Chemical reactivity and aerosol forming potential of anthropic
advertisement

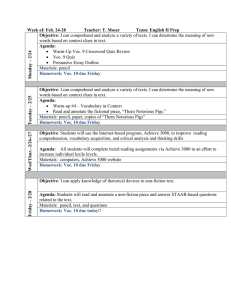
Chemical reactivity and aerosol forming potential of anthropic Volatile Organic Compounds from chemical structure to properties JF Doussin, M. Scarfogliero, L. Chiappini, B.Picquet-Varrault, E. Perraudin « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin • VOCs are a blend of hundreds of volatile organic compounds some of which are chemically reactive, • VOCs are emitted from a variety of sources, – – – – motor vehicles, chemical plants, refineries, other industries, consumer and commercial products, architectural coatings … • Anthropic VOCs emmission are changing with the increasing use of new products, alternatives fuels/solvents, new industrial processes… Because of this diversity, it is necessary to be able to draw rules to link chemical structure and atmospheric effects « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Plan • Oxidizing capacity of the atmosphere • how fast they are removed ? • how many photo-oxidant are produced ? • Secondary aerosol formation « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Atmospheric Oxidation of the VOCs NOx Ozone production ? Secondary products ? Aerosols production VOC VOC + + OH OH → → products products VOC VOC + + OO33 → → products products VOC VOC + + NO NO33 → → products products « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Nitrate Radical (NO3 ) NO3 sources : NO2 + O3 → NO3 + O2 NO3 + NO2 N2O5 HNO3 + OH → NO3 + H2O Puits de NO3 : NO3 + hν → NO2 + O3P NO3 + hν → NO + O2 NO3 + NO → 2 NO2 N2O5 + H2O → 2 HNO3 (het.) ⇒ Daytime lifetime : few seconds only !!! Geyer et al, JGR, 2003 (Ground measurement, « VOC in the urban atmosphere of Europe – sources, transformation and impactsHouston, » Texas) Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin NO3 induce a night-time chemistry… composé τNO3 τOH τO3 at night ([OH]= 2.106 [O3] = 30 ppb [NO3]= 10 ppt molécules.cm-3) 3-methylpentane Acetaldéhyde 2-methylbutanal 23 days 18 days 1,7 days 1 days 10 hours 4 hours 755 years - Pentene Trans-2-butene 6-methyl-5-hepten-2-one Acétate de cis-3-héxényl α-pinene β-pinene 2-carene 3 days 3 hours 9 min. 4,5 hours 11 min. 27 min. 4 min. 4,5 hours 14 min. 53 min. 2 hours 3 hours 2 hours 2 hours 1,7 days 42 min. 1 hours 7 hours 5 hours 1,1 days 2 hours « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin … but NO3 can also be a daytime oxidant τNO3 daytime τOH τO3 [NO3]= 0,2 ppt [OH]= 0.1ppt [O3] = 30 ppb 3,2 years 2,5 years 0.25 years 1 day 10 hours 4 hours 755 years - Pentene Trans-2-butene 152 days 6,3 days 4,5 hours 14 min 1,7 days 42 min 6-methyl-5-hepten-2-one 8 hours 53 min 1 hours Acétate de cis-3-héxényl α-pinene β-pinene 2-carene 10 days 9 hours 23 hours 3 hours 2 hours 3 hours 2 hours 2 hours 7 hours 5 hours 1,1 days 2 hours compounds 3-methylpentane Acétaldehyde 2-methylbutanal « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin What about new compounds ? • Vinyl ethers (new solvents, motor oil additives, manufacturing of coatings, synthesis intermediates…) • Unsaturated acetates (new fumigants, synthesis intermediates, monomer for polymer production, emission from new fuel use…) Only few kinetics and mechanistic studies Need for data to rationalize our understanding of the gas phase