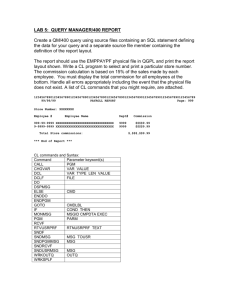
Prior Authorization CPT Look-up
advertisement

*** Any services related to any type of inpatient confinement require authorization *** Any services rendered by a nonpar provider require authorization unless related to emergency services Code Type Code Description CPT 0001F HEART FAILURE ASSESSED CPT 0006M HEPATIC CARCINOMA TUMOR TISSUE MOPATH ASSAY CPT 0007M ONCOLOGY GASTRO 51 GENES NOMOGRAM DISEASE INDEX CPT 0008M ONCOLOGY BREAST MRNA 58 ALGORITHM RISK SCORE CPT 0009M FETAL ANEUPLOIDY 21 18 SEQ ANALY TRISOM RISK CPT 00100 ANESTHESIA FOR PROC ON SALIVARY GLANDS, W/BX CPT 00102 ANESTHESIA, PROC INVOLVING PLASTIC REPAIR OF CLEFT CPT 00103 ANESTHESIA FOR RECONSTRUCTIVE PROC, EYELID CPT 00104 ANESTHESIA, ELECTROCONVULSIVE THERAPY CPT 0010M ONCOLOGY PROSTATE 4 PROTEINS PROBABILITY SCORE CPT 00120 ANESTHESIA, PROC ON EXT, MIDDLE, AND INNER EAR W/B CPT 00124 ANESTHESIA, PROC ON EXT, MIDDLE, AND INNER EAR W/B CPT 00126 ANESTHESIA, PROC ON EXT, MIDDLE, AND INNER EAR W/B CPT 0012F Cap bacterial assess CPT 00140 ANESTHESIA, PROC ON EYE; NOS CPT 00142 ANESTHESIA, PROC ON EYE; LENS SURGERY CPT 00144 ANESTHESIA, PROC ON EYE; CORNEAL TRANSPLANT CPT 00145 ANESTHESIA, PROC ON EYE; VITREORETINAL SURGERY CPT 00147 ANESTHESIA, PROC ON EYE; IRIDECTOMY CPT 00148 ANESTHESIA, PROC ON EYE; OPHTHALMOSCOPY CPT 0014F COMP PREOP ASSESS CATARACT SURG W/ IOL CPT 0015F MELANOMA FOLLOW UP COMPLETED CPT 00160 ANESTHESIA, PROC ON NOSE AND ACCESSORY SINUSES; NO CPT 00162 ANESTHESIA, PROC ON NOSE AND ACCESSORY SINUSES; RA CPT 00164 ANESTHESIA, PROC ON NOSE AND ACCESSORY SINUSES; BX CPT 00170 ANESTHESIA, INTRAORAL PROC, W/BX; NOS CPT 00172 ANESTHESIA, INTRAORAL PROC, W/BX; REPAIR, CLEFT PA CPT 00174 ANESTHESIA, INTRAORAL PROC, W/BX; EXCISION, RETROP CPT 00176 ANESTHESIA, INTRAORAL PROC, W/BX; RADICAL SURGERY CPT 00190 ANESTHESIA, PROC ON FACIAL BONES/SKULL; NOS CPT 00192 ANESTHESIA, PROC ON FACIAL BONES/SKULL; RADICAL SU CPT 0019T EXTRACORPOREAL SHOCK WAVE MUSCULOSKELETAL SYSTEM CPT 00210 ANESTHESIA, INTRACRANIAL PROC; NOS CPT 00211 ANES INTRACRANIAL CRANIOTOMY/CRANIECTOMY HMTMA CPT 00212 ANESTHESIA, INTRACRANIAL PROC; SUBDURAL TAPS CPT 00214 ANESTHESIA, INTRACRANIAL PROC; BURR HOLES CPT 00215 ANESTHESIA, INTRACRANIAL PROC; CRANIOPLASTY/ELEVAT CPT 00216 ANESTHESIA, INTRACRANIAL PROC; VASCULAR PROC CPT 00218 ANESTHESIA, INTRACRANIAL PROC; PROC IN SITTING POS CPT 00220 ANESTHESIA, INTRACRANIAL PROC; CEREBROSPINAL FLUID CPT 00222 ANESTHESIA, INTRACRANIAL PROC; ELECTROCOAGULATION, CPT 00300 ANESTHESIA, HEAD, NECK, POST TRUNK INTEGUMENTARY, CPT 00320 ANESTHESIA, NECK ORGAN PROC; NOS, AGE 1 YR/OLDER CPT 00322 ANESTHESIA, NECK ORGAN PROC; THYROID NEEDLE BX CPT 00326 ANESTHESIA, LARYNX AND TRACHEA PROC; CHILDREN LESS CPT 00350 ANESTHESIA, PROC ON MAJOR VESSELS, NECK; NOS CPT 00352 ANESTHESIA, PROC ON MAJOR VESSELS, NECK; SIMPLE LI CPT 00400 ANESTHESIA, EXTREMITIES, ANTERIOR TRUNK, PERINEUM, CPT 00402 ANESTHESIA, EXTREMITIES, ANTERIOR TRUNK, PERINEUM, CPT 00404 ANESTHESIA, EXTREMITIES, ANT TRUNK, PERINEUM, INTE CPT 00406 ANESTHESIA, EXTREMITIES, ANT TRUNK, PERINEUM, INTE CPT 00410 ANESTHESIA, EXTREMITIES, ANTERIOR TRUNK, PERINEUM, CPT 0042T CEREBRAL PERFUSION ANAYSIS, CT W/CONTRST, POSTPROC CPT 00450 ANESTHESIA, PROC ON CLAVICLE AND SCAPULA; NOS CPT 00454 ANESTHESIA, PROC ON CLAVICLE AND SCAPULA; BX, CLAV CPT 00470 ANESTHESIA, PARTIAL RIB RESECTION; NOS CPT 00472 ANESTHESIA, PARTIAL RIB RESECTION; THORACOPLASTY, CPT 00474 ANESTHESIA, PARTIAL RIB RESECTION; RADICAL PROC CPT 00500 ANESTHESIA, ALL PROC ON ESOPHAGUS CPT 0051T IMPLANT, TOT REPLACEMENT HEART SYSTEM W/RECIPIENT CPT 00520 ANESTHESIA, CLOSED CHEST PROC; NOS CPT 00522 ANESTHESIA, CLOSED CHEST PROC; PLEURA NEEDLE BX CPT 00524 ANESTHESIA, CLOSED CHEST PROC; PNEUMOCENTESIS CPT 00528 ANESTHESIA, CLOSED CHEST PROC, MEDIASTINOSCOPY AND CPT 00529 ANESTHESIA, CLOSED CHEST PROC, MEDIASTINOSCOPY AND CPT 0052T REPLACEMENT/REPAIR, THORACIC UNIT, TOT REPLACEMENT CPT 00530 ANESTHESIA, PERMANENT TRANSVENOUS PACEMAKER INSERT CPT 00532 ANESTHESIA, ACCESS TO CENTRAL VENOUS CIRCULATION CPT 00534 ANESTHESIA, TRANSVENOUS INSERTION/REPLACEMENT, PAC CPT 00537 ANESTHESIA, CARDIAC ELECTROPHYSIOLOGIC PROC W/ RAD CPT 00539 ANESTHESIA, TRACHEOBRONCHIAL RECONSTRUCTION CPT 0053T REPLACEMENT/REPAIR, IMPLANTABLE COMPONENT(S), TOT CPT 00540 ANESTHESIA, THORACOTOMY PROC, W/SURG THORACOSCOPY; CPT 00541 ANESTHESIA, THORACOTOMY PROC, W/SURG THORACOSCOPY; CPT 00542 ANESTHESIA, THORACOTOMY PROC, W/SURG THORACOSCOPY; CPT 00546 ANESTHESIA, THORACOTOMY PROC, W/SURG THORACOSCOPY; CPT 00548 ANESTHESIA, THORACOTOMY PROC, W/SURG THORACOSCOPY; CPT 0054T CPTR-ASST MUSCSKEL NAVIGJ ORTHO FLUOR IMAGES CPT 00550 ANESTHESIA, STERNAL DEBRIDEMENT CPT 0055T CPTR-ASST MUSCSKEL NAVIGJ ORTHO CT/MRI CPT 00560 ANESTHESIA, PROC ON HEART, PERICARD, GRT VESSLS, C CPT 00561 ANESTHESIA, PROC ON