local or systemic inflammation or erosion of the implant. Conclusions. The device effectively closed large PDAs in our model and may overcome the previous limitations of coils. Clinical trials are indicated. (J Am Coll Cardiol 1998;31:677– 83) ©1998 by the American College of Cardiology Several percutaneous transcatheter techniques for closing the persistently patent ductus arteriosus (PDA) in older children and adults have been described (1– 8). The Rashkind occluder is the only system thus far with widespread acceptance. However, size and a relatively large delivery system, as well as high costs and a late incidence of residual shunting, limit its application. Spring coils to occlude arteries were introduced by Gianturco et al. (9) in 1975. These coils, once delivered, are not retrievable into the delivery catheter and therefore carry the risk of improper placement or undesired embolization. Preformed Nitinol snares may be used to improve delivery (10). New developments allow the retrieval of certain coils after positioning and until final release (11,12). These devices can be used with small introduction catheters but because of their physical properties (increasing the diameter of the reconfigured coil decreases its stiffness by threefold, leading to pullthrough and embolization), they were found to be effective only in occluding small PDAs (13–15). The present report focuses on the use of selectively enhanced stiffness of the outer rings of coils to allow safe positioning of the device in medium to large PDAs. The new coil on trial relies on the strong memory effect of certain metals (American National Standards Institute [ANSI] 301/4) to form biconical, “double-disk” coils in which rings inside the PDA cause its mechanical and thrombotic closure (12,16). To evaluate practicability, efficacy and medium-term biocompatibility of the new device in occluding PDAs, we used a new chronic lamb model with high shunting and a minimal inner ductal diameter .5 mm. From the Department of Pediatric Cardiology and Interdisciplinary Center of Clinical Research on Biomaterials, Aachen University of Technology, Aachen; *Division of Cardiology, Children’s Hospital, University of Bonn, Bonn; †Produkte für die Medizin GmbH, Cologne; and ‡Department of Pathology, Aachen University of Technology, Aachen, Germany. This study was supported in part by Grant 01 KS 9503/9 from the German Ministry of Research and Technology, Bonn, Germany. Some coils and catheters were generously donated by Produkte für die Medizin (pfm) GmbH, Cologne, Germany. Dr. Freudenthal owns patents on aspects of the device described in this report and may receive royalties if and when it is marketed. Dr. Boosfeld is a consult to pfm GmbH. Manuscript received February 17, 1997; revised manuscript received August 25, 1997, accepted November 21, 1997. Address for correspondence: Dr. Ralph G. Grabitz, Department of Pediatric Cardiology, Aachen University of Technology, Pauwelsstrasse 30, 52074 Aachen, Germany. E-mail: grabitz@alpha.imib.rwth-aachen.de. ©1998 by the American College of Cardiology Published by Elsevier Science Inc. Downloaded From: https://content.onlinejacc.org/ on 09/29/2016 Methods Occlusion device and delivery system. The PDA occlusion device consists of 1) A double-cone memory-shaped spring coil with enhanced stiffness limited to the larger distal loops by a selective double-helix configuration of the primary wire strand 0735-1097/98/$19.00 PII S0735-1097(97)00025-4 678 GRABITZ ET AL. DOUBLE-HELIX COIL FOR CLOSURE OF LARGE PDA Abbreviations and Acronyms ANSI 5 American National Standards Institute PDA 5 patent ductus arteriosus (stainless steel [DIN 1.4310, 'ANSI 301/4] wire strand 0.20 mm in diameter, 0.80-mm primary coil, 6- to 12-mm reconfigured secondary diameter, minimal inner diameter ,1.5 mm, overall number of loops 8 to 13 [pfm GmbH, Cologne, Germany]) (Fig. 1); 2) a pusher system: a) a pusher wire (a coiled