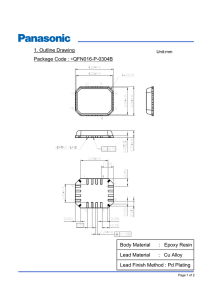
Industrial Zinc Plating Processes
advertisement

Indiana University of Pennsylvania Knowledge Repository @ IUP Theses and Dissertations 5-2015 Industrial Zinc Plating Processes Christopher Eric Sierka Indiana University of Pennsylvania Follow this and additional works at: http://knowledge.library.iup.edu/etd Recommended Citation Sierka, Christopher Eric, "Industrial Zinc Plating Processes" (2015). Theses and Dissertations. Paper 1283. This Thesis is brought to you for free and open access by Knowledge Repository @ IUP. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Knowledge Repository @ IUP. For more information, please contact cclouser@iup.edu. INDUSTRIAL ZINC PLATING PROCESSES A Thesis Submitted to the School of Graduates Studies and Research in Partial Fulfillment of the Requirements for the Degree Master of Science Christopher Eric Sierka Indiana University of Pennsylvania May 2015 Indiana University of Pennsylvania School of Graduate Studies and Research Department of Chemistry We hereby approve the thesis of Christopher Eric Sierka Candidate for the degree of Master of Science ___________________ ____________________________________ Keith Kyler, Ph.D. Professor of Chemistry, Advisor ____________________________________ Jonathan Southard, Ph.D. Professor of Biochemistry ____________________________________ Nathan McElroy, Ph.D. Professor of Chemistry ____________________________________ Jean-Pierre Habets. President of H&W Global Industries ACCEPTED ___________________________________ Timothy P. Mack, Ph.D. Dean School of Graduate Studies and Research _____________________ ii Title: Industrial Zinc Plating Processes Author: Christopher Sierka Thesis Chair: Dr. Keith Kyler Thesis Committee Members: Dr. Jonathan Southard Dr. Nathan McElroy Mr. Jean-Pierre Habets Electrodeposition is a process, which uses an electrical current to reduce cations of a desired material from a solution and coat that material as a thin film onto a conductive substrate surface. In this case, zinc cations will be reduced and coat the steel substrates acting as a sacrificial coating to provide corrosion resistance. Three widely used industrial zinc plating processes will be examined, including acid chloride, alkaline cyanide, and alkaline non-cyanide. These processes will be discussed in terms of throwing power, cathode efficiency, hydrogen embrittlement, deposition mechanisms and salt spray testing. After the zinc plating line is operational, training methods must be documented to adhere to ISO 9001 quality level. iii TABLE OF CONTENTS Chapter I Page INTRODUCTION ........................................................................................................................ 1 1.1 Electroplating Background………………………………………………………………….…………………….....1 1.2 Electroplating Deposition Mechanisms ........................................................................... 10 II ZINC PLATING PROCESSES ..................................................................................................... 12 2.1 Cyanide Zinc Plating ......................................................................................................... 12 2.2 Acid Chloride Plating........................................................................................................ 15 2.3 Alkaline Non-Cyanide Plating .......................................................................................... 17 2.4 Zinc-Alloy Plating ............................................................................................................. 19 III ACID CHLORIDE PROCESS STEPS ............................................................................................ 21 3.1 Chemicals and Equipment ............................................................................................... 22 3.2 Alkaline Cleaning and Electrocleaning............................................................................. 22 3.3 Acid Pickling ..................................................................................................................... 26 3.4 Acid Chloride Zinc Plating ................................................................................................ 27 3.5 Acid Dip / Chromates ....................................................................................................... 33 3.6 Waste Treatment Management ...................................................................................... 34 IV TESTING AND ANALYSIS .......................................................................................................... 38 4.1 Salt Spray ......................................................................................................................... 38 4.2 Hull Cell Analysis .............................................................................................................. 41 4.3 Plating Tank Analysis ....................................................................................................... 44 V TRAINING METHODOLOGIES AND QUALITY ASSURANCE...................................................... 47 5.1 Introduction to Training Methodologies and Quality Assurance .................................... 47 VI COST ANALYSIS....................................................................................................................... 56 6.1 Zinc Plating Chemicals ..................................................................................................................... 56 VII SUMMARY AND CONCLUSION ............................................................................................... 59 REFERENCES…………………………………………………………………………………………………………….……... 60 iv LIST OF TABLES Table Page 1 List of Metals that can be Electroplated................................................................................ 2 2 Cathode Current Efficiencies of Various Plating Solutions .................................................... 5 3 Electromotive Force Series .................................................................................................... 6 4 Alkaline Cyanide Zinc Plating Bath Constituents ................................................................. 12 5 Acid Chloride Zinc Plating Bath Constituents ...................................................................... 16 6 Alkaline Non-Cyanide Bath Constituents............................................................................. 18 7 Miscellaneous Requirements .............................................................................................. 18 8 Surfactant Composition ....................................................................................................... 24 9 Salt Spray Hourly Requirements .......................................................................................... 40 10 Salt Spray Hourly Requirements.......................................................................................... 40 11 Hull Cell Zinc Plating Testing................................................................................................ 43 12 Hull Cell Process Sequence .................................................................................................. 43 13 Sample Work Instruction for Zinc Plating with Type II Colored Chromate Coating ........... 51 14 Sample Work Instruction for Zinc Plating with Type III Colorless Chromate Coating ........ 52 15 Plating Time Calculations for Various Zinc Coating Thicknesses and ASF ........................... 53 16 Chemical Costs..................................................................................................................... 57 17 Zinc Plating Chemical Costs ................................................................................................. 57 18 Maximum Square Footage for 250 Ibs of Zinc Ball Anodes................................................. 58 v LIST OF FIGURES Figure Page 1 Cathode efficiencies of plating processes.1 ......................................................................................... 4 2 Nernst Diffusion Layer2 ........................................................................................................................ 9 3 Step Edge Ion Transfer……………………………………………………………………………………………..……………….11 4 Step Edge Transfer and Diffusion……………………………………………………………………………………………..…..11 5 Terrace Ion-Transfer .......................................................................................................................... 11 6 Simultaneous Metal Deposition. ....................................................................................................... 20 7 Acid Chloride Process Steps. .............................................................................................................. 21 8 Pourbaix diagram of Iron at 25˚C....................................................................................................... 26 9 Pourbaix diagram of Aluminum at 25˚C. ........................................................................................... 26 10 Tank Design. ...................................................................................................................................... 28 11 Polypropylene tank (left) and Stainless Steel tank (right). ................................................................ 29 12 pH Adjustment Tank. ......................................................................................................................... 37 13 Semi-Automatic Waste Treatment System. ...................................................................................... 37 14 Salt Spray Corrosion Theory. ............................................................................................................. 39 15 A Kocour 267 mL Hull Cell. ................................................................................................................ 41 16 Hull Cell Ruler. ................................................................................................................................... 42 17 Structure of metal/EDTA complex..................................................................................................... 44 18 Zinc Plating with Yellow Passivate Coating........................................................................................ 54 19 Zinc Plating with Clear Passivate Coating .......................................................................................... 