HEART, PERICARD, AND GRT VESSL CPT 00562 ANESTHESIA, PROC ON HEART, PERICARD, AND GRT VESSL CPT 00563 ANESTHESIA, PROC ON HEART, PERICARD, AND GRT VESSL CPT 00566 ANESTHESIA, DIRECT CORONARY ARTERY BYPASS GRAFTING CPT 00567 ANES DIRECT CAB GRAFTING W/ PUMP OXYGENATOR Effective Date 1/1/2004 7/1/2014 10/1/2014 10/1/2014 7/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 7/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 10/1/2003 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2009 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2009 Term date 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Is Preauth required Additional Parameters N N N N N N N Y N N N N N N N N Y N N N N N N N N Y N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N Y N N N N N Y N N N N N Y N N N N N N Y N Y Y Y N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 00580 0058T 00600 00604 00620 00625 00626 00630 00632 00635 00640 00670 00700 00702 0071T 0072T 00730 00740 00750 00752 00754 00756 0075T 0076T 00770 00790 00792 00794 00796 00797 00800 00802 0080T 00810 00820 00830 00832 00834 00836 00840 00842 00844 00846 00848 00851 00860 00862 00864 00865 00866 00868 00870 00872 00873 00880 00882 00902 00904 00906 00908 00910 00912 00914 00916 00918 00920 00921 00922 00924 00926 00928 00930 00932 00934 00936 00938 00940 00942 00944 00948 00950 00952 01112 01120 01130 01140 01150 01160 01170 01173 01180 ANESTHESIA, HEART TRANSPLANT/HEART AND LUNG TRANSP CRYOPRSRV REPRDTVE TISS OVARIAN ANESTHESIA, PROC, CERVICAL SPINE AND CORD; NOS ANESTHESIA, PROC, CERVICAL SPINE AND CORD; PROC W/ ANESTHESIA, PROC, THORACIC SPINE AND CORD; NOS ANES: THORACIC, SPINE & C/D ANT APPR W/O 1 LNG VNT ANESTHESIA: THORACIC, SPINE & C/D ANT APPR W/1 LNG ANESTHESIA, PROC, LUMBAR REGION; NOS ANESTHESIA, PROC, LUMBAR REGION; LUMBAR SYMPATHECT ANESTHESIA, PROC, LUMBAR REGION; DIAGNOSTIC/THERAP ANESTHESIA, MANIPULATION, SPINE, CLOSED PROC, CERV ANESTHESIA, EXTENSIVE SPINE AND SPINAL CORD PROC ANESTHESIA, PROC, UPPER ANTERIOR ABDOMINAL WALL; N ANESTHESIA, PROC, UPPER ANTERIOR ABDOMINAL WALL; P FOCUSED ULTRASOUND ABLATION OF UTERINE LEIOMYOMATA FOCUSED ULTRASOUND ABLATION OF UTERINE LEIOMYOMATA ANESTHESIA, PROC, UPPER POSTERIOR ABDOMINAL WALL ANESTHESIA, UPPER GI ENDOSCOPY PROC, PROXIMAL DUOD ANESTHESIA, HERNIA REPAIRS, UPPER ABDOMEN; NOS ANESTHESIA, HERNIA REPAIRS, UPPER ABDOMEN; LUMBAR ANESTHESIA, HERNIA REPAIRS, UPPER ABDOMEN; OMPHALO ANESTHESIA, HERNIA REPAIRS, UPPER ABDOMEN; TRANSAB TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL TCAT PLMT XTRC VRT CRTD STENT RS&IPRQ EA VSL ANESTHESIA, ALL PROC ON MAJOR ABDOMINAL BLOOD VESS ANESTHESIA, INTRAPERITONEAL PROC, UPPER ABDOMEN, W ANESTHESIA, UPPER ABD W/LAPAROSCOPY; PARTIAL HEPAT ANESTHESIA, INTRAPERITONEAL PROC, UPPER ABDOMEN, W ANESTHESIA, INTRAPERITONEAL PROC, UPPER ABDOMEN, W ANESTHESIA, INTRAPERITONEAL PROC, UPPER ABDOMEN, W ANESTHESIA, PROC, LOWER ANTERIOR ABDOMINAL WALL; N ANESTHESIA, PROC, LOWER ANTERIOR ABDOMINAL WALL; P EVASC RPR AAA PSEUDOARYSM ABDL AORTA VISC RS&I ANESTHESIA, LOWER INTESTINAL ENDOSCOPY PROC, DISTA ANESTHESIA, PROC, LOWER POSTERIOR ABDOMINAL WALL ANESTHESIA, HERNIA REPAIRS, LOWER ABDOMEN; NOS ANESTHESIA, HERNIA REPAIRS, LOWER ABDOMEN; VENTRAL ANESTHESIA, HERNIA REPAIRS, LOWER ABDOMEN, NOS, UN ANESTHESIA, HERNIA REPAIRS, LOWER ABDOMEN, NOS, IN ANESTHESIA, INTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, INTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, INTROPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, INTROPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, INTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, INTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, EXTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, EXTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, EXTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, EXTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, EXTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, EXTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, EXTRAPERITONEAL PROC, LOWER ABDOMEN, W ANESTHESIA, LITHOTRIPSY, EXTRACORPOREAL SHOCK WAVE ANESTHESIA, LITHOTRIPSY, EXTRACORPOREAL SHOCK WAVE ANESTHESIA, PROC ON MAJOR LOWER ABDOMINAL VESSELS; ANESTHESIA, PROC ON MAJOR LOWER ABDOMINAL VESSELS; ANESTHESIA, ANORECTAL PROC ANESTHESIA, RADICAL PERINEAL PROC ANESTHESIA, VULVECTOMY ANESTHESIA, PERINEAL PROSTATECTOMY ANESTHESIA, TRANSURETHRAL PROC (W/URETHROCYSTOSCOP ANESTHESIA, TRANSURETHRAL PROC (W/URETHROCYSTOSCOP ANESTHESIA, TRANSURETHRAL PROC (W/URETHROCYSTOSCOP ANESTHESIA, TRANSURETHRAL PROC (W/URETHROCYSTOSCOP ANESTHESIA, TRANSURETHRAL PROC (W/URETHROCYSTOSCOP ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, PROC, MALE GENITALIA(W/OPEN URETHRAL P ANESTHESIA, VAGINAL PROC, W/BX; NOS ANESTHESIA, VAGINAL PROC, W/BX; COLPOTOMY, VAGINEC ANESTHESIA, VAGINAL