stainless steel wire, 90 cm long, with a modified base to fit into hold-back mechanism of the safety handle) that is advanced over the b) core wire (nickel/titanium alloy [Nitinol], 110 cm long, 0.35 mm in diameter, a modified tip to control the coils and a modified base to fit into the hold-back Figure 1. Top, Macroscopic appearance of two coils, nearly identical in appearance, with primary strand diameter 0.2 mm, primary coil diameter 0.8 mm, reconfigured coil diameter 10 mm (in contrast to the double-cone shaped configuration of coils used in the study); the coil on the right is constructed as a double-helix coil. Bottom, Effect on stiffness is demonstrated when the coils are each pulled at 0.11 N. Downloaded From: https://content.onlinejacc.org/ on 09/29/2016 JACC Vol. 31, No. 3 March 1, 1998:677– 83 mechanism of the safety handle); 3) a safety handle (stainless steel, engraved metric scale, two safety mechanisms and one distal ring on its shaft [OccluGrip, pfm, Cologne, Germany]); and 4) a Teflon introduction catheter (60-cm long, 4F or 5F in diameter [1.0/1.3 mm], including tip marker [inner gold ring]). The system itself is described in detail elsewhere (12,17). Briefly, the coil is stretched and mounted on the distal end of the core wire. The proximal end of the core wire is secured into the safety grip, into which the pusher wire is screwed as well, allowing the latter to be moved independently of the core wire or vice versa. The whole system is introduced through the delivery catheter like a conventional guide wire. Advancing the pusher wire or withdrawing the core wire will deploy the ductal coil from the tip of the core wire, allowing it to regain its original shape. As long as at least 0.5 cm of coil is left on the core wire, the whole system can be withdrawn into the delivery catheter. Final release is accomplished by a positive felt action of pushing the coil from the core wire. Because the purpose of the stiffer outer loops is to avoid pull-through from the aortic side into the pulmonary artery, the loops have to be released first, characterizing the system as a transvenous device by design. Animal studies. The animal experiments were conducted according to the guidelines of the German Animal Protection Law and were approved by the state agency supervising animal experimentation. Catheterization of the animals was performed as a sterile procedure under anesthesia with halothane (0.3% to 0.6%) and N2O and using portable, digital, monoplane fluoroscopy (Philips, The Netherlands). A color Doppler echocardiograph (Kontron, France) was used to acquire echocardiographic data. No animal received antibiotics or antithrombotic agents, except for a heparinized flush solution (2 U/ml of normal saline solution). Before and after the investigations the lambs were cared for by the ewe. Lamb model of persistent PDA. Eight neonatal lambs (Morino Mixed) 3 to 30 h old and weighing 3.2 to 6.4 kg (median 4.7) were anesthetized and artificially ventilated. By means of a 6F vascular sheath in the left-sided jugular vein, a pediatric valvuloplasty catheter (balloon diameter 6 to 8 mm, length 20 mm; Dr. Osypka, GmbH, Grenzach, Germany) was passed over a guide wire through the right heart across the ductus arteriosus. The ductus was dilated up to 6 to 8 mm over 10 min. On postpartum day 5 or 6, the procedure was repeated, and an angiogram 10 min after dilation demonstrated ductal patency and dimensions. If control angiography revealed circumscript narrowing on the aortic or pulmonary orifice or the minimal inner diameter of the ductus was ,5 mm, the balloon angioplasty was repeated, as previously described (Fig. 2). Coil delivery. Four to 53 days (mean 20) after the last dilation the lambs were again anesthetized and artificially ventilated. A sheath was placed into the femoral artery and jugular vein. The location and size of the PDA were again demonstrated by aortogram. By the venous