55 vi CHAPTER I INTRODUCTION 1.1 Electroplating Background Many electroplating processes exist to change or enhance existing properties of metals. Almost all metals can be plated, if the correct sequence of steps is taken, Table I gives a list of all possible metals that can be electroplated. The concentration of this research is predominantly on zinc electroplating. Three types of electroplating chemical processes predominantly exist for Zinc plating. These processes are (1) alkaline cyanide, (2) alkaline noncyanide, and (3) acid chloride. The main component of each electroplating chemical process includes a dominant Zn2+ complex which gets reduced to Zn0 at the cathode. Zinc coatings are predominantly coated onto carbon steel cathodes. Furthermore, successful zinc plating does not occur without proper pre-cleaning and post chemical treatments. A whole plating industry exists for commercial and military purposes, and H&W Global Industries is a metal finishing facility interested in installing a moderate size zinc plating line to meet the demands. Areas of my research include; the basics of electroplating, the Zinc Plating process, equipment selection, Analytical Testing, and training of chemical line operators. First and foremost, the basics of electroplating will be explained to understand underlying principles that can be beneficial in an industrial setting. The first equation to be explained in detail will be Faraday’s Law, followed by Cathode Efficiency, Nernst Equation, Throwing Power, and Ion Mobility. 1 Table 1. List of Metals that can be Electroplated Group Number Metals 6 7 8 9 10 11 12 13 14 15 16 Cr Mn, Tc, Re Fe, Ru, Os Co, Rh, Ir Ni, Pt, Pd Cu, Ag, Au Zn, Cd, Hg In, Ti Sn, Pb As, Sb, Bi Se, Te A. Faraday’s Law The definition of electroplating is the “electrodeposition of an adherent metallic coating upon an electrode for the purpose of securing a surface with properties of dimensions different from those of the basis metal1” Electroplating is an electrolytic process which follows the principles of Faradays laws. Faraday’s 1st law states the amount of chemical change produced by an electric current is proportional to the amount of electricity that passes. By measuring the quantity of electricity that passes, one can measure the amount of chemical change that will be produced. Faraday’s 2nd law states the amounts of different substances liberated by a given quantity of electricity are proportional to their chemical equivalent weights. If the equivalent weight of a metal is known (E) then one can predict the amount of substance that can be plated on a specific substrate indicated by Eq 1. G= lET/96000 (1) 2 where G = grams of substance reacting, I = Current (amps), E = chemical equivalent weight, and T = Time (sec). The equation commonly employed in the electroplating industry is Eq 2. ASF = Amps X Surface Area (2) The acronym ASF stands for Amps per Square foot. Most often industry utilizes this acronym for ease of learning. A lesser known measurement used is ADM, which stands for Amps per square decimeter. Surface Area is always measured in ft2 because it is used for larger plating areas used most frequently in the plating industry. Another important parameter to determine the best zinc plating process is Cathode Efficiency represented by Eq 3, denoted as CE. By industry standards, cathode efficiency translates to the speed of a plating solution. For example, the acid chloride process has 95% CE and that of the alkaline cyanide process has 5080% CE. Before taking CE into consideration, both plating processes would take the same amount of time for plating; however cathode efficiency must be accounted, resulting in the acid chloride process to plate Zinc faster than the alkaline cyanide process. By industry standards, plating times are vital to determine if a plating process is worth the monetary risk. B. Cathode Efficiency CE = 100 X Actual/Theoretical (3) Figure 1 shows several cathode efficiencies of varying zinc plating baths. As depicted by Figure 11, the Acid Chloride process shows constant cathode efficiencies above 90% as current density increases, while the cathode efficiencies of the non-cyanide zincate plating solution decrease below 60% as the current density increases. Competing reactions are limited at the cathode for the acid chloride process, while many competing reactions occur at the cathode of the non- 3 cyanide zincate process. This pattern of current efficiencies is seen with other metals as well. For example, Table 2 shows plating efficiencies for a range of different plating metals, such as Gold and Tin. As portrayed, an acid chloride bath shows above 95% cathode current efficiencies. As Cathode efficiency increase, throwing power will decrease. Acid Chloride Acid Sulfate Cyanide Noncyanide Zincate Figure 1. Cathode efficiencies of plating processes.1 4 Table 2. Cathode Current Efficiencies of Various Plating Solutions1 Metal Deposit Ag Au Cd Cr Electrolyte CN Acid, Neutral, CN CN Cu Acid SO4, CN (High efficiency) CN (Low efficiency) P2O7 Fe In Acid Acid CN Acid Acid Acid Acid Alkaline Acid CN CrO3 /H2SO4 CrO3 /SO4-F Ni Pb Rh Sn Zn Cathode Efficiency (%) 100 50-100 85-95 10-15 18-25 97-100 30-45 90-95 100 90-98 30-50 30-50 93-98 95-100 10-50 90-95 70-95 95 50-80 C. The Nernst Equation important in plating of metals. E = E0 + (RT/nF) lna (4) i. E = electrode potential ii. E0 = constant characteristic of material of electrode. Standard electrode potential iii. R = gas constant = 8.3143 J/kmol iv. T = absolute temperature in Kelvins v. F = faraday vi. N = valence change vii. A = activity of the metal E0 = -0.76 volts for zinc without complex Zn2+ + 4(CN)- → [Zn(CN)4] E0 = -1.1 volts for zinc with cyanide complex When the unit activity a; is found to be 1, which rarely happens in nature, then E = E0. When this occurs, the standard electrode potentials can be calculated and tabulated as in Table 5 3. The EMF series is an arrangement of various metals in order of their electrochemical activities based on their standard oxidation-reduction potentials (E0). The most active metals in the series will have high negative E0 values and are located on the bottom of the EMF series table. The noble metals will have positive E0 values, located on the top of EMF series table. For instance, if two metals are coupled together in the EMF series, the species with a larger negative standard potential will act as the anode and corrode compared to the other metal species with a positive standard potential. Several exceptions exist due to environmental factors and passivity. Aluminum exhibits higher corrosion resistance despite having a larger negative E0 due to the presence of an Al2O3 layer present on the surface. As a consequence of the electrode potentials in this series, the Nernst Equation is born. Table 3. Electromotive Force Series Reaction Au3+ + 3e = Au Pt2+ + 2e = Pt O2 + 4H+ + 4e = 2H2O Pd2+ + 2e = Pd Ag+ + e = Ag O2 + 2H2O + 4e =4OHCu2+ + 2e = Cu Sn3+ + 2e = Sn2+ 2H+ + 2e = H2 Pb2+ + 2e = Pb Sn2+ + 2e = Sn Ni2+ + 2e = Ni Co2+ + 2e = Co Cd2+ + 2e = Cd Fe2+ + 2e = Fe Cr3+ + 3e = Cr Zn2+ + 2e = Zn Al3+ + 3e = Al Mg2+ + 2e = Mg Na+ + e = Na E0,V(SHE) +1.42 + 1.2 +1.23 +0.83 +0.799 +0.401 +0.34 +0.154 0.00 -0.126 -0.140 -0.23 -0.27 -0.402 -0.44 -0.71 -0.763 -1.66 -2.38 -2.71 6 D. Throwing Power1 The metal deposit distribution is affected by the variation of the cathode efficiency with current density. In plating solutions in which the cathode efficiencies decrease rapidly as current density increases, excess deposits will plate on edges and corners. This phenomenon is coined throwing power. In this case, little throwing power is available. If throwing power is minimal then longer plating times are necessary to achieve the minimum coating thicknesses in recesses. In plating solutions in which the cathode efficiencies increase as current density increases, excess deposits will plate more evenly, resulting in high throwing power. Less plating time is required to achieve minimum coating thicknesses in low current density areas. The next factor that influences throwing power is the overall geometry of the plating system. Plating tanks can be made to accommodate varying part geometries by moving the anodes to the contour of the parts. Higher cathode polarizations at higher current densities results in decreased cathode efficiency, resulting in higher throwing power. Cathode polarization is commonly known as overpotential; which is the difference in electrode potential between its equilibrium potential and its operating potential when a current is flowing2,10. Throwing power is mistakenly used to describe covering power. Covering power applies to the lowest applied current density at which a plating bath produces a deposit. The inability to plate in low current density areas can be improved by applying a high current density strike to initiate plating into recesses, and then normal current densities may be used to finish plating. E. Ion Mobility The mobility is the ability of ions to move in an electric field. Under the influence of an applied voltage, ions move toward electrodes; cations move toward cathode, anions move toward 7 anode. Each particular ion moves at a particular rate characteristic of that ion, is called its mobility or ionic conductivity. Electrical forces between ions interfere with mobility, along with solvent impediment, solution viscosity and retarding effects of ions of opposite charge. Ion mobility is the velocity that an ion attains per unit of electric field. The ion mobility is given by the following eq2: Ui = |Zi|e/ (6πηri) (5) Where z = ion charge, e = electronic charge, η = solvent viscosity, and r = ion radius. Electrical conductivities will be highest for highly charged small ions in solvents of low viscosity. The consumption of electroactive species close to the electrode results in a concentration gradient and diffusion of the species towards the electrode from the bulk may become ratedetermining as described by Figure 2. Therefore, a large overpotential is needed to produce a given current. For any reaction to proceed, and overpotential is required to overcome the potential barrier at the electrode/surface barrier. Cathodic activation overpotential shifts the energy level of the ions right outside the Nernst diffusion layer nearer to the potential barrier, so more ions can cross the potential barrier at a given time, producing a deposit. 