PROC, W/BX; VAGINAL HYSTERECTO ANESTHESIA, VAGINAL PROC, W/BX; CERVICAL CERCLAGE ANESTHESIA, VAGINAL PROC, W/BX; CULDOSCOPY ANESTHESIA, VAGINAL PROC, W/BX; HYSTEROSCOPY AND/O ANESTHESIA, BONE MARROW ASPIRATION AND/OR BX, ANTE ANESTHESIA, PROC, BONY PELVIS ANESTHESIA, BODY CAST APPLICATION/REVISION ANESTHESIA, INTERPELVIABDOMINAL (HINDQUARTER) AMPU ANESTHESIA, RADICAL PROC, TUMOR, PELVIS, EXCEPT HI ANESTHESIA, CLOSED PROC INVOLVING SYMPHYSIS PUBIS/ ANESTHESIA, OPEN PROC INVOLVING SYMPHYSIS PUBIS/SA ANESTHESIA, OPEN REPAIR, FX DISRUPTION, PELVIS ANESTHESIA, OBTURATOR NEURECTOMY; EXTRAPELVIC 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y N Y Y N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N Y N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 01190 01200 01202 01210 01212 01214 01215 01220 01230 01232 01234 01250 01260 01270 01272 01274 01320 01340 01360 01380 01382 01390 01392 01400 01402 01404 01420 01430 01432 01440 01442 01444 01462 01464 01470 01472 01474 01480 01482 01484 01486 01490 01500 01502 01520 01522 0159T 01610 01620 01622 01630 01634 01636 01638 0163T 0164T 01650 01652 01654 01656 0165T 01670 01680 01682 0169T 01710 01712 01714 01716 0171T 0172T 01730 01732 01740 01742 01744 0174T 01756 01758 0175T 01760 01770 01772 01780 01782 01810 01820 01829 01830 01832 01840 ANESTHESIA, OBTURATOR NEURECTOMY; INTRAPELVIC ANESTHESIA, ALL CLOSED PROC INVOLVING HIP JOINT ANESTHESIA, ARTHROSCOPIC PROC, HIP JOINT ANESTHESIA, OPEN PROC INVOLVING HIP JOINT; NOS ANESTHESIA, OPEN PROC INVOLVING HIP JOINT; HIP DIS ANESTHESIA, OPEN PROC INVOLVING HIP JOINT; TOTAL H ANESTHESIA, OPEN PROC INVOLVING HIP JOINT; REVISIO ANESTHESIA, ALL CLOSED PROC INVOLVING UPPER TWO TH ANESTHESIA, OPEN PROC INVOLVING UPPER TWO THIRDS, ANESTHESIA, OPEN PROC INVOLVING UPPER TWO THIRDS, ANESTHESIA, OPEN PROC INVOLVING UPPER TWO THIRDS, ANESTHESIA, ALL PROC ON NERVES, MUSCLES, TENDONS, ANESTHESIA, ALL PROC INVOLVING VEINS, UPPER LEG, W ANESTHESIA, UPPER LEG ARTERY PROC W/BYPASS GRAFT; ANESTHESIA, UPPER LEG ARTERY PROC W/BYPASS GRAFT; ANESTHESIA, UPPER LEG ARTERY PROC W/BYPASS GRAFT; ANESTHESIA, NERVES, MUSCLES, TENDONS, FASCIA AND B ANESTHESIA, ALL CLOSED PROC ON LOWER ONE THIRD, FE ANESTHESIA, ALL OPEN PROC ON LOWER ONE THIRD, FEMU ANESTHESIA, ALL CLOSED PROC ON KNEE JOINT ANESTHESIA, DX ARTHROSCOPIC PROC, KNEE JOINT ANESTHESIA, ALL CLOSED PROC ON UPPER ENDS, TIBIA, ANESTHESIA, ALL OPEN PROC ON UPPER ENDS, TIBIA, FI ANESTHESIA, OPEN/SURG ARTHROSCOPIC PROC, KNEE JOIN ANESTHESIA, OPEN/SURG ARTHROSCOPIC PROC, KNEE JOIN ANESTHESIA, OPEN/SURG ARTHROSCOPIC PROC, KNEE JOIN ANESTHESIA, ALL CAST APPLICATIONS, REMOVAL/REPAIR ANESTHESIA, PROC ON VEINS, KNEE AND POPLITEAL AREA ANESTHESIA, PROC ON VEINS, KNEE AND POPLITEAL AREA ANESTHESIA, ARTERIES, KNEE AND POPLITEAL AREA; NOS ANESTHESIA, ARTERIES, KNEE AND POPLITEAL AREA; POP ANESTHESIA, ARTERIES, KNEE AND POPLITEAL AREA; POP ANESTHESIA, ALL CLOSED PROC, LOWER LEG, ANKLE, AND ANESTHESIA, ARTHROSCOPIC PROC, ANKLE AND/OR FOOT ANESTHESIA, NERVES/MUSCLES/TENDONS AND FASCIA, LOW ANESTHESIA, NERVES/MUSCLES/TENDONS AND FASCIA, LOW ANESTHESIA, NERVES/MUSCLES/TENDONS AND FASCIA, LOW ANESTHESIA, OPEN PROC, BONES, LOWER LEG/ANKLE/FOOT ANESTHESIA, OPEN PROC, BONES, LOWER LEG,ANKLE AND ANESTHESIA, OPEN PROC, BONES, LOWER LEG/ANKLE/FOOT ANESTHESIA, OPEN PROC, BONES, LOWER LEG/ANKLE/FOOT ANESTHESIA, LOWER LEG CAST APPLICATION, REMOVAL/RE ANESTHESIA, ARTERIES LOWER LEG PROC W/BYPASS GRAFT ANESTHESIA, ARTERIES LOWER LEG PROC W/BYPASS GRAFT ANESTHESIA, PROC ON VEINS, LOWER LEG; NOS ANESTHESIA, PROC ON VEINS, LOWER LEG; VENOUS THROM Cad breast mri ANESTHESIA, NERVES/MUSCLES/TENDONS/FASCIA AND BURS ANESTHESIA, CLOSED PROC, HUMERAL HEAD AND NECK/STE ANESTHESIA, DX ARTHROSCOPIC PROC, SHOULDER JOINT ANESTHESIA, OPEN/SURG ARTHROSCOPIC PROC, HUMERAL H ANESTHESIA, OPEN/SURG ARTHROSCOPIC PROC, HUMERAL H ANESTH, OPEN/SURG ARTHROSCOP PROC, HUMER HEAD AND ANESTHESIA, OPEN/SURG ARTHROSCOPIC PROC, HUMERAL H Lumb artif diskectomy addl Remove lumb artif disc addl ANESTHESIA, PROC ON ARTERIES, SHOULDER AND AXILLA; ANESTHESIA, PROC ON ARTERIES, SHOULDER AND AXILLA; ANESTHESIA, PROC ON ARTERIES, SHOULDER AND AXILLA; ANESTHESIA, PROC ON ARTERIES, SHOULDER AND AXILLA; Revise lumb artif disc addl ANESTHESIA, ALL PROC ON VEINS, SHOULDER AND AXILLA ANESTHESIA, SHOULDER CAST APPLICATION, REMOVAL/REP ANESTHESIA, SHOULDER CAST APPLICATION, REMOVAL/REP Place stereo cath brain ANESTHESIA, NERVES/MUSCLES/TENDONS/FASCIA AND BURS ANESTHESIA, NERVES/MUSCLES/TENDONS/FASCIA AND BURS ANESTHESIA, NERVES/MUSCLES/TENDONS/FASCIA AND BURS ANESTHESIA, NERVE/MUSCL/TENDON/FASCIA AND BURSAE, Lumbar spine proces distract Lumbar spine proces addl ANESTHESIA, ALL CLOSED PROC ON HUMERUS AND ELBOW ANESTHESIA, DX ARTHROSCOPIC PROC, ELBOW JOINT ANESTHESIA, OPEN/SURG ARTHROSCOPIC PROC, ELBOW; NO ANESTHESIA, OPEN PROC ON HUMERUS