route and the right heart, a 4F or 5F end-hole delivery catheter (nylon or Teflon) was placed through the PDA into the descending aorta. Two rings of the occluding coil were freed from the core wire and JACC Vol. 31, No. 3 March 1, 1998:677– 83 GRABITZ ET AL. DOUBLE-HELIX COIL FOR CLOSURE OF LARGE PDA 679 Figure 2. Angiographic appearance of the PDAs used for double-helix coil closure at day of implantation (lateral or left lateral oblique projection; anterior is left, cranial is top; in some instances, a scaling catheter in the aorta is visible, where the smaller distance between marks counts for 10 mm and the larger for 20 mm). extruded into the aorta, regaining their original shape and forming the distal, outer disc. The whole system was then withdrawn into the aortic ampulla of the duct, where additional loops were released. Withdrawing the system further across the PDA into the pulmonary artery, the final loops forming the proximal disk were released and, once a satisfactory position was achieved, the occlusion coil was freed from the core wire. Alternatively, the first two distal loops were primarily configured and placed into the midportion of the ductus; because of their enhanced stiffness, they were not dislocated by the shunt flow across the ductus. The softer middle and proximal loops were then deployed into this ring to occlude the ductus mechanically and through thrombus forma- Downloaded From: https://content.onlinejacc.org/ on 09/29/2016 tion (Fig. 3). An additional aortogram and angiogram of the main pulmonary artery documented positioning of the coils 30 to 60 min after final placement of the device. The catheters, wires and sheath were removed, and anesthesia was discontinued. The animal was returned to the pen to be cared for by the ewe. The lambs were investigated clinically on days 1, 2 and 4 after ductal closure. Additionally, color Doppler echocardiography (transthoracal or transesophageal, or both) and clinical investigation were performed under sedation or anesthesia on day 6 to evaluate the occlusion of the duct and flow disturbances in the pulmonary artery and descending aorta. One to 181 days (mean 64) after coil implantation, a second left and 680 GRABITZ ET AL. DOUBLE-HELIX COIL FOR CLOSURE OF LARGE PDA JACC Vol. 31, No. 3 March 1, 1998:677– 83 Figure 3. Implantation of double-helix coil (lateral projection; anterior left, cranial top) in lamb 5. Top left, Native aortogram showing the PDA. Top center and right, Distal loops of coil with enhanced stiffness are placed inside the lumen of the ductus, and softer loops are added to the center. Bottom left, Conglomerate of softer loops is held by the stiff outer loop in place, obstructing the ductal lumen. Bottom center and right, Coils are released and stay in place. Aortogram demonstrates adequate placement and closure (on motion pictures, a small residual shunt remained on the final angiogram on the day of implantation). right heart catheterization through the jugular vein and femoral artery, including angiography, was performed under general anesthesia before the animals were killed and the ductal block removed. For gross and microscopic examination, the specimens were fixed in formalin, alcohol or glutaraldehyde. The aortic and pulmonary portions were scanned by raster electron microscopy, and the middle portion was prepared for light microscopy. Some portions were embedded in methyl methacrylate and sectioned crosswise at 1-mm intervals; the rest was submitted for routine paraffin embedding after careful coil removal to avoid damage to this section. Results Lamb model of the PDA. In all animals, dilation of the PDA was possible and produced persistent ductal patency. Angioplasty had to be repeated a third time in one animal and a fourth time in another. Four to 53 days (mean 19) after the last dilation at the time of coil implantation, the PDA had a tubular appearance, and the minimal inner diameter ranged