8 Figure 2. Nernst Diffusion Layer2 Whereas COX(X) is the concentration of ions at the surface of the metal and COX is the concentration of ions in the bulk of solution surrounding the metal surface. At the maximum current density, the metal species is reduced as soon as it reaches the electrode. The concentration of the Zn2+ is non-existent at the electrode surface, and is determined by the rate of transport of the ions to the metal surface. If the current density introduced is greater than the maximum current density, the double layer becomes further charged and other processes will occur other than reduction of the Zn2+. F. The Plating Bath3 All plating baths have the basic features. The first part of a plating bath is to provide a source of metal to be deposited. The second feature is to form complexes with ions of the depositing metal. Complex formations are not always required; however deposits formed from complex salts are far superior to those of simple salts (ZnCl2). The next feature of a plating bath is to provide conductivity. Many metal salts are poor conductors (low ionic mobilities), so conducting salts are added to increase conductivity of solution. Two examples of conducting 9 salts are KCl and NH4Cl. The next feature in a plating bath is compounds that stabilize the solution against hydrolysis. When metal salts are subject to hydrolysis, the corresponding metal hydroxides are insoluble in solution. In some alkaline baths, absorption of CO2 from the air would precipitate the metal compounds in solution, unless a CO2 acceptor was present such as NaOH. Another feature in a plating solution is compounds that act as a buffer. pH is critical in neutral pH ranges of 5-8. pH is not critical in plating solutions with high and low pH. The last feature in a plating bath is compounds to aid in dissolving anodes to replenish metal concentrations without having to add Zinc. 1.2 Electroplating Deposition Mechanisms: It is hypothesized that electro deposition occurs in two phases. The first phase involves initiation of a few metal atoms adhering to the substrate. This phase has been proposed to occur by two generally accepted mechanisms. The first mechanism can occur by two pathways4. The mechanism will be determined by the inhomogeneity of the surface. The first of these mechanisms is the Step-Edge Ion-Transfer. An M-adion is an ion adsorbed onto a surface. The first case “ involves a direct transfer to the kink site, as portrayed by Figure 3; the M-adion is in the half crystal position, where it is bonded to the crystal lattice with one half of the bonding energy of the bulk ion, thus the M-adion belongs to the bulk crystal, but it is still hydrated.” The second pathway involves a transfer to the step-edge site and diffuses along the step-edge until it reaches a kink as portrayed by Figure 4. However, in both cases the M-adion is incorporated into the metal crystal lattice. 10 Metal Surface Figure 3. Step Edge Ion Transfer Figure 4. Step Edge Transfer and Diffusion The second proposed mechanism is a Terrace Ion-Transfer Mechanism, 4 as portrayed by Figure 5. The metal ion is transferred from the solution to the flat face of the terrace region. At this position, the metal ion is in the adion state having almost all the water of hydration. The M adion goes to lower energy and diffuses into the kink site. Metal Surface Figure 5. Terrace Ion-Transfer 11 Following this initial first phase is a second phase where crystal growth occurs from the few initially adhering atoms. Two basic mechanisms exist to explain the growth mechanism of plated deposit; Layer growth and three dimensional crystallite growths. In a “layer growth mechanism a crystal enlarges by a spreading of discrete steps one after another across a substrate.” Several growth forms can be made from this method including columnar, whiskers and fiber texture. The second method involves formation of “isolated nuclei and their growth to three dimensional crystallites and then the coalescence of the crystallites then a formation of the linked network and then a formation of a continuous deposit.” 12 CHAPTER II ZINC PLATING PROCESSES 2.1 Cyanide Zinc Plating For plating zinc onto a substrate, the cyanide Zinc plating process was the first commercially available zinc plating chemistry. However, due to strict environmental regulations, the use of “cyanide” zinc plating has decreased exponentially over the years being replaced by other processes. Nevertheless, a brief description of the chemistry of the process is warranted. In the “cyanide” process the reduction of Zn2+ ions occurs by the following this series of reactions.5 The main bath constituents that make the reduction of Zn possible are stated in Table 4. [Zn(CN)4]2- + 2OH- [Zn(OH)2] + 4CN- (6) [Zn(OH)2] + e- [Zn(OH)2]- (7) [Zn(OH)2]- Zn(OH) + OH- (8) ZnOH + e- Zn + OH- (9) Table 4. Alkaline Cyanide Zinc Plating Bath Constituents Chemical Regular (g/L) Mid (g/L) Low (g/L) Zn(CN)2 60 30 10 NaCN NaOH Na2CO3 40 80 15 20 75 15 8 65 15 NaxSy 2 2 - Brightener 1-4 1-4 1-4 12 A key advantage of utilizing this process over the others is the capability of zinc plating parts with low current density areas such as tubes. The reason this process works well is because it has higher throwing power than the other two processes. Another significant issue with the cyanide zinc process is a problem known as Hydrogen Embrittlement11. Only the chloride process seems not to be plagued by Hydrogen Embrittlement and this latter process will be discussed in detail later. Hydrogen Embrittlement is a process in which high strength steels become brittle and fractures after exposure to hydrogen. The introduction of hydrogen to a plating bath is accomplished with the following equation, which occurs at the cathode. 2H+ + 2e- H2 Two proposed models exist to explain the hydrogen absorption onto a metal substrate. Both models incorporate the idea that atomic hydrogen adsorbs onto the surface of the metal substrate, and diffuses into the metal, resulting in brittleness of the metal. The first model suggests that the intermediate stage through which electrolytic hydrogen passes on entry to the metal substrate is the adsorbed state (MHads)) and is identical to hydrogen evolution mechanism. The reaction sequence is as follows below: The first step introduces hydrogen into solution as H3O+ which then adsorbs onto the metal surface (MHads), releasing water as a byproduct as shown below. H3O+ + M + e- MHads + H2O The adsorbed hydrogen then diffuses through the metal surface, forming a metal-hydrogen complex (MHabs) as depicted below. 13 MHads MHabs Adsorbed hydrogen still on the surface of the metal will be released under equilibrium controls, in which hydrogen evolution occurs as shown below. MHads + MHads H2 + 2M The second model suggests the atomic hydrogen enters the metal lattice the same way it is discharged, and that the intermediate stage in which hydrogen enters the metal lattice is not identical to the adsorbed hydrogen mechanism, which results in hydrogen evolution. The first step depicts hydrogen absorbing through the metal surface (MHabs). H3O+ + M + e- MHabs + H2O At the same time hydrogen is adsorbing to the metal surface as shown below. H3O+ + M + e- MHads + H2O Both mechanisms end with the same result in which hydrogen evolution occurs as shown below. MHads + MHads H2 + 2M Secondly, this process has decreased cathode efficiencies compared to the others, which leads to higher plating times. Lastly, the costs of alkaline cyanide chemical process are steep, resulting from the waste treatment needed to reduce cyanide. Cyano-complexes are used to plate Cu, Cd, Au, Ag, Zn and In. All cyano plating solutions are alkaline in nature. If acid is added to a cyanide plating solution, poisonous cyanide gas would be produced. One exemption is the cyano-gold [cyanoaurate (I)] complex, which is stabilized in low pH solutions. 14 Hydrogen embrittlement may occur as a result of acid pickling, electroplating, and aqueous corrosion, which involve the discharge of hydrogen ions. One key way to remove atomic hydrogen is derived from its mobility at high temperatures. A bake cycle is employed between the temperatures of 350-400◦F. This allows the diffusion of the hydrogen out of the metal or metal-alloy. 2.2 Acid Chloride Plating Zinc may be plated from several types of acid solutions, based on zinc sulfate or zinc chloride complexes. The acid chloride process is relatively new in comparison with the alkaline cyanide and alkaline non-cyanide zinc plating processes. Acid Chloride process has rapidly changed the zinc plating industry and constitutes about 50% of all zinc baths in most developed nations. Cast iron, malleable iron, and carbonitrided are readily plated in acid zinc plating processes and not the others. The reduction of Zn2 at the cathode occurs in the following manner5. ZnCl2 + 2KCl K2ZnCl4 (10) K2ZnCl4 2K+ + ZnCl4 (11) ZnCl2 Zn2+ + 2Cl- (12) The first advantages for selecting the acid chloride process is the high cathode efficiencies, resulting in less side reactions and faster plating times. Secondly, minimal waste treatment is needed dependent on the Acid Chloride process selected as depicted by Table 5. The only chemical that can cause waste treatment issues is an abundant amount of NH4Cl. 15 A disadvantage of this process is the corrosive nature of the chemical used, resulting in solution laying in recesses, which could be detrimental to the coating if proper rinsing procedures are not followed. Three types of chloride baths exist; all NH4Cl, mixed NH4Cl / KCl, and all KCl. Each has a distinct advantage over the other; however the mixed NH4Cl / KCl bath gets the best of both worlds. The proprietary ingredients in zinc plating solutions include Carrier Brighteners, Primary Brighteners, and surfactants/carriers. Primary Brighteners reduce the roughness of zinc plated surface to maximize optical reflecting power (ammonium bisulfitezetaplus maintenance). These brighteners tend not to be highly soluble in solution. Carrier Brighteners keep Primary Brighteners from crashing out of solution (Sodium Benzoate-Zetaplus Merit Make-up). Most chloride systems contain at least 2 primary brighteners and 4-8 surfactants. Surfactants are compounds that lower the surface tension of a liquid and a solid, which allow the Primary and Carrier Brighteners to reach the surface of the substrate easily. Table 5. Acid Chloride Zinc Plating Bath Constituents5 Chemical All NH4OH (g/L) All KCl (g/L) Mixed Bath-KCl (g/L) Mixed Bath-KCl (g/L) Zn 15-30 22-38 15-30 15-30 NH4Cl 120-180 - 30-45 30-45 KCl - 185-225 120-150 - NaCl - - - 120 H3BO3 - 22-38 - - Carrier brightener 4% b/v 4% b/v 4% b/v 4% b/v Primary brightener 0.25% b/v 0.25% b/v 0.25% b/v 0.25% b/v 16 2.3 Alkaline Non-Cyanide Plating The last process which bears some discussion is the alkaline non-cyanide process which still finds current use in Industry today. The alkaline non-cyanide zinc plating process is a reliable, cost efficient method, similar to that of the acid chloride zinc plating process. The electrodeposition reaction of plating zinc on a steel substrate occurs in the pathway shown below.5 [Zn(OH)4]2- [Zn(OH)3]- + OH- (13) [Zn(OH)3]- + e- [Zn(OH)2] + OH- (14) [Zn(OH)2]- Zn(OH) + OH- (15) ZnOH + e- Zn + OH- (16) The first drawback of an alkaline non-cyanide process is the presence of high levels of carbonates found in solution. Carbonate build-up occurs in solution due to elevated levels of CO2 entering the solution by the following reaction. 