Presentation - Setting specifications, statistical considerations
advertisement

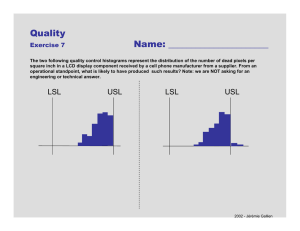
Setting Specifications Statistical considerations Enda Moran – Senior Director, Development, Pfizer Melvyn Perry – Manager, Statistics, Pfizer B i St ti ti Basic Statistics Population p distribution (usually unknown). Normal distribution described by μ and σ. 15 1.5 True batch assay 1.0 Distribution of possible values 0.5 0.0 98 99 100 We infer the population from samples by calculating x and s. 1.5 1.0 1.5 1.5 Sample 1 Average 98.6 True batch assay y True batch assay y 1.0 Sample 2 Average 98.7 1.0 Distribution of possible values 0.5 99 100 Distribution of possible values 0.5 0.0 0.0 98 True batch assay y Distribution of possible values 0.5 0.0 Sample 3 Average 99.0 98 99 100 98 99 100 2 I t Intervals l 30 28 26 24 Population average 22 20 18 16 0 10 20 30 40 50 60 70 80 90 100 •100 samples of size 5 taken from a population with an average of 23.0 and a standard deviation of 2.0. •The highlighted intervals do not include the population average (there are 6 of them). •For For a 95% confidence level expect 5 in 100 intervals to NOT include the population average average. •Usually we calculate just one interval and then act as if the population mean falls within this interval. 3 I t Intervals l Point Estimation The best estimate; eg MEAN Interval Estimation A range which contains the true population parameter or a future observation to a certain degree of confidence. Confidence Interval - The interval to estimate the true population parameter (e.g. the population mean). mean) Prediction Interval - The interval containing the next single response. Tolerance Interval - The interval which contains at least a given proportion of the population. 4 F Formulae l ffor Intervals I t l Intervals are defined as: x ± ks A Assuming i a normall di distribution t ib ti • Confidence (1-α) interval 0 .5 ⎛ 1⎞ CI = x ± ⎜ ⎟ t α s ⎝ n ⎠ 1− 2 ,n −1 • Prediction (1-α) (1 α) interval for m future observations 0. 5 1⎞ ⎛ PI = x ± ⎜ 1 + ⎟ t α s n ⎠ 1− 2m ,n −1 ⎝ • Tolerance interval for confidence (1-α) that proportion ( ) is (p) i covered d (n − 1)⎜⎛ 1 + 1 ⎞⎟ z(21− p ) TI = x ± ⎝ χ n⎠ 2 α ,n −1 2 s 5 P Process Capability C bilit 3s Process capability is a measure of the risk of failing specification. The spread of the data are compared with the width of th specifications. the ifi ti 3s The distance from the mean to the nearest specification relative to half the process width (3s). The index measures actual performance. Which may or may not be on target i.e., centred. LSL ⎧USL − x x − LSL ⎫ Ppk = min⎨ , ⎬ s 3s ⎭ 3 ⎩ x x − LSL USL − x USL 6 P Process Capability C bilit – Ppk P k and dC Cpk k • Ppk should be used as this is the actual risk of failing specification specification. • Cpk is the potential capability for the process when free of shifts and drifts. Random data of mean 10 and SD 1, thus natural span 7 to 13. Added shifts to simulate trends around common average average. With specs at 7 and 13 process capability should be unity. Process Capability of Shifted I Chart of Shifted 15 LSL 1 14 UCL=13.589 13 Ind dividual Value When data is with trend Ppk less than Cpk due to method of calculation of std dev. 12 11 USL Within Ov erall P rocess D ata LS L 7 Target * USL 13 S ample M ean 10.0917 S ample N 30 S tD ev (Within) 1.16561 S tD ev (O v erall) 1.95585 P otential (Within) C apability Cp 0.86 C P L 0.88 C P U 0.83 C pk 0.83 O v erall C apability _ X=10.092 10 Pp PPL PPU P pk C pm 9 0.51 0.53 0.50 0.50 * 8 7 6 LCL=6.595 6 1 4 7 10 13 16 19 Observation 22 25 O bserv ed P erformance P P M < LS L 0.00 P P M > U S L 100000.00 P P M Total 100000.00 28 10 12 14 E xp. O v erall P erformance P P M < LS L 56966.34 PPM > USL 68512.70 P P M Total 125479.03 UCL=13.038 13 12 11 LSL USL Within Ov erall P rocess Data LS L 7 Target * USL 13 S ample M ean 10.0917 S ample N 30 S tD ev (Within) 0.982187 S tD ev (O v erall) 1.14225 P otential (Within) C apability Cp 1.02 C P L 1.05 C P U 0.99 C pk 0.99 _ X=10.092 10 O v erall C apability Pp PPL PPU P pk C pm 9 8 LCL=7.145 7 1 4 7 10 13 16 19 Observation Cpk C k uses average moving i range SD (same as for control chart limits). Cpk p is close to 1 at 0.83. Process Capability of Raw I Chart of Raw IIndividual Value 8 E xp. Within P erformance P P M < LS L 3995.30 PPM > USL 6296.59 P P M Total 10291.88 Ppk uses sample SD. Ppk less than 1 at 0.5. 22 25 28 7 O bserv ed P erformance P P M < LS L 0.00 P P M > U S L 0.00 P P M Total 0.00 8 E xp. Within P erformance P P M < LS L 822.51 P P M > U S L 1533.13 P P M Total 2355.65 9 10 11 12 E xp. O v erall P erformance P P M < LS L 3397.73 P P M > U S L 5446.84 P P M Total 8844.57 13 0.88 0.90 0.85 0.85 * When data is without trend Ppk is same as Cpk. Only small differences are seen. Cpk effectively 1 at 0.99. Ppk close to 1 at 0.85. 7 M Measurement t Uncertainty U t i t LSL Good parts t almost always passed p Bad parts almost always rejected j t d ±3σmeasurement USL σmeasurement σtotal Bad parts almost always rejected ±3σmeasurement The grey areas highlighted represent those parts of the curve with the potential for wrong decisions, or mis-classification. 