Downloaded From: https://content.onlinejacc.org/ on 09/29/2016 between 5.2 and 7.8 mm (mean 6.3) and the overall length between 6.5 and 15 mm (mean 10). Coil placement. Placement of coils in the PDA was possible in all lambs. To achieve complete obstruction of the PDA in the first animal, two coils had to be implanted at the same time in a crossover technique from the aorta and the pulmonary artery. This procedure was necessary to provide enough loops to obstruct the ductal flow entirely. The whole system had to be pulled back into the delivery catheter between 1 and 12 times for repositioning or exchange. In one case the distal ring was loosened by mistake, and the coil was unintentionally freed, leading to its embolization into the left pulmonary artery. Retrieval by a snare into an 8F catheter was possible without difficulty. Ductal occlusion and follow-up. The final aortogram 30 to 60 min after implantation revealed a complete occlusion in two cases. Clinical follow-up and color Doppler echocardiography on day 6 after implantation confirmed a complete occlusion of the ductus in four additional lambs, and no turbulent flow in the aorta or pulmonary artery was seen. One animal had to be Downloaded From: https://content.onlinejacc.org/ on 09/29/2016 10.7 15.0 6.5 6.3 7.8 5.0 28.1 66.0 9.0 10.4 21.0 6.5 19.0 53.0 4.0 4.7 6.4 3.2 Mean Max Min 8 7 8 7 8 10 10 10 10 10 2 2 3 2 2 16 23 9 9 17 10.2 6.6 6.5 7.4 7 9 18 4 5 11 5.3 4.5 5 4.9 3.2 4/M 5/M 6/F 7/F 8/F Diam 5 diameter; Explant 5 explantation; F 5 female; ID 5 inner diameter; Implant 5 implantation; M 5 male; Max 5 maximum; Min 5 minimum (minimal); PDA 5 patent ductus arteriosus; Wt 5 weight. 22.3 46.0 6.4 64.4 181.0 1.0 98 13 7 62 52 Tubular Tubular Tubular Tubular Tubular 9 13 9 10 6.5 7.8 6 5.5 5 5.5 13 10 8 9 8 11 3 9 937 736 736 736 235 165 110 120 120 15 14 6.2 6.5 13 12 11.8 12.6 66 27 4 2 10 10 10 8 21 58 2 10 7 29 23 3.7 6.4 2/F 3/M #606523 #606900 1pre 9510/7001 #606528 #606514 #606507 #606502 #606503 11 3 9 12 3 12 210 240 28.5 8.4 6.4 21.4 18 Minute shunt 2nd coil for definitive closure Closed Closed Closed Closed Closed 11.8 46 1 181 37.8 101 Slight stenosis of aortic orifice Tubular Tubular, very long 9 7.5 160 8 12 3 10 #605910 4.5 1/M 53 Implant ID Overall Coil Overall No. Length if Coil Size of Straightened Min ID Length (mm) Loops (mm) (mm) (mm) 681 Duration Birth PDA Diam at of Last Age at Wt at Days After Wt Last Dilation Dilation No. of Implant Implant Last Dilation Lamb (kg) (mm) (min) Dilations (days) (kg) at Implant Transcatheter occlusion of the PDA is now a well established method of treatment that started with the pioneering work of Porstmann et al. (18) and was continued with the Rashkind PDA occluder (7,8), probably the most extensively investigated device. High costs, size limitations, a large transvascular sheath, a late incidence of residual shunting and risks of left pulmonary artery stenosis have led to a search for alternatives in experimental models (19 –21) and clinical trials (11,17,22,23). High cumulative occlusion rates have been accomplished with various coils in different protocols in PDAs of small to moderate size; however, attempts to occlude large PDAs with coils have either not succeeded (13,15,24 –27) or have led to increased side effects (28). Table 1. Summary of Coil Implantation in Lambs Discussion PDA killed prematurely 1 day after coil placement because of recurrent arterial bleeding at the femoral puncture site. Premortem angiography demonstrated a small residual shunt across the PDA. In one animal a hemodynamically insignificant shunt persisted clinically and on color Doppler echocardiography, and a standard coil was added 3 months after primary implantation, leading to subsequent complete ductal closure. Seven to 181 days after primary implantation the final angiograms confirmed persistent ductal occlusion in all seven remaining animals. Postmortem macroscopic examination