2NaOH + CO2 Na2CO3 +H2O (17) Carbonates increase with an increase in solution agitation and solution temperature, which leads to a decrease in solution conductivity, thus hindering the electrodepostion process. The maximum amounts of carbonates allowed in solution range from 50-100 g/L which is customary with absorption of CO2 from air. A number of methods exist to crash carbonates out of solution. The first technique is to cool the solution to 5-10°C, until the carbonates freeze, then the carbonates can be filtered out of solution. A less frequent method employed is the precipitation of carbonates by calcium hydroxide. 17 Table 6. Alkaline Non-Cyanide Bath Constituents Chemical Range (g/L) Zn metal 6.0-17.0 NaOH 75-112 Additives As recommended by Manafacturer The three processes that were presented; cyanide, acid chloride, and alkaline noncyanide require other additional conditions in order to be successful. A comprehensive discussion of all the conditions required for each process is beyond the scope of this paper. Table 7 provides a condensed version of some of the specific requirements for each process. For example, the acid chloride process required air agitation while the others do not. Air agitation would be detrimental to the zinc coating for the cyanide and alkaline non-cyanide processes due to CO2 being produced with air agitation. Table 7. Miscellaneous Requirements Requirements Anode Polarization Conduct. of Bath Sol’n Air Agitation Heating Required Filtration Required pH Adjustment Req’d Purifier needed to treat impurities Chromate Receptivity Waste Treatment Iron treatment by Oxid. Acid Chloride Zinc Alkaline NonCyanide Zinc Cyanide Zinc Rarely Excellent Yes Dependent on C.D. Yes Fair Required Required Yes Yes No Not required Required Yes No Yes Not required Required No No Yes Good Simple Yes Dependent on C.D. Simple No Excellent Complex No 18 Furthermore, the acid chloride solution has greater conductivity due to KCl. The conductivity of the alkaline non-cyanide plating solution is poor at low current density regions and excellent at high current density regions. pH adjustment of the acid chloride process solution is required to keep Fe2+ in solution. However, the initial plating solution is in need of oxidation treatment with 30% H2O2, followed by filtration to discard any Fe2+ impurities in KCl, ZnCl2, and NH4Cl. 2.4 Zinc-Alloy Plating Recent demands for higher quality finishes, more specifically, longer lasting coatings, have lead into a newer process known as zinc-alloy plating. The most notable zinc alloying elements are Iron, Cobalt, Nickel, and Tin. Two metals may be plated simultaneously if the potentials are similar, and if the ionic activities of two dissimilar metals are different. Alloys cannot be deposited unless the activity of the more noble metal ion in a solution is greatly decreased by a stable complex formation. The current density vs potential graphs will look similar for alloying elements as depicted by Figure 6. As stated earlier, the single deposition potentials are similar, but the ionic activities must be different in order for simultaneous deposition to occur. So, M1 and M2 may be plated simultaneously. Zinc-nickel alloys4 can be plated from acid or alkaline non-cyanide solutions. Typically, the acid solution provides a nickel content of 10-14% while the alkaline non-cyanide solution provides 5-8% nickel or 10-17% nickel content. The corrosion resistance increases as nickel content increases to 17%, after this level the zinc-nickel deposit becomes nobler than the substrate, losing its corrosion properties. The zinc-nickel alloy plating process is a quite bit more 19 expensive than all other zinc-alloy plating processes, however the added corrosion resistance more than makes up for the increase in cost. Zinc-Cobalt alloys are employed as acid chloride solutions with little or no ammonium present. The cobalt content is generally 1%. The acid chloride bath has increased cathode efficiencies and plating speeds. Figure 6. Simultaneous Metal Deposition. 20 CHAPTER III ACID CHLORIDE PROCESS STEPS A series of zinc plating steps is required to successfully apply a metallic coating to a steel substrate. Figure 7 depicts a typical zinc plating sequence. Each chemical process shown will be explained in detail with the mixing procedures. Figure 7. Acid Chloride Process Steps. 21 3.1 Chemicals and Equipment All equipment and chemicals were purchased by H&W Global Industries. Almost all chemicals were purchased through Trichem Technologies. All necessary equipment for a pilot scale zinc plating line including Industrial Tanks (Polypropylene and SSTL), Heaters, Filtration Systems, Air Agitation lines, Rectifiers, Fume Hoods, Cranes, Racks, Load bars, Copper Cathodes, Zinc Anodes, and Anode baskets were provided by H&W Global Industries. Furthermore, a Kocour 267 mL Hull Cell with built-in heater and air agitation was purchased by H&W Global Industries. Also, commodity chemicals such as Hydrochloric acid and Nitric Acid were purchased from Interstate Chemicals in large quantities. All titration reagents such as Ammonium Hydroxide, Ammonium Chloride, Sodium Cyanide, 10% Formaldehyde, 0.153 N Silver Nitrate, and Eriochrome Black T Indicator were purchased through Trichem Technologies without further purification. 3.2 Alkaline Cleaning and Electrocleaning Cleaning Variables include Time, Temperature, Concentration, pH, and agitation. A cleaning process needs to balance these variables to obtain optimal cleaning at a reasonable cost. For example, longer time of exposure to the cleaning solution will typically produce a cleaner surface. But the customer may only have limited time in which to clean. Likewise, higher temperatures usually help clean better. But higher temperature will increase customer energy costs, and can damage some substrate. This is true as well for cleaner bath concentration. A more concentrated cleaning bath may remove soils better, but will add to chemical costs, and strongly alkaline or strongly acidic chemicals may attack the substrate. At higher concentrations, cleaning chemicals may be more difficult to rinse off and present a 22 higher risk of contaminating subsequent stages. Spray application cleans better but may not get into complicated areas of fabricated metal parts. Immersion application can penetrate complicated shapes but has less mechanical energy acting on the soils. Chemical cleaners can be divided into solvent-based and aqueous. Solvent-based cleaners can be chlorinated hydrocarbons, glycol ethers, alcohols, etc. Due to VOC and safety issues, the popularity of solvent-based cleaners is diminishing, although for organic soils and greases they can be highly effective. Aqueous cleaning can be further divided into acid cleaning, neutral cleaning and alkaline cleaning. Acid cleaners are good at removing oxides and corrosion products, but can attack and dissolve the substrate, so they have limited use. Cleaners dedicated for aluminum are frequently acidic. Neutral cleaners are generally mild and soap-like; they don’t usually attack the base substrate, due to their neutral pH, but this also makes them less aggressive to soils. Alkaline cleaning is the most popular type of cleaning. Like acid cleaning, however, they can attack certain substrates, particularly zinc and aluminum. Other methods include vapor degreasing, sand blasting, electrocleaning, and ultrasonic cleaning. Blasting removes bulk soils on the surface, however care must be taken so no substrate contamination occurs. Aqueous cleaners contain one or more soap-like chemicals6, known as “surfactants” which include Anionic, Cationic, and Non-ionic compounds, referred to in Table 8. In addition, they usually contain other materials known as “builders”; these can be phosphates, silicates, hydroxides, carbonates, or acids, which form insoluble compounds with metals that would adversely affect the electroplating process such as Iron. Many aqueous cleaners will also contain small amounts of solvents, such as glycol ethers. 23 Alkaline cleaner’s work in a series of steps6: 1) Displacement, which removes soils from the part physically. 2) Emulsification and Dispersion, which chemically absorbs soils into solution and disperses the soil throughout and prevents re-depositing, back onto the part. 3) Saponification, which makes a chemical “soap” out of organic acids in the oils and caustic from the cleaner. 4) Dissolution, which removes a small layer of metal oxides by the alkaline strength of the cleaner. Table 8. Surfactant Composition6 Anionic (ionic) (Carboxylic Acids and Salts, Sulfuric Acids Derivates, Sulfonic Acids and Salts, Phosporic Acids Esters and Salts and, Acylamino Acids and Salts) Negatively charged. Oldest and most common surfactant. Excellent dispersive action. Sensitive to water hardness ions. Produced in high volumes. The majority are inexpensive. Used in most detergent systems. Sulfates and sulfonates are common anionic surfactants. Cationic (ionic) Positively charged. Prompt to change surface properties making a hydrophilic surface act as a hydrophobic and vice (Alkyl Amines, Alkylimidazolines, versa. Poor dispersive action. Used as germicides. Great Quaternary Ammonium emulsifying capacity. Used mostly as fabric softeners. Compounds, Ethoxylated Alkyl Amines and, Esterified Quaternaries) Amphoteric (ionic) Can act as cationic or anionic detergents, depending on the pH of the solution. Work best at neutral pH. Used in (Acyl Ethylenediamines and combination with Anionic or Cationic surfactants to enhance Derivatives, N-Alkyl Amino Acids certain properties (foam or detergency). Commonly used in or Imino Acids) personal care products (shampoos, foam baths, etc). Electrocleaning chemistries work the same way as alkaline cleaning chemistries, except electrocleaning processes tend to create much more foam, formed by electrolysis, which is increased by solution agitation. Certain compounds are placed in the tank to lessen the amount 24 of soap bubbles formed. Some cleaner chemistry allows the alkaline cleaner and electrocleaner to be used interchangeably, which saves time, space and money. Two methods of electrocleaning exist, anodic and cathodic. Oxygen evolution occurs with anodic electro cleaning which helps loosen any particles that may still be on the surface. Hydrogen