AND ELBOW; OSTEOT ANESTHESIA, OPEN PROC ON HUMERUS AND ELBOW; REPAIR Cad cxr with interp ANESTHESIA, OPEN PROC ON HUMERUS AND ELBOW; RADICA ANESTHESIA, OPEN PROC ON HUMERUS AND ELBOW; EXCISI Cad cxr remote ANESTHESIA, OPEN PROC ON HUMERUS AND ELBOW; TOTAL ANESTHESIA, PROC ON ARTERIES, UPPER ARM AND ELBOW; ANESTHESIA, PROC ON ARTERIES, UPPER ARM AND ELBOW; ANESTHESIA, PROC ON VEINS, UPPER ARM AND ELBOW; NO ANESTHESIA, PROC ON VEINS, UPPER ARM AND ELBOW; PH ANESTHESIA, NERVES/MUSCLES/TENDONS/FASCIA AND BURS ANESTHESIA, ALL CLOSED PROC ON RADIUS, ULNA, WRIST ANESTHESIA, DX ARTHROSCOPIC PROCEDURES, WRIST ANESTHESIA, OPEN/SURG ARTHROSCOPIC/ENDOSCOPIC PROC ANESTHESIA, OPEN/SURG ARTHROSC/ENDOSCOP PROC, DISTL RADI ANESTHESIA, PROC ON ARTERIES, FOREARM, WRIST, AND 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 01842 01844 0184T 01850 01852 01860 0188T 0189T 0190T 01916 0191T 01920 01922 01924 01925 01926 01930 01931 01932 01933 01935 01936 01951 01952 01953 01958 0195T 01960 01961 01962 01963 01965 01966 01967 01968 01969 0196T 0198T 01990 01991 01992 01996 01999 0200T 0201T 0202T 0205T 0206T 0207T 0208T 0209T 0210T 0211T 0212T 0213T 0214T 0215T 0216T 0217T 0218T 0219T 0220T 0221T 0222T 0223T 0224T 0225T 0228T 0229T 0230T 0231T 0232T 0233T 0234T 0235T 0236T 0237T 0238T 0240T 0241T 0243T 0244T 0249T 0253T 0254T 0255T 0262T 0263T 0264T 0265T 0266T ANESTHESIA, PROC ON ARTERIES, FOREARM, WRIST, AND ANESTHESIA, VASCULAR SHUNT/SHUNT REVISION, ANY TYP RECTAL TUMOR EXCISION TRANSANAL ENDOSCOPIC ANESTHESIA, PROC ON VEINS, FOREARM, WRIST, AND HAN ANESTHESIA, PROC ON VEINS, FOREARM, WRIST, AND HAN ANESTHESIA, FOREARM/WRIST/HAND CAST APPLICATION, R VIDEOCONFERENCED CRITICAL CARE FIRST 30- 74 MIN VIDEOCONFERENCED CRITICAL CARE EA ADDL 30 MIN INTRAOCULAR RADIATION SRC APPLICATOR PLACEMENT ANESTHESIA, DX ARTERIOGRAPHY/VENOGRAPHY ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT ANESTHESIA, CARDIAC CATHETERIZATION W/CORONARY ART ANESTHESIA, NON-INVASIVE IMAGING/RADIATION THERAPY ANESTHESIA, THERAPEUTIC INTERVENTIONAL RADIOL, ART ANESTHESIA, THERAPEUTIC INTERVENTIONAL RADIOL, ART ANESTHESIA, THERAPEUTIC INTERVENTIONAL RADIOL, ART ANES, THERAPEUTIC INTERVEN RADIOL, VENOUS/LYMPHATI ANES, THERAP INTERVEN RADIOL, VENOUS/LYMPHATIC SYS ANES, THERAP INTERVEN RADIOL, VENOUS/LYMPHATIC SYS ANES, THERAP INTERVEN RADIOL, VENOUS/LYMPHATIC SYS ANESTHESIA PERQ IMG GID SPINE DIAGNOSTIC ANESTHESIA PERQ IMG GID SPINE THERAPEUTIC ANESTHESIA 2ND AND 3RD DEGREE BURN EXCISION/DEBRID ANESTHESIA 2ND AND 3RD DEGREE BURN EXCIS/DEBRIDEME ANESTHESIA 2ND AND 3RD DEGREE BURN EXCISION/DEBRID ANESTHESIA, EXTERNAL CEPHALIC VESION PROC ARTHRODESIS, PRE-SACRAL INTERBODY TECHNIQUE, DISC SPACE PREPARATION, DISCECTOMY ANESTHESIA FOR VAGINAL DELIVERY ONLY ANESTHESIA FOR CESAREAN DELIVERY ONLY ANESTHESIA FOR URGENT HYSTERECTOMY FOLLOWING DELIV ANESTHESIA FOR CESAREAN HYSTERECTOMY W/O ANY LABOR ANES INC/MISSED ABORT ANES INDUCED ABORT NEURAXIAL LABOR ANALGESIA/ANESTHESIA, PLANNED VAGI ANESTHESIA FOR CESAREAN DELIVERY FOLLOWING NEURAXI ANES FOR CESAREAN HYSTERECTOMY FOLLOWING NEURAXIAL ARTHRODESIS, PRE-SACRAL INTERBODY TECHNIQUE, DISC SPACE PREPARATION, DISCECTOMY OCULAR BLOOD FLOW MEASUREMENT PHYSIOLOGICAL SUPPORT, HARVESTING, ORGAN(S), BRAIN ANES DX/THER NRV BLK/NJX OTH/THN PRONE POS ANES DX/THER NERVE BLOCK/INJECTION PRONE POS DAILY HOSPITAL MANAGEMENT, EPIDURAL/SUBARACHNOID C UNLISTED ANESTHESIA PROC(S) PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> NDL PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> NDLS POST VERTEB JOINT ARTHROPLASTY SINGLE LUMBAR IVASC VESSEL/GRAFT SPECTROSCOPY+-TX INVTJ W/RSI CPTR DBS ALYS MLT CYCLS CAR ELEC DTA 2/> ECG LDS EVAC MEIBOMIAN GLANDS W/HEAT& INTMT PRESSURE PURE TONE AUDIOMTRY THRESHOLD COMPUTER DEV AIR PURE TONE AUDIOMTRY THRESHOLD CPTR DEV AIR&BONE SPEECH AUDIOMTRY THRESHOLD CPTR DEVICE AIR&BONE SPEECH AUDIOMTRY THRESHOLD AIR&BN SPEECH RECOG COMPREHNS AUDIOMTRY THRESHOLD EVAL&SPEECH RECOG INJECT PARAVERT FACET JT/NERVE CERV/THORAC 1LVL INJECT PARAVER FACET JT/NERVE CERV/THORAC LVL 2 INJECT PARAVER FACET JT/NERVE CERV/THORAC 3+LVL INJECTION PARAVERT FACET JT/NERVE LMBR/SAC 1LVL INJECTION PARAVERT FACET JT/NERVE LMBR/SAC LVL2 INJECTION PARAVER FACET JT/NERVE LMBR/SAC 3+LVL PLACE POSTR INFACET IMPLANT GRAFT/DEV 1LVL CERV PLACE POSTR INFACET IMPLANT GRAFT/DEV 1LVL THOR PLACE POSTR INFACET IMPLANT GRAFT/DEV 1LVL LMBR PLACE POSTR INFACET IMPLANT GRAFT/DEV 1LV +LVL ACOUSTIC/ELECTR CARDGRPHY COMB SINGLE W/INT&REPT ACSTIC/ELECT CARDGRPHY MULTI AV/VV W/INT&REPT ACSTIC/ELECT CARDGRPHY MULTI AV+VV W/INT&REPT INJ ANES AGT&/STRD TFRML EDRL US CRV/THRC 1LVL INJ ANES AGT&/STRD TFRML EDRL US CRV/THRC + LVL INJ ANES AGT&/STRD TFRML EDRL US LMBR/SAC 1LVL INJ ANES AGT&/STRD TFRML EDRL US LMBR/SAC + LVL NJX PLTLT PLASMA W/IMG HARVEST/PREPARATION SKN AGE MEAS MLT-WVELNGTH FLUOR SPCTRSCPY TRLUML PERIPHERAL