of the ductal orifices showed little if any protrusion of the outer rings of the coils into the lumen of the aorta or the pulmonary artery. Depending on the duration of implantation, the coils were partially or completely covered by thin, shiny tissue (Fig. 4). Further details are summarized in Table 1. Scanning electron microscopic and histologic studies. At higher magnification, most of the coils were covered by a monolayer of cells resembling endothelial cells, starting as early as 13 days after implantation (Fig. 5). When different intervals after implantation were compared, endothelial coverage was more pronounced at longer intervals of implantation. A foreign body reaction was not noticeable at any time (13, 62, 80, 98 and 181 days after implantation). Segmental areas of the media showed the destruction of elastic fibers and the replacement by fibrous tissue with focal calcification that was not related to the implant (Fig. 6). General Description Follow-Up After Wt at Implant Explant (days) (kg) Figure 4. Aortic ductal orifice 101 days after coil implantation in lamb 1, demonstrating adequate ingrowth. Closed GRABITZ ET AL. DOUBLE-HELIX COIL FOR CLOSURE OF LARGE PDA Comment JACC Vol. 31, No. 3 March 1, 1998:677– 83 682 GRABITZ ET AL. DOUBLE-HELIX COIL FOR CLOSURE OF LARGE PDA JACC Vol. 31, No. 3 March 1, 1998:677– 83 Figure 5. Scanning electron micrograph in lamb 5, 13 days after coil implantation. The surface is covered partially with a fibrin net on which single endothelial cells are growing. Scale 5 0.1 mm; ;36,000, reduced by 35%. Technical aspects. Larger and stiffer coils are needed for use in larger PDAs to withstand high flow and avoid embolization. For a given material and diameter of the primary strand, increasing the diameter of a reconfigured coil will result in a threefold decrease in its stiffness. Enlarging the diameter of the primary strand would cause a fourfold increase in the stiffness of the coil but make it very difficult to control the small-diameter loops in the center of the double-cone coil. Shaping the primary strands of the outer loops in a doublehelix configuration leads to a fourfold increase in the stiffness of this selected portion of the coil because the double-helix portion will act as two coil springs at the same place. Chronic lamb model of PDA. Dilating the ductus after a protocol of angioplasty procedures provided a chronic model of a large tubular PDA without operation (19) or implantation of stents (29), thus avoiding unwanted interactions with the device. Despite the PDA large size, we encountered no acute problems with overcirculation of the lungs, most likely due to the immediate onset of a right to left shunt on the day of birth, which allowed gradual adaptation parallel to the physiologic decrease in pulmonary resistance. Our dilated ducts are similar in shape to the long PDAs found in humans and are thought to be difficult to close because of high flow, a large minimal inner diameter and lack of tapering. Their long-term patency is at least comparable (if not superior) to other animal models of PDA (30,31). Functional results. Appropriate placement of coils was possible in all lambs. In one animal, a coil embolized because of entrance into the pulmonary artery by mistake, but it was successfully retrieved by a snare. The overall success rate for cumulative PDA closure with appropriate follow-up rose to 100% after implantation of an additional standard coil in one animal. However, because of the small number of animals tested, the 95% confidence interval for failure still extends to 43% (32). Histopathologic examination. No clinical, bacteriologic or histologic signs of inflammation were found in any of the lambs. Raster electron microscopy revealed endothelial cover- Figure 6. Central ductal portion in lamb 4, 98 days after coil implantation. Fixation with methyl methacrylate, toluidin blue stain. Black areas show coil implantation. No histiocytic reaction is visible. Arrows indicate area with calcification beneath the implant. 