evolution occurs with cathodic electro cleaning which consequently leads to hydrogen embrittlement issues. A quick method to determine if a part is clean is by the water break test. A mist of water is sprayed onto part and a visual check is done. If water beads on the surface, then the part fails the water break test and must be re-cleaned. Water beading means oil is still present on the surface. If the water forms a continuous streak then the surface of the part is deemed to be a water break free surface. Surface Oxides that form on the surface of different metals in cleaners are pH dependent. A common example is a steel part, in which cleaners operate within a pH window of passivation of 2-14. Therefore, highly alkaline chemicals may be used, such as NaOH as shown by Figure 8. Aluminum cleaners cannot use caustic chemicals in their formulation, because the part would corrode, in reference to Figure 9. However, less caustic chemicals may be used, including carbonates, or acids.6 25 Figure 8. Pourbaix diagram of Iron at 25˚C. Figure 9. Pourbaix diagram of Aluminum at 25˚C. 3.3 Acid Pickling7 After cleaning the part to be plated, next step in the pre-plating process is acid pickling. Acid pickling is an important step that removes oxides and rust, as well as activating the surface for plating. Pickling activates the surface by neutralizing and solubilizing the residual alkaline films and micro-etching the surface1. Several acids can be used for pickling, depending specifically on the substrate. Sulfuric acid and Hydrochloric acid are predominantly used for 26 acid pickling. The concentration of acids can vary as long as the tanks are made of appropriate materials. Pickling tanks require no heat, because they work well enough without them. The last parameter for pickling is immersion times. The immersion times differ from 30 seconds to 5 minutes. Lower immersion times are used when no rust is on the surface of the part, and higher immersion times are used for parts that have elevated amounts of rust present. The mixing procedure for creating a pickling bath was as follows. Approximately 50% of the tank was filled with DI water, then 48.75 gallons of 20 degree Hydrochloric acid was added, which equated to 25% by volume hydrochloric acid. The tank was filled to the operating range with DI water. Tank is now ready for production. 3.4 Acid Chloride Zinc Plating Tank Schematic: A schematic of a plating tank is shown below. Several key factors that need examined for a plating tank; include A) tank design B) tank material C) solution agitation D) filtration E) cathode/anode placement F) heating elements, G) source of DC current and H) Plating racks. 27 G D F B E C Figure 10. Tank Design. A. Tank Design Tank design is the most important part of development of a plating line. Tank design parameters include size, location, and set-up of plating tanks. The size of the plating tank is beneficial in determining the amount of plating that can be achieved in an 8 hour shift. For instance, smaller plating tanks are adequate for running small batches of parts, however if the parts are large in size, then less can be plated at a time, which leads to longer lead time for customers. The area of the plating tank is also important in determining the type of parts that can fit. If the plating tank 8’ X 2’ then only long parts can fit in the tank, and if the plating tank is 6’ X 4’ then only wide parts can fit in the tank. The size of the tank is based on customer needs, which is why each plating facility has different size specifications. The next important step in the development of a zinc plating tank is the design, which includes location of anodes, 28 cathodes, air lines, filter pump, and heater. Plating tanks can be designed in a variety of ways, but the wrong design can lead to devastating results. For instance, if the anodes are placed too far apart in the plating bath, then the coating thicknesses of the parts will differ greatly from one end to the other. If the heater is not large enough to heat the solution, then it will take more time to operate heat up the plating solution. B. Tank Material Tank material is selected by two criteria. A first criterion is the pH of solution being used, for example, alkaline or acidic. Polypropylene tanks can withstand acidic solutions, but can only withstand alkaline solutions to a certain degree. A second criterion is the temperature of the solution, for example SSTL tanks cannot withstand excessive temperatures, whilst polypropylene tanks can. A mixture of these two criteria will afford the correct tank material. The two most notable tank materials are polypropylene and SSTL 302 or 316 series. Figure 11 below depicts the two types. The left is polypropylene and the right is a SSTL series. In this case of zinc plating, a polypropylene tank is the best choice. Stainless Steel tanks have the chance of stray currents which affect the plating process and could be a potential danger. Figure 11. Polypropylene tank (left) and Stainless Steel tank (right). 29 C. Solution Agitation The main objective of solution agitation is to replenish ions to the substrate by one of two ways; air agitation or mechanical agitation. Most facilities utilize air agitation because of its simplicity and low cost. The air agitation lines in Figure 10 give good solution agitation close to the substrate. Size and spread of holes in air agitation is important for size of bubbles (want small, large quantities of bubbles). The specific gravity of solution, chemical, and temperature play key roles in determining air agitation requirements. The specific equations used to determine air agitation holes and spread are beyond the scope of this paper. D. Filtration The purpose of filtration is to discard any unwanted material present in the plating bath. Unwanted material can unknowingly deposit on the part causing adhesion failures. The selection of filtration pump systems is selected by two variables; solution turnover and filter size. Solution turnover refers to the number of gallons of solution filtered per hour. A larger filter pump is required for larger tanks and vice versa. Filter size refers to the micron rating of filters. Micron ratings can range from 5 microns – 200 microns. Most plating solutions will utilize this micron range, however larger micron ratings are not as effective as removing metallic impurities as smaller micron ratings. The type of material being filtered must be known to determine the type of filtration required. E. Cathode/Anode Placement The type of anode material is indicative of the metal being plated. First and foremost the anode completes the electrical circuit and introduces current into the plating bath. In the instance of zinc plating, titanium baskets work to hold the anodes. The anode material is high 30 purity zinc ball anodes which replenish the plating bath, eliminating zinc metal additions, when a current is applied. F. Heating Element Most plating tanks require heat to speed up plating operations. The # of kilowatts required for the heater is based on the chemical composition, required temperature of solution, ambient temperature at which the tank will be used, total cubic feet of tank, total # gallons, and heat up time desired, as shown by Eq 18, however a surface temperature loss factor must be added. The surface temperature loss factor is based on the temperature of the plating solution, which can be found in any engineering handbooks. The equation below is the example calculation for determine kilowatts of heating a solution of water. K = A x 1.0* x 8.35** x B (18) 3412 x C A = Total gallons of solution B = Temperature difference between ambient temperature and expected temperature C = # of hours required to heat up solution 1.0* = Specific heat of water or chemical solution 8.35** = Specific weight of water or chemical solution After the # of kilowatts is determined, the correct heater can be purchased. G. DC current A direct current is required for the plating operation to succeed. The tool that converts an AC current to a DC current is a rectifier. A rectifier comes in a wide range of Amps and Volts, and is chosen based on the size of the plating tank and the total surface area that can be plated and type of plating solution used. As mentioned earlier by Eq 2, the amount of Amps can be 31 determined by the surface area and current density. In this way, the size of the rectifier can be determined. After the size of the rectifier is found, the + feed must be connected to the anodes, and the – feed must be connected to the cathode, as shown in Figure 10. H. Plating Racks Next, size and types of racks are important to prohibit solution entrapment and for cost efficiency. The rack material is important, because racks could be a potential source of contaminants in plating baths if the right material is not chosen correctly. Zinc plating racks can be Steel or Stainless Steel. Not only are tanks and materials needed for the zinc plating tank, but every step in the process has different requirements. Zinc Plating Tank Mixing Procedure15 Before adding chemicals to the polypropylene tank, a pretreatment of the plating tank was necessary. The tank was cleaned and leeched with 1-2% hydrochloric acid for 2-4 hours, and then cleaned with DI water. The plating tank was then filled with water approximately to 75% of its final volume. While stirring, 63.4 pounds of ZnCl2 was added, this equates to 39.0 grams per liter. After the ZnCl2 was dissolved, 271.9 pounds of KCl was added, this equates to 167.3 grams per liter. Next, while stirring, 84.1 pounds of NH4Cl was added, this equates to 51.75 grams per liter. All chemicals were completely dissolved in solution. The bath was then diluted to approximately 90% of its final volume with cold water, and mixed to ensure uniform composition of the solution. The pH of zinc plating solution was adjusted to 5.0 – 5.3. Next, 737.1 milliliters of 30% H2O2 was added, which was 0.1% of the bath concentration. The purpose of H2O2 was to oxidize any iron impurities that were present in the Potassium Chloride. The solution was filtered for 3-4 hours to remove the oxidized iron. Next, 530 mL 32 Zetaplus Merit Maintenance was added and mixed into solution. The purpose of Zetaplus Maintenance is to brighten the zinc deposit. Then, 9.75 gallons of Zetaplus Merit Make-up was added to solution. The purpose of the Zetaplus Merit Make-up is too solubilize the brightener in solution. The bath was diluted to its final volume and mixed well. The solution was ready for production use. 3.5 Acid Dip / Chromates Directly after the plating step, a brown blume is present on the parts, which decreases the brightness of the parts. A post plating nitric dip is required to remove the brown blume, resulting in an increase in deposit brightness. The nitric acid step not only removes the brownish tint to the coating, it also lowers the pH of the coating to activate it for the chromate/passivate. The scope of this research was based on the use of the trivalent passivates. The use of hexavalent chromates is beyond the scope of this paper, however a little detail on the matter is warranted due to its importance in the past. Acid Dip Tank Mixing Procedure The last tank needed for the zinc plating line is the nitric acid dip tank. Add 75% DI water to tank and add 185.2 mL of 42◦ nitric acid. Add the final volume of DI water to tank. Tank was ready for production. Clear Passivate Tank Mixing Procedure The next tank that required chemicals was the clear passivation step9. Approximately 50% of DI water was added to the tank, and with constant agitation 15% of Lanthane 316 or 33 29.25 gallons was added. Lanthane 316 is composed of Cr3+ complexes and cobalt which increase the corrosion resistance of the zinc deposit. The final operating level was filled with DI water, and the pH was adjusted to 2 with dilute Nitric acid or Sodium Hydroxide. The temperature of the solution was verified to be within the operating range. The clear passivation tank was ready for production. Yellow Passivate Tank Mixing Procedure The next tank was the yellow passivation tank9. Approximately 50% DI water was added to tank, and with constant agitation, 10% by volume of Lanthane 316 or 19.5 gallons was added. 