chemistry of new oxigenated compounds « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Kinetic and mechanistic studies of the NO3 induced oxidation scheme • Use of simulation chambers • Relative and absolute studies • Products and pathways identification « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin A serie of vinyl ether … • A homogeneous serie of vinyl ether O methyl vinyl ether O ethyl vinyl ether O propyl vinyl ether O butyl vinyl ether • Some studies (Thiault 2002, Klotz et al. 2004, Zhou, 2006) « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Vinyl ether experiment 3 Concentrations (ppm) MVE isoprène N2O5 2 1 0 4000 6000 8000 10000 t (sec.) 12000 14000 « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin 16000 Kinetics Composé MVE k (∗∗) (∗ ∗) 7,6 ± 0,4 6,1 ± 1,1 O kmoyen 7,2 ± 2,9 6,3 ± 0,5 EVE 15,1 ± 3,3 O 13,8 ± 1,5 13,1 ± 5,3 14,5 ± 2,5 PVE (∗ ∗) 4,68 (Grosjean et al., 1992) (Estimation) 17 ± 13 (Pfrang et al., 2006) 14.0 ± 3.5 (Zhou et al., 2006) 13,3 ± 1,8 12,8 ± 1,6 O Autres travaux 12,4 ± 2,0 13,3 ± 5,9 18.5 ± 5.3 (Zhou et al., 2006) 17,0 ± 7,3 21.0 ± 5.4 (Zhou et al. 2006) 15,1 ± 1,7 BVE 15,8 ± 1,8 O 18,9 ± 1,6 16,5 ± 1,4 (∗) : 10-13 cm3.molecule-1.s-1 Scarfogliero et al, 2006 Journal of Physical Chemistry A « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Products Identification from FTIR monitoring PVE alone RONO2 + ROONO2 5 min after N2O5 injection Formaldehyde ROONO2 RONO2 Propyl Formate 1 hour after N2O5 injection 3300 3200 3100 3000 2900 2800 2700 2600 σ (cm-1) 1500 1400 1300 1200 1100 1000 900 800 700 σ (cm-1) « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Chemical Scheme O2NO H RO OH O2NO H R-C(O)-R' + O2 H OONO2 H H RO H + HO2 H C RO ONO2 H H HO H RO ONO2 H H H ONOO 2 RO ONO2 H H R-C(O)-R' + O2 + O2 R-CHO2.-R' O2NO NO2 H RO O2NO O NO3 H C H H OO. H NO3 O2NO H RO + + O2 R-CHO2.-R' O2NO H RO H RO H + O2 H RO H .OO RO ONO2 H H NO3 NO2 + O2 H O H H .O RO NO2 NO2 + O2 ONO2 H H + O2 O RO H R-O-CHO + ONO2 H H HO2 + HCHO + NO2 « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Products study experiments méthyl vinyl ether formaldehyde formate de méthyle 0,8 concentrations (ppm) ) concentration (ppm) 1,0 0,6 0,4 0,2 0,0 1,0 0,8 0,6 nitrates organiques (unités arbitraires) 0,4 0,2 0,0 0 30 60 90 120 150 180 0 30 tem ps (m inutes ) 10 N2O5 acide ntrique 0,8 0,6 0,4 0,2 0,0 0 30 60 90 90 120 150 180 150 180 tem ps (m inutes ) concentrations (ppm) O2]) concentrations (ppm) 1,0 60 120 150 NO 8 NO2 6 4 2 0 180 tem ps (m inutes ) 0 30 60 90 120 tem ps (m inutes ) NO injection at the end to destroy peroxynitrates « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Products study experiments : Yield 0,5 0,5 formaldéhyde 0,4 0,3 0,2 y = 0,39x + 0,03 R2 = 0,99 0,1 concentrations (ppm) concentrations (ppm) méthyl formate 0,0 0,3 0,2 y = 0,41x + 0,02 R2 = 0,99 0,1 0,0 0,0 1,2 0,2 0,4 - ∆ [MVE] (ppm) 0,6 0,8 0,0 0,2 0,4 - ∆ [MVE] (ppm) 0,6 0,8 0,6 0,8 1,2 péroxynitrates 1,0 concentrations (unités arbitraires) concentrations (unités arbitraires) 0,4 0,8 0,6 0,4 0,2 0,0 nitrates organiques 1,0 0,8 0,6 0,4 0,2 0,0 0,0 0,2 0,4 - ∆ [MVE] (ppm) 0,6 0,8 0,0 0,2 0,4 - ∆ [MVE] (ppm) « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Products Yields vinyl ether Primary yield (%) final yield (%) HCHO alkyl formate HCHO alkyl formate methyl 