ATHERECTOMY RENAL ARTERY EA TRLUML PERIPHERAL ATHERECTOMY VISCERAL ARTERY EA TRLUML PERIPH ATHRC W/RS&I ABDOM AORTA TRLUML PERIPH ATHRC W/RS&I BRCHIOCPHL EA VSL TRLUML PERIPHERAL ATHERECTOMY ILIAC ARTERY EA ESOPH MOTILITY 3D PRESSURE TOPOGRAPHY W/I&R ESOPH/GASTROESOPH MOTILITY W/STIM/PERFU W/I&R INTERMIT MEAS WHEEZE RATE BRONCHODIL DX W/I&R CONT MEAS WHEEZE RATE BRONCHODIL SLEEP 3-24 HRS LIGATION HEMORRHOID BUNDLE W/US INSERT ANT SGM DRAINAGE DEV W/O RESERVR INT APPR EVASC ILIAC ART BIFURC W/ENDOPROSTH UNI EVASC ILIAC ART BIFURC W/ENDOPROSTH UNI RS&I IMPLTJ CATH-DLVR PRSTHC PULM VLV EVASC APPR IM AUTOL B1 MRW CEL THER 1 LEG COMPL INCL HRVST IM AUTOL B1 MRW CEL THER 1 LEG COMPL XCL HRVST IM AUTOL B1 MRW CEL THER UNI/BI HRVST ONLY IMPLTJ/RPLCMT CRTD SNS BRORFLX ACTV DEV TOT SYS 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 7/1/2008 7/1/2008 7/1/2008 1/1/2004 7/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 7/1/2009 7/1/2009 7/1/2009 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 7/1/2010 7/1/2010 7/1/2010 7/1/2010 7/1/2010 7/1/2010 7/1/2010 7/1/2010 7/1/2010 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 7/1/2011 7/1/2011 7/1/2011 7/1/2011 7/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 0267T 0268T 0269T 0270T 0271T 0272T 0273T 0274T 0275T 0278T 0281T 0282T 0283T 0284T 0285T 0286T 0287T 0288T 0289T 0290T 0291T 0292T 0293T 0294T 0295T 0296T 0297T 0298T 0299T 0300T 0301T 0302T 0303T 0304T 0305T 0306T 0307T 0308T 0309T 0310T 0311T 0312T 0313T 0314T 0315T 0316T 0317T 0329T 0330T 0331T 0332T 0333T 0335T 0336T 0337T 0338T 0339T 0340T 0341T 0342T 0345T 0346T 0347T 0348T 0349T 0350T 0351T 0352T 0353T 0354T 0355T 0356T 0357T 0358T 0359T 0360T 0361T 0362T 0363T 0364T 0365T 0366T 0367T 0368T 0369T 0370T 0371T 0372T 0373T 0374T 0375T IMPLTJ/RPLCMT CRTD SNS BRORFLX ACTV DEV LEAD UNI IMPLTJ/RPLCMT CRTD SNS BRORFLX ACTV DEV PLS GEN REV/REMVL CRTD SNS BRORFLX ACTV DEV TOT SYS REV/REMVL CRTD SNS BRORFLX ACTV DEV LEAD UNI REV/REMVL CRTD SNS BRORFLX ACTV DEV PLS GEN INTERROGATION EVAL CRTD SNS BRORFLX ACTV SYS INTERROGATION EVAL CRTD SNS BRORFLX W/PROGRMG PERC LAMINO-/LAMINECTOMY IMAGE GUIDE CERV/THORAC PERC LAMINO-/LAMINECTOMY INDIR IMAG GUIDE LUMBAR TRNSCUT ELECT MODLATION PAIN REPROCES EA TX SESS PERC TRANSCTH CLOSR LT ATRIAL APPNDGE IMPLNT S&I PERC/OPEN IMPLNT NEUROSTIM ELECTRODE SUBQ TRIAL PERC/OPEN IMPLNT NEUROSTIM ELECTRODE SUBQ PERM REV/REMVL PG/ELCTRODES IMAGNG ADDN NEW ELCTRODES ELEC ANLYS IMPLANT SUBQ FIELD STIM PG REPROGRAMM NEAR INFRARED SPECTROSCPY STUDIES LOW EXT WOUNDS NEARINFRED GUIDANCE VASC ACES RL TIME DIG VISU ANSCPY W/DELVRY THERMAL ENERGY MUSCLE ANAL CANAL CORNEA INCISNS DONOR CORNEA W/LASR KERTPLSTY CORNEA INCISNS RECIPIENT CORNEA W/LASR KERTPLSTY INTRAV OPTCL CHERNCE TMGRPHY W/S&I INJ INTL VESL INTRAV OPTCAL COHRNCE TMGRPHY W/S&I ADL VESL INS LT ATRL HEMODYN MOTR CMPLETE SYST W/S&I INS LT ATRL HEMDYN MTR PRSR SENSR LEAD W/S&I EXT ECG > 48HR TO 21 DAY RCRD SCAN ANLYS REP R&I EXT ECG > 48HR TO 21 DAY RCRD W/CONECT INTL RCRD EXT ECG > 48HR TO 21 DAY SCAN ANALYSIS W/REPORT EXT ECG > 48HR TO 21 DAY REVIEW AND INTERPRETATN ESW H ENERGY W/TOPCAL APP &DRESNG CARE 1ST WOUND ESW H ENERGY W/TOPCAL APP &DRESNG CARE ADL WOUND DEST/REDUC MALIG BRST TUMR W/US THRMORX GUIDANCE INSJ/RMVL RPLCMT ICAR ISCHM MNTRNG SYS COMPL INSJ/RMVL RPLCMT ICAR ISCHM MNTRNG SYS ELTRD INSJ/RMVL RPLCMT ICAR ISCHM MNTRNG SYS DEVICE PROGRAM EVAL ICAR ISCHM MNTRNG SYS INTERROGATION EVAL ICAR ISCHM MNTRNG SYS RMVL INTRACARDIAC ISCHEMIA MONITORING DEVICE INSJ OCULAR TELESCOPE PROSTH ARTHRODESIS PRESACRAL INTRBDY W/INSTRUMENT L4/L5 MOTOR FUNCTION MAPPING NAVIGATED TMS TX PLAN N-INVAS CAL & ALYS CNTRL ARTL PRESSURE WAVEFORM LAPS IMPLTJ NSTIM ELTRD ARRAY&PLS GEN VAGUS NRV LAPS REVJ/REPLCMT NSTIM ELTRD ARRAY VAGUS NRV LAPS RMVL NSTIM ELTRD ARRAY & PLS GEN VAGUS NRV REMOVAL PULSE GENERATOR VAGUS NERVE REPLACEMENT PULSE GENERATOR VAGUS NERVE ELEC ALYS NSTIM PLS GEN VAGUS NRV W/REPRGRMG MNTR INTRAOCULAR PRESS 24HRS/> UNI/BI W/INTERP TEAR FILM IMAGING UNILATERAL OR BILATERAL W/I&R MYOCRD SYMPATHETIC INNERVAJ IMG PLNR QUAL&QUANT MYOCRD SYMP INNERVAJ IMG PLNR QUAL&QUANT W/SPECT VISUAL EVOKED POTENTIAL ACUITY SCREENING AUTO EXTRA-OSSEOUS JOINT IMPLANT TALOTARSAL STABILJ LAPS ABLATJ UTERINE FIBROIDS W/ INTRAOP US GDNC ENDOTHELIAL FUNCTION ASSESSMENT NON-INVASIVE TRANSCATHETER RENAL SYMPATH DENERVATION UNILAT TRANSCATHETER RENAL SYMPATH DENERVATION BILAT ABLATE PULM TUMORS W/ PLEURA/CHEST WALL EXTSN QUANT PUPILLOMETRY W/ INTERP&REPORT UNILAT/BILAT THERAPEUTIC APHERESIS W/ SELECTIVE HDL DELIP TRANSCATH MITRAL VALVE REPAIR VIA CORONARY SINUS ULTRASOUND ELASTOGRAPHY PLACE INTERSTITIAL DEVICE(S) IN BONE FOR RSA RADIOSTEREOMETRIC ANALYSIS SPINE EXAM RADIOSTEREOMETRIC ANALYSIS UPPER EXTREMITY EXAM RADIOSTEREOMETRIC ANALYSIS LOWER EXTREMITY EXAM INTRAOP OCT BREAST OR AXILL NODE EACH SPECIMEN OCT BREAST OR