350, reduced by 35%. Downloaded From: https://content.onlinejacc.org/ on 09/29/2016 JACC Vol. 31, No. 3 March 1, 1998:677– 83 GRABITZ ET AL. DOUBLE-HELIX COIL FOR CLOSURE OF LARGE PDA age of the device that was visible as early as 13 days after implantation. In contrast to a previous study in piglets utilizing the same alloy (12), we found no foreign body reaction to the implant. This finding could be explained either by species differences or by thermal modeling of the new device in the present study, which was done without oxygen to avoid scaling on the surface of the coils. Clinical implications and conclusions. Coils can be deployed through 4F or 5F catheters, reducing potential hazard to vessels and permitting application in very small infants. Attempts to minimize the risk of inadvertent embolization have recently led to the introduction of retrievable coils (12,17,24). Reduced costs and a comparatively easy procedure, together with a high cumulative PDA closure rate, have led to widespread clinical trials limited to small PDAs. This limitation is almost solely due to physical properties because larger coils lack appropriate stiffness. Our new coil device offers increased stiffness of the outer rings without loss of any of the advantages attributed to retrievable coil systems. Like other coils it is made of a ferruginous alloy, leading at least theoretically to limited nuclear magnetic resonance imaging compatibility. Our device may be difficult to place in very short ducts because despite being safely anchored on the aortic side through the enforced loops, the softer parts may pull through to the pulmonary side and not leave enough material to obstruct the narrowest portion. The cost of the present device will most likely range between the recently commercially available detachable coils and the Rashkind double umbrella. The device can be manufactured in different shapes and sizes and has the potential to overcome the previous limitations of coils for use in the occlusion of large PDAs. Clinical trials of the device are warranted. We thank the Departments of Electron Microscopy and Experimental Animal Research, Medical Faculty, Aachen University of Technology, for their technical support and expertise. References 1. Porstmann W, Wierny L, Warnke H. Closure of ductus arteriosus persistens without thoracotomy. Radiol Diagn Berl 1968;9:168 –9. 2. Rao PS, Wilson AD, Sideris EB, Chopra PS. Transcatheter closure of patent ductus arteriosus with buttoned device: first successful clinical application in a child. Am Heart J 1991;121:1799 – 802. 3. Warnecke I, Frank J, Hohle R, Lemm W, Bucherl ES. Transvenous double-balloon occlusion of the persistent ductus arteriosus: an experimental study. Pediatr Cardiol 1984;5:79 – 83. 4. Magal C, Wright KC, Duprat GJ, Wallace S, Gianturco C. A new device for transcatheter closure of the patent ductus arteriosus: a feasibility study in dogs. Invest Radiol 1989;24:272– 6. 5. Bridges ND, Perry SB, Parness I, Keane JF, Lock JE. Transcatheter closure of a large patent ductus arteriosus with the Clamshell septal umbrella. J Am Coll Cardiol 1991;18:1297–302. 6. Nazarian GK, Qian Z, Vlodaver Z, et al. Evaluation of a new vascular occlusion device. Invest Radiol 1993;28:1165–9. 7. Rashkind WJ, Cuaso CC. Transcatheter closure of patent ductus arteriosus: successful use in a 3.5 kilogram infant. Pediatr Cardiol 1979;1:3–7. 8. Rashkind WJ, Mullins CE, Hellenbrand WE, Tait MA. Nonsurgical closure of patent ductus arteriosus: clinical application of the Rashkind PDA Occluder System. Circulation 1987;75:583–92. Downloaded From: https://content.onlinejacc.org/ on 09/29/2016 683 9. Gianturco C, Anderson JH, Wallace A. Mechanical devices for arterial occlusion. Am J Roentgenol 1975;124:428 –35. 