Six Basic Forms of Energy
advertisement

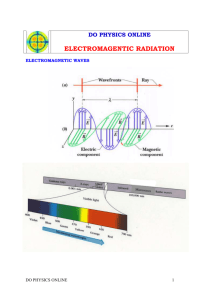
Forms of Energy Six Basic Forms of Energy Energy exists in a number of different forms, all of which measure the ability of an object or system to do work on another object or system. There are six different basic forms in which we use energy in our day-to-day life: Mechanical Energy (Kinetic) Energy that a body possesses by virtue of its motion. A few examples are a baseball player pitching a ball, a plow being pulled by a tractor, and a hammer that is being used to pound nails. In the United States, we use about a third of our total energy for transportation or movement of people and goods. Mechanical Energy (Potential) Energy that a body possesses by virtue of its position relative to a reference point. A few examples of mechanical energy include a pendulum, a bow (archery), a spring, and a hammer that is raised in preparation to pound nails. Let's investigate further... A book sitting on a shelf in the library is said to have potential energy because if it is nudged off, gravity will accelerate the book, giving the book kinetic energy. Because the Earth's gravity is necessary to create this kinetic energy, and because this gravity depends on the Earth being present, we say that the Earth-book system is what really possesses this potential energy, and that this energy is converted into kinetic energy as the book falls. Chemical Energy Energy locked in the bonds of molecules in the form of microscopic potential energy, which exists because of the electric and magnetic forces of attraction exerted between the different parts of each molecule: o It is the same attractive force involved in thermal vibrations. o The molecular parts get rearranged in the chemical reactions, releasing or adding to this potential energy. Some examples include a battery, burning wood, and glucose in the body. Approximately 85% of the energy used in the U.S. comes from fossil fuels such as coal, oil, and natural gas. o All of these fuels store energy in the form of chemical energy. o When they are burnt, these fuels release energy in the form of heat or thermal energy. The glucose (blood sugar) in your body is said to have "chemical energy" because the glucose releases energy when chemically reacted (combusted) with oxygen. Your muscles use this energy to generate mechanical force (work) and also heat. Thermal or Heat Energy: Energy that combines microscopic, kinetic and potential energy of the molecules. Some examples of this include a hot beverage and boiling water. Temperature is really a measure of how much thermal energy something has: The higher the temperature, the faster the molecules are moving around and/or vibrating, i.e., the more kinetic and potential energy the molecules have. Fuels (chemical energy) are oftentimes burnt and converted to thermal or heat energy, which is then converted to motion in an automobile or electricity. Thermal Energy A hot cup of coffee is said to possess "thermal energy," or "heat energy," because it has a combination of kinetic energy (its molecules are moving and vibrating) and potential energy (the molecules have a mutual attraction for one another) - much the same way that the book on the bookshelf and the Earth have potential energy because they attract each other. Electrical Energy: Energy created through the movement of electrons among the atoms of matter. Although electricity is seldom used directly, it is one of the most useful and versatile forms of energy. Following are some examples. o When put into a toaster, it can be converted to heat; o When put into a stereo, it is converted into sound; o When put into an electric bulb, it converts into light; o When put into a motor it converts into motion or movement (mechanical energy). Due to its versatility, it is in high demand; in the US about 40% of the total primary energy used is converted into electricity for various uses. Remember This! All matter is made up of atoms, and atoms are made up of smaller particles, called protons (which have positive charge), neutrons (which have neutral charge), and electrons (which are negatively charged). The electrons orbit around the nucleus which contains protons and neutrons, just like the planets orbit the sun. Certain metals have electrons that are only loosely attached to their atoms, so they can easily be made to move from one atom to another if an electric field is applied to them. When those electrons move among the atoms of matter, a current of electricity is created. Nuclear Energy: Energy produced when reactions occur in an atom, resulting in some type of structural change in the nuclei. Fusion occurs when two small nuclei join together to create one large nucleus or particle, and during this process, energy is released in the form of light and heat An example is in the Sun, hydrogen nuclei fuse (combine) together to make helium nuclei which releases energy. Fission occurs when the nucleus of one big atom splits into two new atoms, and during this process, a tremendous amount of energy is released in the form of light and heat. An example is in a nuclear reactor or the interior of the earth, uranium nuclei split apart causing energy to be released. Did You Know? In both fusion and fission, some of the matter making up the nuclei is converted into energy, represented by the famous equation: E = mc2 Energy = Mass x (Speed of Light)2 This formula indicates that energy intrinsically stored in matter at rest equals its mass times the speed of light squared. When matter is destroyed, the energy stored is released. This equation suggests that an incredibly huge amount of energy is released when a small amount of matter is converted to energy. Radiation: Energy radiated or transmitted in the form of rays, waves or particles. Some examples include: o Visible light that can be seen by naked eye; o Infrared radiation; o Ultraviolet radiation (UV) that cannot be seen with the naked eye; o Long wave radiation, such as TV waves and radio waves; o Very short waves, such as x-rays and gamma rays. Electromagnetic Radiation o Energy from the sun comes to the earth in the form of Electromagnetic radiation, which is a type of energy that oscillates (side to side) and is coupled with electric and magnetic fields that travel freely through space. o Electromagnetic radiation is composed of photons or particles of light, which are sometimes referred to as packets of energy. o Photons, like all particles, have properties of waves. Photons make the world a brighter place! Photons are created when electrons jump to lower energy levels in atoms, and absorbed when electrons jump to higher levels. Photons are also created when a charged particle, such as an electron or proton, is accelerated. An example of this phenomenon is a radio transmitter antenna that generates radio waves. Electromagnetic Spectrum o The “Electromagnetic spectrum“ is a representation of the wide range of wavelengths of electromagnetic radiation. o Photons are associated with visible light, which accounts for only a very limited part of the electromagnetic spectrum. o A great discovery of the nineteenth century was that radio waves, x-rays, and gamma-rays are just forms of light, and that light is electromagnetic waves. As depicted in the image above, the lower the energy, the longer the wavelength and lower the frequency, and vice versa. The reason that sunlight can hurt your skin or your eyes is because it contains "ultraviolet light," which consists of high energy photons. These photons have short wavelength and high frequency, and pack enough energy in each photon to cause physical damage to your skin if they get past the outer layer of skin or the lens in your eye. Radio waves, and the radiant heat you feel at a distance from a campfire, for example, are also forms of electromagnetic radiation, or light, except that they consist of low energy photons (long wavelength and high frequencies - in the infrared band and lower) that your eyes can't perceive. This was a great discovery of the nineteenth century - that radio waves, x-rays, and gamma-rays are just forms of light, and that light is electromagnetic waves. About 20% of the electricity used in the US is used to produce visible light for lighting purposes. Author: Dr. Sarma Pisupati, Associate Professor of Energy and Mineral Engineering, College of Earth and Mineral Sciences, The Pennsylvania State University.