AXILL NODE SPECIMEN I&R OCT OF BREAST SURG CAVITY REAL TIME INTRAOP OCT BREAST SURG CAVITY REAL TIME/REFERRED I&R GI TRACT IMAGING INTRALUMINAL COLON WITH I&R 7INSERT DRUG IMPLANT INTO LACRIMAL CANAL FOR IOP CRYOPRESERVATION IMMATURE OOCYTE(S) BIA WHOLE BODY SUPINE POSTION WITH I&R BEHAVIORAL IDENTIFICATION ASSESSMENT OBSERVATIONAL BEHAV ASSESSMENT FIRST 30 MIN OBSERVATIONAL BEHAV ASSESSMENT ADDL 30 MIN EXPOSURE BEHAV ASSESSMENT FIRST 30 MIN EXPOSURE BEHAV ASSESSMENT ADDL 30 MIN BEHAVIOR TREATMENT BY PROTOCOL FIRST 30 MIN BEHAVIOR TREATMENT BY PROTOCOL ADDL 30 MIN GROUP BEHAVIOR TREATMENT FIRST 30 MIN GROUP BEHAVIOR TREATMENT ADDL 30 MIN BEHAVIOR TREATMENT WITH MODIFICATION 30 MIN BEHAVIOR TREATMENT WITH MODIFICATION ADDL 30 MIN FAMILY BEHAVIOR TREATMENT GUIDANCE MULTIPLE FAMILY GROUP BEHAV TREATMENT GUIDANCE BEHAVIOR TREATMENT SOCIAL SKILLS TRAINING GROUP EXPOSURE BEHAVIOR TREATMENT FIRST 30 MIN EXPOSURE BEHAVIOR TREATMENT ADDL 30 MIN TOTAL DISC ARTHRP ANT APPR W/DISCECTOMY CRV 3+ 7/1/2011 7/1/2011 7/1/2011 7/1/2011 7/1/2011 7/1/2011 7/1/2011 7/1/2011 7/1/2011 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 7/1/2012 7/1/2012 7/1/2012 7/1/2012 7/1/2012 7/1/2012 7/1/2012 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 7/1/2013 7/1/2013 7/1/2013 7/1/2013 7/1/2013 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 7/1/2014 1/1/2015 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 0376T 0377T 0378T 0379T 0380T 0381T 0382T 0383T 0384T 0385T 0386T 0387T 0388T 0389T 0390T 0391T 0392T 0393T 0394T 0395T 0396T 0397T 0398T 0399T 0400T 0401T 0402T 0403T 0404T 0405T 0406T 0407T 0500F 0501F 0502F 0503F 0505F 0507F 0513F 0514F 0516F 0517F 0518F 0519F 0520F 0521F 0525F 0526F 0528F 0529F 0535F 0540F 0545F 0550F 0551F 0555F 0556F 0557F 0575F 0580F 0581F 0582F 0583F 0584F 098PO 1000F 10021 10022 1002F 10030 10035 10036 10040 10060 10061 10080 10081 1010F 1011F 10120 10121 1012F 10140 1015F 10160 10180 1018F 1019F 1022F 1026F 1030F ANT SEGMENT INSERT DRAIN W/O RESERVOIR EA ADDL ANOSCOPY W/BULKING AGENT INJ FOR FECAL INCONT VISUAL FIELD ASSESSMENT PHYS REVIEW AND REPORT VISUAL FIELD ASSESSMENT TECH SUPPORT W/INSTRUCT COMP ANIMATION RETINA IMAGE TIME SERIES ANALYSIS EXT HEART RATE FOR EPI SZ UP TO 14 DAYS COMPLETE EXT HEART RATE FOR EPI SZ UP TO 14 DAYS R&I ONLY EXT HEART RATE FOR EPI SZ UP TO 30 DAYS COMPLETE EXT HEART RATE FOR EPI SZ UP TO 30 DAYS R&I ONLY EXT HEART RATE FOR EPI SZ OVER 30 DAYS COMPLETE EXT HEART RATE FOR EPI SZ OVER 30 DAYS R&I ONLY TRANSCATH INSERT OR REPLACE LEADLESS CA PM VENTR TRANSCATH REMOVAL LEADLESS CA PM VENTR PROG DEVICE EVAL IN PERSON LEADLESS PM SYSTEM PERI-PROC DEVICE EVAL IN PERS LEADLESS PM SYSTEM INTERROG DEVICE EVAL IN PERSON LEADLESS PM SYST LAPAROSCOPY, SURGICAL, ESOPHAGEAL SPHINCTER AUGMENTATION PROCEDURE, PLACEMEN REMOVAL OF ESOPHAGEAL SPHINCTER AUGMENTATION DEVICE HIGH DOSE RATE ELECTRONIC BRACHYTHERAPY, SKIN SURFACE APPLICATION, PER FRACTION, HIGH DOSE RATE ELECTRONIC BRACHYTHERAPY, INTERSTITIAL OR INTRACAVITARY TREATMEN INTRA-OPERATIVE USE OF KINETIC BALANCE SENSOR FOR IMPLANT STABILITY DURING KNEE R ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY (ERCP), WITH OPTICAL ENDOMIC MAGNETIC RESONANCE IMAGE GUIDED HIGH INTENSITY FOCUSED ULTRASOUND (MRGFUS), S MYOCARDIAL STRAIN IMAGING (QUANTITATIVE ASSESSMENT OF MYOCARDIAL MECHANICS U MULTI-SPECTRAL DIGITAL SKIN LESION ANALYSIS OF CLINICALLY ATYPICAL CUTANEOUS PIGME MULTI-SPECTRAL DIGITAL SKIN LESION ANALYSIS OF CLINICALLY ATYPICAL CUTANEOUS PIGME COLLAGEN CROSS-LINKING OF CORNEA (INCLUDING REMOVAL OF THE CORNEAL EPITHELIUM A PREVENTIVE BEHAVIOR CHANGE, INTENSIVE PROGRAM OF PREVENTION OF DIABETES USING A TRANSCERVICAL UTERINE FIBROID(S) ABLATION WITH ULTRASOUND GUIDANCE, RADIOFREQU OVERSIGHT OF THE CARE OF AN EXTRACORPOREAL LIVER ASSIST SYSTEM PATIENT REQUIRING NASAL ENDOSCOPY, SURGICAL, ETHMOID SINUS, PLACEMENT OF DRUG ELUTING IMPLANT; NASAL ENDOSCOPY, SURGICAL, ETHMOID SINUS, PLACEMENT OF DRUG ELUTING IMPLANT; W INITIAL PRENATAL CARE VISIT(REPORT OF 1ST PRENATAL PRENATAL FLOW SHEET DOCUMENTED IN MEDICAL RECORD B SUBSEQUENT PRENATAL CARE VISIT POSTPARTUM CARE VISIT Hemodialysis plan documented Periton dialysis plan documented ELEVATED BLOOD PRESSURE PLAN OF CARE DOCUMENTED PLAN/CARE INCRSD HGB LVL DOCD PT ON ESA TH ANEMIA PLAN OF CARE DOCUMENTED GLAUCOMA PLAN OF CARE DOCUMENTED FALLS PLAN OF CARE DOCUMENTED PLAND CHEMO REG DOCD B/4 START NEW TXMNT RAD DOS LIMTS ESTABD B/4 3D RAD FOR MIN 2 TISS PLAN OF CARE TO ADDRESS PAIN DOCD INITIAL VISIT FOR EPISODE SUBSEQUENT VISIT FOR EPISODE RCMND FLW-UP 10 YRS DOCD INTRVL 3+YRS PTS CLNSCP DOCD DYSPNEA MNGMNT PLAN DOCD GLUCO MNGMNT PLAN DOCD PLAN FOR FOLLOW-UP CARE FOR MDD DOCD CYTOPATH REPORT ON NONGYN SPECIMEN - 2 WKNG DAYS CYTOPATH REPORT - NONGYN SPCMN DOCD NON-ROUTINE SYMPTOM MANAGEMENT PLAN OF CARE DOCUMENTED PLAN OF CARE TO ACHIEVE LIPID CONTROL