46 ± 9 45 ±9 52 ± 14 53 ± 9 ethyl 54 ± 14 46 ± 8 68 ±13 60 ±7 propyl 51 ±16 45 ± 15 50 ±16 48 ± 6 butyl 44 ± 8 44 ± 7 47 ± 8 50 ± 4 « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin X 100 Kinetics Alkyle Alkenes (a) Vinyl ethers (a) group (R-CH=CH2) (R-O-CH=CH2) CH3 (9,5 ± 0,8).10-15 (b) (7,2 ± 1,5).10-13 C2H5 (13,5 ± 4,1).10-15 (c) (13,1 ± 2,7).10-13 C3H7 (15 ± 5).10-15 (d) (13,3 ± 3,0).10-13 C4H9 (18 ± 7).10-15 (d) (17,0 ± 3,7).10-13 « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin 2,00E-010 1,80E-012 k NO3 1,60E-012 k OH ?? 1,80E-010 1,60E-010 1,40E-012 1,40E-010 1,20E-012 1,20E-010 1,00E-012 1,00E-010 8,00E-013 8,00E-011 6,00E-013 6,00E-011 4,00E-013 4,00E-011 3 -1 -1 k (cm molecule s ) 2,00E-012 2,00E-013 O O O O 0,00E+000 2,00E-011 0,00E+000 Methyl Ethyl Propyl Butyl Alkyl group Electron donor (mesomeric and inductive) effects increase the reactivity of the double bond « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin A serie of unsaturated acetates … • A homogeneous serie O O Vinyl acetate O O Isopropenyl acetate O O • Very few studies Allyl acetate (Grosjean et al. 1992 (SAR)) « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin kacetate + NO3 O O O (115 O ±8)x10-15 cm3.molecule-1.s-1 O ONO 2 C δ+ δ O ONO 2 × 10 O O O (11.4 ±1)x10-15 cm3.molecule-1.s-1 O O ONO 2 C ÷ 10 O O O (1.0 ±0.6)x10-15 cm3.molecule-1.s-1 O C ONO 2 « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin δ+ δ O ONO 2 Atmospheric Lifetimes τNO3 τNO3 τOH τO3 [NO3] = 50 ppt (polluted) [NO3] = 2 ppt (rural) [OH] = 2×106 moléc.cm-3 Methyl vinyl ether 20 min 8 hours 3 hours (a) Ethyl vinyl ether 9 min 4 hours 2 hours (a) Propyl vinyl ether 10 min 4 hours 1.5 hours (a) Butyl vinyl ether 8 min 3 hours 1.5 hours (a) Vinyl Acetate 20 hours 21 days 3 hours (b) 70 hours (c) Isopropényle Acetate 2 hours 2 days 2 hours (e) - Allyl Acétate 7 days 160 days 5 hours (f) - [O3] = 50 ppb 1 hours (a) a : Thiault et al., 2002, b: Grosjean et al., 1992, c: Grosjean et al., 1998, d: Fantechi et al., 1998, e: Ferrari 1995, f: Picquet 2000 •Vinyl Ethers very reactive with NO3 •Acetate lifetime can vary over a wide range with NO3 « VOC in the urban atmosphere of Europe – sources, transformation and impacts » but not really with OH Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Secondary aerosol formation • Organic material contributes from 10 to 90% to total fine aerosol mass • SOA formed by gas-to-particle conversion of oxidation products of VOC. • Complexe mixtures (dicarboxylic acids, ketoacids, polyols …) • Environmental effects : direct and indirect radiative forcing visibility reducing toxicity Good knowledge of their formation processes and chemical composition is needed ! « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin SOA from Aromatic compounds • Intensively emitted by anthropogenic sources • Known to be precursors of SOA (Odum et al., 1997, Forstner et al. 1997) •Two target compounds : 2-methyl Styrene and Indene polymer combustion, biomass burning, cigarette smoke •Strong similarities in chemical structures •Only one kinetic study (Kwok et al., 1996) – no mechanistic study « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin SOA production from indene and Me-Styrene : comparative study • Production of SOA in simulation chamber… •… from ozonolysis reaction … • … under