0.5% of Tri-Yellow Dye or 0.975 gallons was added. DI water was added to fill solution level to optimum operating Hydrogen embrittlement may occur as a result of acid pickling, electroplating, and aqueous corrosion, which involve the discharge of hydrogen ions. The hydrogen is then chemisorbed onto the metal surface, and if the hydrogen is not evolved as a molecular product, can enter the metal. One key way to remove atomic hydrogen is derived from its mobility at high temperatures. A bake cycle is employed between the temperatures of 350-400◦F. This allows the diffusion of the hydrogen out of the metal or metal-alloy. 3.6 Waste Treatment Management The waste treatment system is an important part of the surface finishing industry. Many state and government regulations exist to limit the amount of harmful substances being introduced into the environment. Three major factors influence the size, complexity, and cost of conventional wastewater treatment systems. The type of pollutant substance plays a key 34 role in the complexity of the wastewater system. Hexavalent chromium reduction and cyanide oxidation complicate the water treatment. The second factor that influences conventional wastewater systems is the size of the plant. The use of larger tanks and more chemical processes would require bigger set-ups, furthermore the water flow rate and volume of water treated plays a huge impact on the size of the set-up. The last factor is strict environmental regulations. Facility location and environmental laws governing that area will ultimately affect the type of wastewater treatment system. The main purpose of the waste treatment system at H&W Global is to limit the amount of metals that get introduced into the environment. Wastewater is introduced into the 2000 gallon holding tanks found in Figure 12. After the holding tanks reach their highest capacitance, wastewater is transferred to a smaller 1400 gallon tank where the pH adjustment occurs depicted by Figure 12. The pH of the wastewater must be adjusted to the range of 6.5-8.5, before further steps are taken. After the pH is adjusted, 200 gallons of wastewater at a time is introduced into the semi-automatic flocculent system depicted by Figure 13. The flocculent is then added to the wastewater to bind any metals present and precipitates them out of solution. The wastewater is then transferred on a polypropylene filter media in which water is gravity filtered out and transferred to the final holding tank. The water is now clean and can be placed into the environment or reused. The metal bound flocculent material is placed in the garbage for disposal. Hexavalent chrome is a known carcinogen and must be reduced to trivalent chromium before further processing can occur. Hexavalent chromium can be reduced with SO2 gas, 35 sodium metabisulfite, ferrous sulfate, ferrous chloride, or ferrous hydrosulfide. The reaction of hexavalent chromium and sodium metabisulfite must occur at a pH range of 2.5-3. A pH higher than 4 slows the reaction to impractical limits. Another drawback of the reaction is the noxious acidic vapors. Ferric sulfate will reduce hexavalent chromium near neutral pH, and the ferric ion becomes an excellent coagulant to bind other metals. H&W Global Industries utilizes a proprietary blend primarily made of ferric sulfate. Many plating shops still utilize cyanide in their chemical processes in spite of strict environmental regulations. Sodium hypochlorite or chlorine gas is used to reduce cyanides to carbon dioxide and nitrogen. First stage Cyanide oxidation is carried out at pH of 10.5 or higher to reduce Cyanide to Cyanate. The reaction ceases below pH 9. Second stage Cyanide oxidation is carried out at pH 8.0-8.5 and additional chlorine is added to complete conversion to CO2 and Nitrogen. Second stage cyanide oxidation is rarely used in Industry because most environmental regulations do not require total oxidation of Cyanide. 36 Figure 12: pH Adjustment Tank (Courtesy of H&W Global Industries, inc). Figure 13: Semi-Automatic Waste Treatment System (Courtesy of H&W Global Industries, inc). 37 CHAPTER IV TESTING & ANALYSIS An analysis of the plating process is important to ensure consistent plating results. Several analysis procedures include Salt Spray testing, Hull Cell Analysis, and titrimetric determination of plating bath constituents; including Zinc metal concentration and total chlorides in solution. Salt spray testing is a determination of the corrosion protection of the plated deposit. The Hull Cell gives rapid information on the brightness levels, uniformity, throwing power, and plating bath chemistry. Titrimetric determination of solution constituents gives an idea of how close the concentrations of Zinc and chlorides are to the optimal ranges. Titrimetric determination and Hull Cell Analysis will be explained in detail later. Salt spray testing theory is covered in detail below. 4.1 Salt Spray A very well defined set of tests exist to check the corrosion properties of many finishing processes, including Anodizing, Chemical Conversion Coatings, Passivation, Black Oxide, Zinc Phosphate, Zinc Plating, and paint. The most commonly employed test is the Salt Spray test. Other less common tests include Humidity testing, Acetic Acid Salt Spray, Copper Accelerated Salt Spray, Corrodkote, and Sulfur Dioxide testing. Each test mimics a certain aspect of the environment at an accelerated pace. However, since salt spray testing is used predominantly on coupons, not the parts themselves, it will be explained in depth. 38 If a water droplet rests on metal surface, there exists a difference in the volume of oxygen, available to specimen relative to position of metal surface within the water droplet. At center of drop, metal is in contact with dissolved oxygen in water droplet and with oxygen in OH- due to water dissociation. —Frank Altmayer 12 At edge of drop, additional oxygen from air is available; an [O2] gradient is produced from edge of drop to center. The difference in oxygen creates an oxidation potential of 0.3 V. Center of drop/metal surface become anodic, as metal dissolves from water, leaving behind electrons that flow to the edge of drop. Metal ions form at center of water droplet, and a pit develops depicted by Figure 11. Figure 14. Salt Spray Corrosion Theory. Each chemical process such as Anodizing, Chemical Conversion, and Plating require coupons to be subject to salt spray solution for a given standard amount of hours until first signs of corrosion are seen. Some typical salt spray times are shown in Tables 9 and 10. For example, anodizing requires 336 hours of salt spray (MIL-A-8625F). Zinc plating salt spray hours with a clear chromate conversion coating requires a minimum of 96 hours of salt spray before 39 signs of corrosion occur as stated by Table 9. Several parameters must be followed in accordance with ASTM B11713. The specific gravity, pH, collection rate, chamber temperature, cabinet temperature, and pressure must be recorded daily and kept within range. The salt spray solution must be kept between 4-6%. A benefit of salt spray testing is the troubleshooting capabilities. Problems in the process such as in the pre-cleaning, chemical treatment, and posttreatment steps can be identified and eliminated. If test coupons fail the salt spray test, one variable at a time can be changed until the test coupons pass salt spray. A possible deterrent to the salt spray test is the evident time factor. Anodize test coupons take up to two weeks in the salt spray. If the test fails, all production parts must be recalled until a solution is found and the test coupons re-tested. Table 9. Salt Spray Hourly Requirements IAW ASTM B 633-13 Type I Description As-plated without supplementary treatments With colored chromate coatings With colorless chromate conversion coatings With Phosphate conversion coatings With colorless passivate With colored passivate II III IV V VI Minimum Salt Spray (hours) NA 96 12 NA 72 120 Table 10. Salt Spray Hourly Requirements Chemical Process Salt Spray Hours Anodizing Chemical Conversion Coating Black Oxide Primer 336 hours 168 hours 96 hours 336 hours 40 4.2 Hull Cell Analysis Analysis of any plating bath can be analyzed by what is known as a Hull Cell. A Hull Cell is a miniature version of a plating bath with the same parameters. Most Hull Cells come with equipped air agitation, heat, and timers. A Hull cell is shown in Figure 15, which includes a heat source and air agitation. The Hull Cell was patented by R.O. Hull in 19391. A Hull Cell is described as a “Trapezoidal box of a non-conducting material; an anode is laid against the sloping side, connected to a current source with alligator clips”. A current is passed through the Hull Cell; the current density along the sloping side varies in a known manner. The character of the Hull Cell Panel varies with varying current densities. The highest current density is ascertained when the panel is closest to the Anode. The lowest current density is ascertained when the panel is farthest away from the Anode. A Hull cell ruler is used to determine the plating characteristics for a range of current densities as depicted in Figure 16. Figure 15. A Kocour 267 mL Hull Cell. 41 One end of Hull Cell lined up here One end of Hull Cell line up here Figure 16. Hull Cell Ruler. A Hull Cell can either hold 267 ml or 1000 ml of plating solution. Chemical additions or pH adjustments can be made in the Hull Cell, and then scaled up to be made in the actual plating tank. Two grams of material added to the sample corresponds to a 1 ounce per gallon addition to the plating tank. The current density of at any point on the cathode is represented by Equation 6, where L = length (cm) along the cathode, I = Current in amperes, and the current density can be found in Amps per meter squared, which is converted to Amps per foot squared because of its simplicity in Industry. Eq 19 is not used readily to determine the current density in industry; however a tool that is readily used is a Hull Cell ruler. After the Hull Cell panel is plated, it is lined up along the Hull Cell as depicted by Figure 16, and the current density range is shown at 2 amps. Current Density (A/m2) = 100I (5.102 – 5.24 log L) (19) The Hull Cell produces a deposit that is a true reproduction of the plating chemistry obtained at various current densities within the operating range of a particular system. 