DOCUMENTED PLAN OF CARE TO MANAGE ANGINAL SYMPTOMS DOCD HIV RNA CONTROL PLAN OF CARE DOCD MULTIDISCIPLINARY CARE PLAN DEVELOPED/UPDATED PT TRANSFERRED FROM ANESTHETIZING TO CC UNIT PT NOT TRANSFERRED FROM ANESTHETIZING TO CC UNIT TRANSFER OF CARE CHECKLIST USED TRANSFER OF CARE CHECKLIST NOT USED SRVC PROC&/SURG PRVD OFF-CAMPUS PRVD-BSD OP DEPT TOBACCO USE, SMOKE, ASSESSED FINE NEEDLE ASIPIRATION;WITHOUT IMAGING GUIDANCE FINE NEEDLE ASPIRATION; W/IMAGING GUIDANCE ANGINAL SYMPTOMS&LVL ACTV ASSESSED IMAGE-GUIDED CATHETER FLUID COLLECTION DRAINAGE PLACEMENT OF SOFT TISSUE LOCALIZATION DEVICE(S) (EG, CLIP, METALLIC PELLET, WIRE/NEE PLACEMENT OF SOFT TISSUE LOCALIZATION DEVICE(S) (EG, CLIP, METALLIC PELLET, WIRE/NEE ACNE SURGERY INCISION AND DRAINAGE, ABSCESS; SIMPLE/SINGLE INCISION AND DRAINAGE OF ABSCESS; COMPLICATED OR M INCISION AND DRAINAGE, PILONIDAL CYST; SIMPLE INCISION AND DRAINAGE OF PILONDIAL CYST; COMPLICAT SEVERITY OF ANGINA ASSESSED BY LEVEL OF ACTIVITY ANGINA PRESENT INCISION AND REMOVAL, FB, SUBQ TISSUES; SIMPLE INCISION AND REMOVAL, FB, SUBQ TISSUES; COMPLICATE ANGINA ABSENT INCISION AND DRAINAGE, HEMATOMA, SEROMA/FLUID COLL Copd symptoms assess PUNCTURE ASPIRATION, ABSCESS, HEMATOMA, BULLA/CYST INCISION & DRAINAGE, COMPLEX POST OPERATIVE Assess dyspnea not present Assess dyspnea present Pneumo imm status assess Co-morbid condition assess Influenza imm status assess 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2010 7/1/2011 7/1/2011 1/1/2012 1/1/2012 1/1/2012 10/1/2008 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2015 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2014 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 1031F 1032F 1033F 1034F 1035F 1036F 1038F 1039F 1040F 1050F 1052F 1055F 1090F 11000 11001 11004 11005 11006 11008 11010 11011 11012 1101F 11042 11043 11044 11045 11046 11047 11055 11056 11057 11100 11101 1118F 1119F 11200 11201 1121F 1123F 1124F 1125F 1126F 1127F 1128F 11300 11301 11302 11303 11305 11306 11307 11308 1130F 11310 11311 11312 11313 1134F 1135F 1136F 1137F 11400 11401 11402 11403 11404 11406 11420 11421 11422 11423 11424 11426 11440 11441 11442 11443 11444 11446 11450 11451 11462 11463 11470 11471 1150F 1151F 1152F 1153F 1157F SMOKING-2ND HAND SMOKE IN THE HOME ASSESSED CURRENT SMOKER/EXPOSED TO SECONDHAND SMOKE TOBACCO NON-SMOKER +NO 2NDHAND SMOKE EXPOSURE Current tobacco smoker Smokeless tobacco user CURRENT TOBACCO NON-USER CAD CAP COPD PV DM Persistent asthma Intermittent asthma DSM-IV(TM) CRITERIA MDD DOCD AT THE INITIAL EVAL History of mole changes TYPE ANATOMIC LOCATION AND ACTIVITY ALL ASSESSED Visual funct status assess Presence or absence of urinary incontinence assessed (GER) DEBRIDEMENT, EXTENSIVE ECZEMATOUS/INFECTED SKIN; U DEBRIDEMENT, EXTENSIVE ECZEMATOUS, EACH ADDL 10% DEBRIDEMENT, EXTERNAL GENITALIA AND PERINEUM DEBRIDEMENT, ABDOMINAL WAL, WITH/WO FASCIAL CLOSUR DEBRIDEMENT, EXTERNAL GENITALIA, PERIEUM , AND ABD REMOVAL OF PROSTHETIC MATERIAL OR MESH DBRDMT W/RMVL FM FX&/DISLC SKN&SUBQ TISS DEBRIDEMENT W/REMOVAL FOREIGN MATL, OPEN FX/DISLOC DBRDMT FX&/DISLC SUBQ T/M/F BONE PATIENT SCREENED FOR FUTURE FALL; DOCUMENT OF NO FALLS IN PAST YEAR DEBRIDEMENT SUBCUTANEOUS TISSUE 20 SQ CM/< DEBRIDEMENT MUSCLE & FASCIA 20 SQ CM/< DBRDMT BONE M&/F 20 SQ CM/< DBRDMT SUBCUTANEOUS TISSUE EA ADDL 20 SQ CM DBRDMT M&/F EA ADDL 20 SQ CM DEBRIDEMENT BONE EA ADDL 20 SQ CM/< PARING/CUTTING, BENIGN HYPERKERATOTIC LESION; SING PARING/CUTTING, BENIGN HYPERKERATOTIC LESION; 2-4 PARING/CUTTING, BENIGN HYPERKERATOTIC LESION; GREA BX, SKIN, SUBQ/MUCOUS MEMBRANE; SINGLE LESION BX, SKIN, SUBQ/MUCOUS MEMBRANE (SEP PROC); ADDL LE GERD SYMPTOMS ASSESSED AFTER 12 MONTHS OF THXPY INITIAL EVALUATION FOR CONDITION REMOVAL, SKIN TAGS, MULTIPLE FIBROCUTANEOUS TAGS, REMOVAL, SKIN TAGS, MULTIPLE FIBROCUTANEOUS TAGS, SUBSEQUENT EVALUATION FOR COND ADV CARE PLN TLKD & ALT DCSN MAKER DOCD ADV CARE PLN/ NO ALT DCSN MKR DOCD OR REFUSAL PAIN SEVERITY QUANTIFIED; PAIN PRESENT PAIN SEVERITY QUANTIFIED; NO PAIN PRESENT NEW EPISODE FOR CONDITION SUBSEQUENT EPISODE FOR CONDITION SHAVING SKIN LESION, TRUNK/ARMS/LEGS; DIAMETER 0.5 SHAVING SKIN LESION, TRUNK/ARMS/LEGS; DIAMETER 0.6 SHAVING SKIN LESION, TRUNK/ARMS/LEGS; DIAMETER 1.1 SHAVING SKIN LESION, TRUNK/ARMS/LEGS; DIAMETER OVE SHAVING SKIN LESION, SCALP/NECK/HANDS/FEET/GENITAL SHAVING SKIN LESION, SCALP/NECK/HANDS/FEET/GENITAL SHAVING SKIN LESION, SCALP/NECK/HANDS/FEET/GENITAL SHAVING SKIN LESION, SCALP/NECK/HANDS/FEET/GENITAL BK PAIN & FXN ASSESSED CERTAIN ASPECTS OF CARE SHAVING SKIN LESION, FACE/EARS/EYELIDS/NOSE/LIPS/M SHAVING SKIN LESION, FACE/EARS/EYELIDS/NOSE/LIPS/M SHAVING SKIN LESION, FACE/EARS/EYELIDS/NOSE/LIPS/M SHAVING SKIN LESION, FACE/EARS/EYELIDS/NOSE/LIPS/M EPISODE OF BACK PAIN LASTING SIX WEEKS OR LESS EPISODE OF BACK PAIN LASTING LONGER THAN SIX WK EPISODE OF BACK PAIN LASTING 12 WEEKS