10. Sommer RJ, Gutierrez A, Lai WW, Parness IA. Use of preformed nitinol snare to improve transcatheter coil delivery in occlusion of patent ductus arteriosus. Am J Cardiol 1994;74:836 –9. 11. Reidy JF, Qureshi SA. Interlocking detachable platinum coils, a controlled embolization device: early clinical experience. Cardiovasc Intervent Radiol 1996;19:85–90. 12. Grabitz RG, Neuss M, Coe JY, Handt S, Redel D, von Bernuth G. A small interventional device to occlude persistently patent ductus arteriosus in neonates: evaluation in piglets. J Am Coll Cardiol 1996;28:1024 –30. 13. Lloyd TR, Fedderly R, Mendelsohn AM, Sandhu SK, Beekman RH. Transcatheter occlusion of patent ductus arteriosus with Gianturco coils. Circulation 1993;88:1412–20. 14. Cambier PA, Kirby WC, Wortham DC, Moore JW. Percutaneous closure of the small (less than 2.5 mm) patent ductus arteriosus using coil embolization. Am J Cardiol 1992;69:815– 6. 15. Moore JW, George L, Kirkpatrick SE, et al. Percutaneous closure of the small patent ductus arteriosus using occluding spring coils. J Am Coll Cardiol 1994;23:759 – 65. 16. Tometzki AJ, Houston AB, Redington AN, Rigby ML, Redel DA, Wilson N. Closure of Blalock-Taussig shunts using a new detachable coil device. Br Heart J 1995;73:383– 4. 17. Tometzki A, Chan K, de-Giovanni J, et al. Total UK multi-centre experience with a novel arterial occlusion device (Duct Occlud pfm). Heart 1996;76: 520 – 4. 18. Porstmann W, Wierny L, Warnke H, Gerstberger G, Romaniuk PA. Catheter closure of patent ductus arteriosus: 62 cases treated without thoracotomy. Radiol Clin North Am 1971;9:203–18. 19. Sharafuddin MJA, Gu X, Titus JL, et al. Experimental evaluation of a new self-expanding patent ductus arteriosus occluder in a canine model. J Vasc Interv Radiol 1996;7:877– 87. 20. Liu C, Shiraishi H, Kikuchi Y, Yanagisawa M. Effectiveness of a thermal shape-memory patent ductus arteriosus occlusion coil. Am Heart J 1996;131: 1018 –23. 21. Grifka RG, Mullins CE, Gianturco C, et al. New Gianturco-Grifka vascular occlusion device: initial studies in a canine model. Circulation 1995;91: 1840 – 6. 22. Rosenthal E, Qureshi SA, Reidy J, Baker EJ, Tynan M. Evolving use of embolisation coils for occlusion of the arterial duct. Heart 1996;76:525–30. 23. Grifka RG, Vincent JA, Nihill MR, Ing FF, Mullins CE. Transcatheter patent ductus arteriosus closure in an infant using the Gianturco-Grifka vascular occlusion device. Am J Cardiol 1996;78:721–3. 24. Tometzki AJP, Arnold R, Peart I, et al. Transcatheter occlusion of the patent ductus arteriosus with Cook detachable coils. Heart 1996;76:531–5. 25. Uzun O, Hancock S, Parsons JM, Dickinson DF, Gibbs JL. Transcatheter occlusion of the arterial duct with Cook detachable coils: early experience. Heart 1996;76:269 –73. 26. Weber HS, Cyran SE, Gleason MM, White MG, Baylen BG. Transcatheter vascular occlusion of the small patent ductus arteriosus: an alternative method. Pediatr Cardiol 1996;17:181–3. 27. Galal O, de-Moor M, al-Fadley F, Hijazi ZM. Transcatheter closure of the patent ductus arteriosus: comparison between the Rashkind occluder device and the anterograde Gianturco coils technique. Am Heart J 1996;131:368 – 73. 28. Hijazi ZM, Geggel RL. Transcatheter closure of large patent ductus arteriosus (.4 mm) with multiple Gianturco coils: immediate and mid-term results. Heart 1996;76:536 – 40. 29. Rosenthal E, Qureshi SA, Kakadekar AP, et al. Comparison of balloon dilation and stent implantation to maintain patency of the neonatal arterial duct in lambs. Am J Cardiol 1993;71:1373– 6. 30. Weber HS, Baylen BG, Banaszak P, Abt AB. Transvascular catheter infiltration of the ductus arteriosus with formalin in the newborn lamb: a novel method of establishing and maintaining ductal patency. J Am Coll Cardiol 1996;28:241– 6. 31. Lund G, Cragg A, Rysavy J, et al. Patency of the ductus arteriosus after balloon dilatation: an experimental study. Circulation 1983;68:621–7. 32. Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 1983;249:1743–5.