dry conditions. • Chemical/Physical analysis … Particles number and size monitored by SMPS (TSI 3022A/3081) … in gas phase : long-path FTIR … in particulate phase : sampling on glass-fiber filters + Supercritical Fluid extraction with BSTFA + direct coupling with GC/MS (SFE-GC-MS) « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin SOA production from indene and Me-Styrene : comparative study 2-méthylstyrène Ozone 4000 Aérosols 3000 Y = 0.07 2000 1000 Indène 2500 -3 Concentrations ( µg m ) -3 Concentrations ( µg m ) 5000 Ozone 2000 Aérosols 1500 Y = 0.17 1000 500 0 0 0 1000 2000 3000 4000 5000 600 0 7000 Time (s) 0 1000 2000 3000 Time (s) « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin 4000 5000 SOA production from indene and Me-Styrene : comparative study Indene: distribution en taille 3 Millions Millions MeStyrene: distribution en taille 2.5 0.8 0.7 1.5 1 0.5 dN/dlogD Nombre dN/dlogd 2 dN/dlogD Nombre dN/dlogd 0.6 0.4 0.3 0.2 0.5 0.1 0 0 0 50 100 150 200 250 Diamètre (nm) diametre (nm) 0 100 200 300 Diametre (nm) Diamètre « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin 400 (nm) 500 Composition of SOA produced from Me-Styrene ozonolysis H HO O HO O H O O O H O HO OH O OH 18 Ions: 73+75+117+147 19 20 Time (mn) 21 22 Total Ions « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin 23 Composition of SOA produced from Indene ozonolysis O H O O O OH O H O O OH H 16 O OH H H 10 H O OH O 12 OH O H OH 14 OH H O O O O O O O OH H O O OH H O OH O HO O 8 6 4 2 0 15 16 17 18 19 20 21 22 23 24 Time (mn) « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Composition of SOA produced from Me-Styrene ozonolysis « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Composition of SOA produced from Indene ozonolysis « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin SOA production from indene and Me-Styrene : comparative results Me-Styrene Indene Rate Constant with O3 (molec-1.cm3.s-1) (1.5±0.3)x10-17 (14±2)x10-17 Mass yield (%) 7% 17 % Aerosol Mass (mg.m-3) 310 850 Max size (nm) 100 250 Analyzed SOA (%) 54 ±17 % 36 ±7 % Mono-functionnal Poly-functionnal Structure Typical identified species HO O O O H O H H O H O OH OH O OH « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin SOA production from indene and Me-Styrene : comparative results Indene ozonolysis forms more particles (in mass) 2-méthylstyrène Indène O O O • Because it leads to more polar compounds O + O3 O O O3 + • Because of the endocyclic double bond C O H O HC O O + O H C O O H O • Because of the hydroperoxide channel Hydroperoxide pathway Di-carboxylic acids « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Conclusion •Small change in the structure may induce large effects on the impacts of the considered VOC •Simple structure analysis rules can be used to roughtly forecast the reactivity/impact of new VOCs : ex use of allyl acetate may be an alternative to vinyl acetate at local scale •These rules may be useful for the building of StructureActivity Relationship « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin Acknowledgment MOST «Multiphase Chemistry of Oxygenated Species in the Troposphere » Introp EVK2-. 2001-00114 French Ministry of Environment « VOC in the urban atmosphere of Europe – sources, transformation and impacts » Wroclaw, Poland, 25-26 Sept. 2007 - JF Doussin