8 Misclassification with less variable i bl process σtotal LSL ±3σ 3σmeasurement USL σmeasurement ±3σmeasurement 9 M Measurement t Uncertainty U t i t Precision to Tolerance Ratio: How much of the tolerance is taken up by measurement error. This estimate may be appropriate for evaluating how well the measurement system can perform with respect to specifications. Gage R&R (or GRR%) What percent of the total variation is taken up by measurement error (as SD and thus not additive). 6 × σ MS P /T = Tolerance Tolerance = USL − LSL USL = Upper pp Spec p Limit LSL = Lower Spec Limit σ MS = Std. Dev. of Measurement Sys. %R & R = σ MS × 100 σ Total Use Measurement Systems Analysis to assess if the assay method is fit for purpose. It is unwise to have a method where the specification interval is consumed by the measurement variation alone. 10 Specification example – T l Tolerance IInterval t l Data from three sites used to set specifications specifications. 16.5 16.0 Tolerance interval found from pooled data of 253 batches. Tolerance interval chosen as 95% probability that mean ± 3 standard deviations are contained. Sample size: n=253 Mean ± 3s Mean = 14.77 Tolerance interval ((95% %/ 99.7%) s=0.58 R Range 13 03 - 16.51 13.03 16 51 12 89 - 16.65 12.89 16 65 If sample size was smaller, difference between these calculations increases. As the sample size approaches infinity the TI approaches mean ± 3s. 15.5 15.0 14.5 14.0 13.5 13.0 1 2 3 Code N 5 10 15 30 ∞ k multiplier 6.60 4.44 3 89 3.89 3.35 2.58 Table of values for 95% probability of interval containing 99% of population values values. Note at ∞ value is 2.58 which is the z value for 99% coverage of a normal population 11 Specification Example St bilit Stability Batch history (process and Total SD (p measurement) Stability batch with SD (measurement) Shelf life set from 95% CI on slope from three clinical batches. Need to find release criteria for high probability of production batches meeting shelf life based on individual results being less than specification. Review R i off batch b t h history hi t will ill llead d tto a process capability bilit statement t t t against release limit. 12 Specification Example St bilit Stability Release limit is calculated from the rate of change and includes the uncertainty in the slope estimate (rate of change of parameter) and the measurement variation). 8 7 6 ⎛ s2 ⎞ RL = Spec - ⎜ Tm + t T 2sm2 + a ⎟ ⎜ n ⎟⎠ ⎝ Specification = 8 Shelf life ((T)) = 24 Parameter slope (m) = 0.04726 Variation in slope (sm) = 0.00722 Variation around line (sa) = 0.86812 t on 126 df approx approx. = 2 N= 1 for single determination Release limit = 5.1 5 4 3 2 1 0 0 6 12 18 24 30 MONTHS A similar approach can be taken in more complex situations; e.g,, after reconstitution of lyophilised product product. Product might slowly change during cold storage and then rapidly change on reconstitution. Allen et al., (1991) Pharmaceutical Research, 8, 9, p1210-1213 36 13 C i with Coping ith lilimited it d d data t Problems with n<30. • • • Difficult to calculate a reliable estimate of the SD. Difficult to assess distribution. Difficult to assess process stability. With very limited data the specifications will be wide due to the large multiplier multiplier. • This reflects the uncertainty in the estimates of the mean and SD. Is there a small scale model that matches full production scale? How variable is the measurement system? Alternatives are: Example with 4 values • Min / max values based on scientific rationalisation • • • Mean ± 3s Mean ± 3s 13.2 27.0 Mean ± 4s or minimum Ppk p = 1.33. Mean ± 4s 10.9 29.3 Tolerance interval approach TI (95/99.7) -1.5 41.7 N(20 4) – 19.9, N(20,4) 19 9 16 16.9, 9 22 22.1, 1 21 21.3 3 14 Updating p g Specifications p When, why, and a scenario for consideration • Specifications for CQAs might require revision during the product lifecycle. For example, new clinical data and i t lli intelligence on continued ti d use off a product d t might i ht d drive i a change h tto a CQA specification ifi ti • Scenario: What if a process producing a new product approaching regulatory approval is ‘incapable’ of meeting the clinicallytested CQA in the long-term? For example......... Proposed Spec. Process capability estimate CQA Proposed Spec. If spec. required t be to b sett at the Process capability estimate CQA Clinical experience / New spec. Clinical experience clinicallyclinically tested level......... Clinical / Validation Lots Clinical / Validation Lots An ‘incapable’ process • How could we manage this situation? Complex. Many factors need to be considered if setting specifications at clinically tested level (will ( be discussed through this workshop)) • IF it must be done for risk reduction purposes, and if there is sufficient uncertainty that the Proposed Spec. for the CQA could cause harm, a possible approach may be to move towards the clinically-tested level in stages. - Accrue approx. pp n=25-30 batches/lots to consider estimates of future p process capability p y acceptable. p Adjust spec. to a revised process capability estimate. - Accrue further lots of the stabilised process to approx. n=85. Make FINAL spec. adjustment to a revised process capability estimate. 15 Summary • Process capability & measurement uncertainty • Setting spec.s - large number of datapoints • Setting spec.s – small number of datapoints 16