42 Specifically, it mimics operational variables in the actual plating bath, such as pH, current density, temperature, and air agitation2. Other plating factors that can be monitored include organic and metallic contamination, addition agents, covering power, and brightness range of plating deposit. Adjustments can be made in a Hull Cell before chemical additions are made to the plating bath. Table 11 shows a typical Hull Cell sequence of steps. A dull, hazy hull cell panel will be evident if no brighteners are added. However, after numerous additions of Zetaplus Merit Make-up and Zetaplus Merit Maintenance (brightener), the Hull Cell panel was found to be bright throughout, including the low and high current density areas. Table 11. Hull Cell Zinc Plating Testing Panel # 1 2 3 4 ZetaPlus Merit Make-up Hull Cell Additions (mL) None 1.5 1.5 1.5 ZetaPlus Merit Maintenance Hull Cell Additions (mL) None None 1.0 1.5 Table 12. Hull Cell Process Sequence Process Time (sec) Temperature (◦F) Strip (HCl) Rinse Alkaline Cleaner Rinse Pickle (HCl) Rinse Zinc Plating Rinse Acid Dip (HNO3) Rinse Chromate Coating Rinse 60 30 300 30 60 30 300 30 30 30 60 30 Ambient Ambient 150 Ambient Ambient Ambient 87 Ambient Ambient Ambient 85 Ambient 43 4.3 Plating Tank Analysis The zinc metal concentration of the plating bath is mostly determined by EDTA titrations. EDTA is particularly valuable as a titrant because the reagent combines with metal cations in a 1:1 ratio. This hexadentate ligand is shown in Figure 17. EDTA is not only valuable because it forms chelates with metal cations, but also that it is stable for titrations, which makes it useful for the analysis of zinc. Figure 17. Structure of metal/EDTA complex. The reaction between zinc and EDTA is mentioned below. As you can see, the formation constant for the complex is very large at 3.2 X 1016, which means this reaction will take place rapidly. Zn2+ (aq) + EDTA4- (aq) Zn (EDTA)2- (aq) (20) Kf = 3.2 × 1016 The actual titration procedure is stated below. 1. Pipette 5 milliliters of plating solution into 250 milliliter Erlenmeyer flask. 2. Add 50 milliliters of DI water 3. Add approximately 25 milliliters of buffer solution to produce pH of 5.15. (To ensure consistency of results, buffers are used to stabilize the pH). a. Buffer solution- 90 grams of NaOAc / 500 milliliters of DI water, 44 b. 15 milliliter of Acetic Acid & Dilute to 1.0 liter 4. Add a small amount of Xylenol indicator to produce violet color. 5. Titrate immediately with 0.0575 M EDTA to a yellow endpoint. 6. Calculate concentration of zinc metal. (oz/gal) The second titration to determine the zinc metal concentration is stated below. 1. Pipette 2 milliliters of zinc plating solution into a 250 milliliter flask and dilute with 100 milliliter of DI water. 2. Add 20 milliliter of Ammonium Hydroxide/Chloride Buffer. a. 53.5 grams Ammonium Chloride b. 10 grams Sodium Cyanide c. 350 milliliters Ammonium Hydroxide d. Diluted to 1000 milliliter with DI water. 3. Add 10 milliliter of 10% Formaldehyde and 0.5 grams of Eriochrome Black T Indicator. 4. Titrate with 0.0575M EDTA to a blue endpoint. 5. Calculation: mLs 0.0575M EDTA X 7.5 = g/L Zinc Metal Total chlorides in the zinc plating bath is important because the conductivity of the solution increases with chlorides, specifically KCl and NH4Cl. Several ways exist to determine the total chlorides in the plating bath, however a precipitation reaction is the most feasible. A specific precipitation reaction is known as the Mohr method, in which sodium chromate is used as the indicator. The specific sequence of reactions is outlined below. 45 Ag+ + Cl- AgCl (s) 2Ag+ + CrO42- (21) Ag2CrO4(s) (22) After all the chlorides have been precipitated as Silver Chloride, the first excess of Silver Nitrate results in the formation of Silver Chromate precipitate, which indicates the endpoint. The actual titration procedure is outline below. 1. Pipette 1.0 milliliter sample of zinc plating solution into a 500 milliliter flask and dilute with 100 milliliters of DI water. 2. Add 10 milliliters of 10% sodium chromate solution. 3. Titrate with 0.153 N AgNO3 to a reddish-brown endpoint. 4. Factor: milliliters of 0.153 N AgNO3 X 7.5 = g/L Total Chlorides 46 CHAPTER V TRAINING METHODOLOGIES AND QUALITY ASSURANCE 5.1 Introduction to Training Methodologies and Quality Assurance The next step after the development of a plating process is the training methodologies and quality assurance. Proper training procedures and quality documents must be implemented for chemical line operators to follow for consistent results. A quality management system must be in place to comply with ISO 9001. This standard is based on a number of quality management principles, which include strong customer focus, the process approach, and continual improvement. Utilization of ISO 9001 helps ensure that customers get consistent, high quality products and services. A big part of ISO 9001 is training. Operators must have procedures to follow to obtain high quality products and services. Two types of documents that control a process are general work instructions and specific work instructions. General Work Instructions take information directly from the process specification; in this case, ASTM B633-13. This standard specification is used across the country as an industry standard for zinc plating. A customer would typically refer to this specification with vital information that includes coating thickness requirements14, type of chromate coating14, and plating times14. Furthermore, it is not feasible for a line operator to look over a specification in great detail due to time restrictions. This time restriction ultimately leads to the creation of General Work Instructions and Specific Work Instructions that follow industry standards. An example of a General Work Instruction includes 1) reference to specification 2) purpose of General Work Instruction 3) General procedure for chemical line 47 operators to follow for a specific plating process, as well as general guidelines that govern a process, which include 4) calculations, and 5) level of quality expected. A more detailed oriented work instruction is referred to as specific work instructions, which clearly define each process step, indicated by Tables 13 & 14. The time, temperature, and amps are clearly defined in each step (if applicable). Sample General Work Instruction GENERAL WORK INSTRUCTIONS FOR ZINC PLATING ON STEEL SUBSTRATES IAW ASTM B633-13 1) Specification 2) Purpose of General Work Instruction Purpose: To electrodeposit zinc coatings on iron or steel parts to protect them from corrosion. General Procedures: 3 1. The work order will specify one of four standard thickness classes and the type of finish required as indicated in Table 1 and Table 2 attached. 2. Work Instructions xxxx are for zinc plating with clear chromate coatings at four varying thicknesses. 3. Work Instructions xxxx are for zinc plating with yellow chromate coatings at four varying thicknesses. 4. After the Work Instruction to be used is selected, the surface area must be determined for the parts and racks to be ran through the production line. The surface area can be measured in square inches and then converted to square feet. 5. A Hull Cell must be run prior to production to verify the Zinc plating bath constituents are correct. 6. Fill out the Amps, Date, Load #, and Immersion Time on paper to develop a chemical addition baseline. (Zetaplus Merit Make-up & Maintenance) Example: 48 X in2 / 144 in2 = ft2 X = wetted surface area in square inches 5. The immersion time in the zinc plating TK-X must be determined by the following equation: Immersion Time = (Desired Thickness x 14.3 x 60 min) / ASF / 0.97 ASF range = 20-40 ASF Desired Thickness = 0.2- 1.0 mil (5-25 µM), as specified on Work Order 6. The total Amperage must be calculated. Example: ASF X ft2 = Amperage 7. The Work Instructions can now be followed. Table 1: Thickness Classes for Coatings per ASTM B-633-13 Service Condition Classification Number & Conversion Coating Suffix Thickness Range in Mils (µM) SC 4 (Very Severe) Fe/Zn 25 1-2 (25) SC 3 (Severe) Fe/Zn 12 0.5-1 (12) SC 2 (Moderate) Fe/Zn 8 0.3-0.5 (8) SC 1 (Mild) Fe/ Zn 5 0.2-0.3 (5) Table 2: Finish Type and Corrosion Resistance Requirements per ASTM B-633-13 Type Description Minimum Salt Spray (Hours) I As-Plated without supplementary treatments NA II With Colored chromate coatings 96 III With colorless chromate conversion coatings 12 4 49 Plating Time Calculations for Various Zinc Coating Thicknesses and ASF Example Calculations: Assume 97% cathode efficiency for acid chloride process. 20 ASF at varying thicknesses: 1. (1 mil X 14.3 X 60 min) / 20 ASF = 42.9 minutes /0.97 = 44.2 minutes 2. (0.5 mil X 14.3 X 60 min) / 20 ASF = 21.5 minutes /0.97 = 22.1 minutes 3. (0.3 mil X 14.3 X 60 min) / 20 ASF = 12.9 minutes /0.97 = 13.3 minutes 4. (0.2 mil X 14.3 X 60 min) / 20 ASF = 8.6 minutes / 0.97 = 8.8 minutes 25 ASF at varying thicknesses: 1. (1 mil X 14.3 X 60 min) / 25 ASF = 34.3 minutes /0.97 = 35.4 minutes 2. (0.5 mil X 14.3 X 60 min) / 25 ASF = 17.2 minutes /0.97 = 17.7 minutes 3. (0.3 mil X 14.3 X 60 min) / 25 ASF = 10.3 minutes /0.97 = 10.6 minutes 4. (0.2 mil X 14.3 X 60 min) / 25 ASF = 6.9 minutes / 0.97 = 7.1 minutes 30 ASF at varying thicknesses: 1. (1 mil X 14.3 X 60 min) / 30 ASF = 28.6 minutes /0.97 = 29.5 minutes 2. (0.5 mil X 14.3 X 60 min) / 30 ASF = 14.3 minutes/0.97 = 14.7 minutes 3. (0.3 mil X 14.3 X 60 min) / 30 ASF = 8.6 minutes/0.97 = 8.8 minutes 4. (0.2 mil X 14.3 X 60 min) / 30 ASF = 5.7 minutes/0.97 = 5.9 minutes 40 ASF at varying thicknesses: 1. (1 mil X 14.3 X 60 min) / 40 ASF = 21.5 minutes / 0.97 = 22.1 minutes 2. (0.5 mil X 14.3 X 60 min) / 40 ASF = 10.7 minutes / 0.97 = 11.1 minutes 3. (0.3 mil X 14.3 X 60 min) / 40 ASF = 6.4 minutes / 0.97 = 6.6 minutes 4. (0.2 mil X 14.3 X 60 min) / 40 ASF = 4.3 minutes / 0.97 = 4.4 minutes Coating Requirements: 5 50 1. Unless specified otherwise by the purchaser, a bright, semi-bright, or dull finish shall be acceptable. Table 13. Sample Work Instruction for Zinc Plating with Type II Colored Chromate Coating (Courtesy of H&W Global Industries, inc) SAMPLE Spec: ASTM B633-Latest Revision Customer: Type: Part #: SC #: Type II (Colored Chromate) SC 1 Zinc Plating 0.2 - 0.3 mil 20-40 ASF WI #: Surface Area: Coating: Qty./ Rack: Thickness: Racks/Load: ASF: Qty./Load: SEQUENCE # TANK SOLUTION 1 5 Alkaline Cleaner 2 5 Electrocleaner 3 4 Electrocleaner Rinse 4 6 Pickling 5 7 Pickling Rinse 6 8 Zinc Plating 7 9 Zinc Plating Rinse 8 10 Acid Dip 9 11 Acid Dip Rinse 10 14 11 12 TIME Dwell Time = Time in solution TEMP ° F Set Point = control setting 5-10 minutes 140 - 180 Set Point: 150 30 sec-2 minutes 140 - 180 Set Point: 150 1-2 minutes AMBIENT 1-5 minutes AMBIENT 1-2 minutes AMBIENT 4-9 minutes 65-95 Set Point: 90 1-2 minutes AMBIENT 30 sec-1 minute AMBIENT AMBIENT Colored Chromate 45 sec-1.5 minutes Optimal: 60 sec 68-86 Set Point: 75 Colored Chromate Rinse 1-2 minutes AMBIENT 15 Compressed Air Dry Varies AMBIENT X REMARKS 1-2 minutes Date: Date Created By: Rev. Date: By: Approved By: 51 REMARKS 15-20 ASF 3-6 Volts Remove all rust on parts-if applicable 20-40 ASF 3-9 Volts Table 14. Sample Work Instruction for Zinc Plating with Type III Colorless Chromate Coating (Courtesy of H&W Global Industries, inc) SAMPLE WI #: Spec: Customer: Type: Part #: SC #: Surface Area: Coating: Qty./ Rack: Thickness: Racks/Load: ASF: ASTM B633-Latest Revision Type III (Colorless Chromate) SC 1 Zinc Plating 0.2 - 0.3 mil 20-40 ASF Qty./Load: SEQUENCE # TANK SOLUTION 1 5 Alkaline Cleaner 2 5 Electrocleaner 3 4 Electrocleaner Rinse 4 6 Pickling 5 7 Pickling Rinse 6 8 Zinc Plating 7 9 Zinc Plating Rinse 8 10 Acid Dip 9 11 Acid Dip Rinse TIME Dwell Time = Time in solution TEMP ° F Set Point = control setting 5-10 minutes 140 - 180 Set Point: 150 30 sec-2 minutes 140 - 180 Set Point: 150 1-2 minutes AMBIENT 1-5 minutes AMBIENT 1-2 minutes AMBIENT 4-9 minutes 65-95 Set Point: 90 1-2 minutes AMBIENT 30 sec-1 minute AMBIENT 1-2 minutes AMBIENT 45 sec-1.5 minutes Optimal: 60 sec 68-86 Set Point: 75 12 Clear Chromate AMBIENT 13 Clear Chromate Rinse 1-2 minutes 11 AMBIENT X Compressed Air Dry Varies 12 REMARKS 10 Date: Date Created By: Rev. Date: By: Approved By: 52 REMARKS 15-20 ASF 3-6 Volts Remove all rust on parts- if applicable 20-40 ASF 3-9 Volts Using the General Work Instruction for reference, a chemical line operator can utilize the calculations given to determine the plating time in the bath, derived by Faraday’s Law. The line operator would not be responsible for determining the plating time using Faraday’s law, but would have to determine the plating time based on the current density. The calculation derived by Faraday’s Law is shown by Eq 23. Where Time (min) = Plating time required for a specific thickness and current density, Factor (F) = Faraday’s Factor which is 14.3 for zinc plating processes, Thickness (mils) = Required thickness in accordance with ASTM B 633, Current Density = Amps/ft2 which varies from 20-40, and C.E = Cathode Efficiency of a specific plating process, which is 97% for an acid chloride process. Plating time calculations for various coating thicknesses within a range of Current Densities are shown in Table 15, as expected, coating thickness increases with plating time. Furthermore, increases in current density, decrease plating times. Time = (Factor (F) x Thickness x 60) / Current Density (ASF) / C.E (23) Table 15. Plating Time Calculations for Various Zinc Coating Thicknesses and ASF Amps/Ft2 (ASF) 20 Plating Thickness (mils) 0.2 0.3 0.5 1.0 Plating Time (min) 8.8 13.3 22.1 44.2 25 0.2 0.3 0.5 1.0 7.1 10.6 17.7 35.4 30 0.2 0.3 0.5 5.9 8.8 14.7 53 40 1.0 29.5 0.2 0.3 0.5 1.0 4.4 6.6 11.1 22.1 The quality assurance aspect of the zinc plating process is ultimately to make a good looking part that is capable of meeting the requirements of ASTM B-633. Figure 18 depicts a zinc plated part with a yellow passivate coating, and Figure 19 depicts a rack of zinc plated parts with clear passivate coatings. Figure 18. Zinc Plating with Yellow Passivate Coating. 54 Figure 19. Zinc Plating with Clear Passivate Coating 55 CHAPTER VI COST ANALYSIS 6.1 Zinc Plating Chemicals The acid chloride zinc process is a relatively low cost plating process15, as compared to other processes such as Nickel plating, Silver plating, and Gold Plating. Zinc is a low cost metal that requires very few supplementary additions. Tables 16 and 17 gives a cost analysis of each chemical and the total cost of all the chemicals. Zinc chloride would only need to be purchased for the initial charge, because the zinc ball anodes replenish the zinc metal ions in solution. Furthermore, ammonium chloride and potassium chloride require seldom additions, because the only loss of chemical is due to drag-out. Constant additions of Zetaplus Make-up and Zetaplus Maintenance are required because the chemicals are plated onto the substrate, which result in a larger chemical use. These chemical prices are for the initial charge only, these costs do not include the maintenance of the plating bath. 56 Table 16. Chemical Costs Chemical Concentration Cost per Pound/Gallon Cost ($) $14.55 Size of Number of Container Pounds/Gallons Needed 5 gal 15 gal $11.57 5 gal 5 gal $57.9 39.0 g/L 51.75 g/L $3.27 $0.69 50 # 50 # 100 # 100 # $327.2 $69.2 167.3 g/L $0.75 55 # 330 # $248.0 15% b/v 10% b/v 0.5% b/v $17.63 $17.63 $30.35 5 gal 5 gal 5 gal 35 gal 20 gal 5 gal $617.0 $352.5 $151.8 NA $1.55 250 # NA $387.6 NA NA NA NA $2429.5 ZetaPlus Merit 5% b/v Make-up ZetaPlus Merit 0.07% b/v Maintenance Zinc Chloride Ammonium Chloride Potassium Chloride Lanthane 316 Lanthane 316 + Tri-Yellow Dye Zinc ball Anodes (99% Purity) Total Cost Table 17. Zinc Plating Chemical Costs Chemical ZetaPlus Merit Maintentance Cost ($) 57.85 Container size 5 Gal ZetaPlus Merit Make-up 72.77 5 Gal Lanthane 316 88.14 5 Gal Tri-Yellow Dye 151.77 5 Gal Zinc Chloride 163.60 50 # Ammonium Chloride 34.59 50 # Potassium Chloride 41.34 55 # Zinc Ball Anodes 387.55 250 # 57 $218.3 Zinc Ball Anodes Cost Make-up: Table 18. Maximum Square Footage for 250 Ibs of Zinc Ball Anodes SC level Plating Thickness (mils) Weight per Area (g/ft2) Maximum Square footage Allowed (ft2) 1 0.2 3.3 34,293.5 2 0.3 5.3 21,405.9 3 0.5 7.93 13,702.4 4 1.0 16.6 6,851.2 Eq 24 is used to determine the weight (g) per square foot of zinc electro deposition. Zinc ball anodes are a cost that must be accounted. The Density of metallic zinc = 7.14 g/cm3, the zinc ball anodes come in increments of 250 Ibs, which costs $387.5. The amount of zinc ball anodes used does not account for plating solution lost due to drag-out. Weight (grams) = Density (g/cm3) X Area (ft2) X Thickness (mil) (24) Where Density = 7.14 g/cm3 = 445 Ib/ft3 = 201,848.44 g/ft3, Thickness = 25 microns (1 mil) = 8.202 X 10-5 ft, Area = 1 ft2, Weight = 16.6 g/ft2. It would take approximately 6,851.2 ft2 plated at 1 mil to use 250 pounds of Zinc ball Anodes as portrayed by Table 18. These results would be included in the total price for running customer parts. 58 CHAPTER VII SUMMARY AND CONCLUSION All three zinc plating processes have pros and cons; however the acid chloride process is perhaps the best in terms of brightness, cost and plating times. Acid chloride processes make up approximately 50% of the three processes. The alkaline cyanide process is still being used today, but is slowly getting phased out, due to strict environment regulations. The alkaline noncyanide process is the cheapest process, because only NaOH is used, however the finish is much duller than the acid chloride process. Also, the cathode efficiencies for the alkaline non-cyanide and alkaline cyanide are low, which leads to competing reactions, resulting in slower plating times. The other aspect of my research was the pretreatment and post-treatment. Each plating process requires a clean surface, or adhesion issues will arise. Also, a post-treatment is a necessity to add corrosion resistance. Each process requires the correct immersion time, concentration, temperature, and pH to be successful. All these key parameters play a pivotal role in operating a zinc plating line to ultimately make a profit. 59 References 1. Lowenheim, F.A. Electroplating; McGraw-Hill: New York, 1978. 2. Lou, H; Huang, Y. “Electroplating” Department of Chemical Engineering, Lamar University, Beaumont, Texas, U.S.A, 2003, pgs 1-10. 3. Schwartz, M. Deposition from Aqueous Solutions: An Overview. Handbook of Deposition Technologies for Films and Coatings - Science, Technology and Applications, 2nd edition. Bunshah, R.F. Ed.; Park Ridge, New Jersey: Noyes Publications, pg 506. 4. Mordechay, S. “Modern Electroplating” Wiley & Sons, 2010. 20-35. 5. Winand, R. “Electrodeposition of Zinc and Zinc Alloys.” John Wiley & Sons, Inc. 2010, 285302. 6. Sylvester, K. Cleaning and Pretreatment. Presented at PPG Industries Coating Tech, Allison Park, PA, June 19, 2014. 7. Rudy, S.F. Picking and Acid Dipping. In Metal Finishing [Online]; Tucker, R., Ed.; Elsevier, Inc: New York, 2012; 110, pp 81-85. http://metalfinishing.epubxp.com/t/12238-metalfinishing-guide-book (accessed April 2, 2014). 8. Altmayer, F. AESF Foundation, Piitsburgh, PA. Zinc plating personal online class, 2013. 9. Lanthane 316 Technical Data Sheet. Coventya. 10. West, D.M.; Holler. J.F.; Crouch, S.R. Chapter 20: A Brief Look at Some Other Electroanalytical Methods. Analytical Chemistry: An Introduction, 7th Ed; Skoog, Douglas A. Thomson Learning, 2000. pp 511-14. 11. Barnoush, A. Hydrogen Embrittlement. Ph.D Dissertation, Saarland University, 2011. 12. Altmayer, F. Critical Aspects of the Salt Spray Test. Plat. Surf. Finish. 1985. pp 36-40. 60 13. ASTM B117-11, Standard Practice for Operating Salt Spray (Fog) Apparatus, ASTM International, West Conshohocken, PA, 2011, www.astm.org 14. ASTM B633-13, Standard Specification for Electrodeposited Coatings of Zinc on Iron and Steel, ASTM International, West Conshohocken, PA, 2013, www.astm.org 15. Zetaplus Merit Technical Data Sheet. Coventya. 61