OR LESS EPISODE OF BACK PAIN LASTING LONGER THAN 12 WKS EXCISE, BENIGN SKIN LESION, INCL MARGINS, EXCEPT S EXCISE, BENIGN SKIN LESION, INCL MARGINS, EXCEPT S EXCISE, BENIGN SKIN LESION, INCL MARGINS, EXCEPT S EXCISE, BENIGN SKIN LESION, INCL MARGINS, EXCEPT S EXCISE, BENIGN SKIN LESION, INCL MARGINS, EXCEPT S EXCISE, BENIGN SKIN LESION, INCL MARGINS, EXCEPT S EXCISE BENIGN SKIN LESION W/MARG, EXCPT SKIN TAG S EXCISE BEN SKIN LESION W/MARG, EXCEPT SKIN TAG SCA EXCISE BEN SKIN LESION W/MARG, EXCEPT SKIN TAG SCA EXCISE BEN SKIN LESION W/MARG, EXCEPT SKIN TAG SCA EXCISE BEN SKIN LESION W/MARG, EXCEPT SKIN TAG SCA EXCISE BENIGN SKIN LESION W/MARG, EXCEPT SKIN TAG EXCISE, BENIGN SKIN LESION, INCL MARGINS, FACE/EAR EXCISE, BENIGN SKIN LESION, INCL MARGINS, FACE/EAR EXCISE, BENIGN SKIN LESION, INCL MARGINS, FACE/EAR EXCISION, BENIGN LESION, DIAMETER 2.1 TO 3.0 CM EXCISE, BENIGN SKIN LESION, INCL MARGINS, FACE/EAR EXCISE, BENIGN SKIN LESION, INCL MARGINS, FACE/EAR EXCISION, SKIN AND SUBQ TISSUE, HIDRADENITIS, AXIL EXCISION, SKIN ANDSUBQ TISSUE, HIDRADENITIS, AXILL EXCISION, SKIN AND SUBQ TISSUE, HIDRADENITIS, INGU EXCISION, SKIN AND SUBQ TISSUE, HIDRADENITIS, INGU EXCISION, SKIN AND SUBQ TISSUE, HIDRADENITIS, PERI EXCISION, SKIN AND SUBQ TISSUE, HIDRADENITIS, PERI DOC PT RSK DEATH W/IN 1YR DOC NO PT RSK DEATH W/IN 1YR DOC ADVNCD DIS COMFORT 1ST DOC ADVNCD DIS CMFRT NOT 1ST ADVNC CARE PLAN IN RCRD 1/1/2012 1/1/2012 1/1/2012 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2012 1/1/2007 1/1/2010 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 7/1/2011 7/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 1158F 1159F 11600 11601 11602 11603 11604 11606 1160F 11620 11621 11622 11623 11624 11626 11640 11641 11642 11643 11644 11646 1170F 11719 11720 11721 11730 11732 11740 11750 11752 11755 1175F 11760 11762 11765 11770 11771 11772 1180F 1181F 1182F 1183F 11900 11901 11920 11921 11922 11950 11951 11952 11954 11960 11970 11971 11976 11980 11981 11982 11983 12001 12002 12004 12005 12006 12007 1200F 12011 12013 12014 12015 12016 12017 12018 12020 12021 12031 12032 12034 12035 12036 12037 12041 12042 12044 12045 12046 12047 12051 12052 12053 12054 ADVNC CARE PLAN TLK DOCD MED LIST DOCD IN RCRD EXCISION, MALIGNANT LESION, INCL MARGINS, TRUNK/AR EXCISION, MALIGNANT LESION, DIAMETER 0.6 TO 1.0 CM EXCISION, MALIGNANT LESION, DIAMETER 1.1 TO 2.0 CM EXCISION, MALIGNANT LESION, DIAMETER 2.1 TO 3.0 CM EXCISION, MALIGNANT LESION, INCL MARGINS, TRUNK/AR EXCISION, MALIGNANT LESION, INCL MARGINS, TRUNK/AR RVW MEDS BY RX/DR IN RCRD EXCISION, MALIGNANT LESION, INCL MARGINS, SCALP/NE EXCISION, MALIGNANT LESION, INCL MARGINS, SCALP/NE EXCISION, MALIGNANT LESION, INCL MARGINS, SCALP/NE EXCISION, MALIGNANT LESION, INCL MARGINS, SCALP/NE EXCISION, MALIGNANT LESION, INCL MARGINS, SCALP/NE EXCISION, MALIGNANT LESION, INCL MARGINS, SCALP/NE EXCISION, MALIGNANT LESION, INCL MARGINS, FACE/EAR EXCISION, MALIGNANT LESION, DIAMETER 0.6 TO 1.0 CM EXCISION, MALIGNANT LESION, DIAMETER 1.1 TO 2.0 CM EXCISION, MALIGNANT LESION, DIAMETER 2.1 TO 3.0 CM EXCISION, MALIGNANT LESION, INCL MARGINS, FACE/EAR EXCISION, MALIGNANT LESION, INCL MARGINS, FACE/EAR FXNL STATUS ASSESSED TRIMMING, NONDYSTROPHIC NAILS, ANY NUMBER DEBRIDEMENT, NAIL(S), ANY METHOD(S); 1-5 DEBRIDEMENT, NAIL(S), ANY METHOD(S); OVER 6 AVULSION, NAIL PLATE, PARTIAL/COMPLETE, SIMPLE; SI AVULSION, NAIL PLATE, PARTIAL/COMPLETE, SIMPLE; AD EVACUATION, SUBUNGUAL HEMATOMA EXCISION, NAIL/NAIL MATRIX, PERMANENT REMOVAL EXCISION, NAIL/NAIL MATRIX, PERMANENT REMOVAL; W/A BX, NAIL UNIT (SEP PROC) FUNCTIONAL STATUS DEMENTIA ASSESS RESULTS RVWD REPAIR, NAIL BED RECONSTRUCTION, NAIL BED W/GRAFT WEDGE EXCISION, SKIN, NAIL FOLD EXCISION, PILONIDAL CYST/SINUS; SIMPLE EXCISION, PILONIDAL CYST/SINUS; EXTENSIVE EXCISION, PILONIDAL CYST/SINUS; COMPLICATED THROMBOEMB RISK FACTORS ASSESSED NEUROPSYCHIATRIC SYMPTS ASSESSED RESULTS REVIEWD NEUROPSYCHIATRIC SYMPTOMS NE OR MORE PRESENT NEUROPSYCHIATRIC SYMPTOMS ABSENT INJECTION, INTRALESIONAL; UP TO AND INCL 7 LESIONS INJECTION, INTRALESIONAL; MORE THAN LESIONS TATTOOING TO CORRECT COLOR DEFECTS; 6.0 SQ CM/LESS TATTOOING TO CORRECT COLOR DEFECTS; 6.1-20.0 SQ CM TATTOOING TO CORRECT COLOR DEFECTS; ADDL 20.0 SQ C SUBQ INJECTION, FILLING MATL; 1 CC/OR LESS SUBQ INJECTION, FILLING MATL; 1.1 TO 5.0 CC SUBQ INJECTION, FILLING MATL; 5.1 TO 10.0 CC SUBQ INJECTION, FILLING MATL; OVER 10.0 CC INSERTION, TISSUE EXPANDER(S), OTHER THAN BREAST, REPLACEMENT, TISSUE EXPANDER W/PERMANENT PROSTHESI REMOVAL, TISSUE EXPANDER(S) W/O INSERTION, PROSTHE REMOVAL, IMPLANTABLE CONTRACEPTIVE CAPSULES IMPLANTATION, HORMONE PELLET, SUBCUTANEOUS INSERTION, NON-BIODEGRADABLE DRUG DELIVERY IMPLANT REMOVAL, NON-BIODEGRADABLE DRUG DELIVERY IMPLANT REMOVAL W/REINSERTION, NON-BIODEGRADABLE DRUG DELI SIMPLE REPAIR, SUPERFICIAL WOUNDS, SCALP/NECK/AXIL SIMPLE REPAIR, SUPERFICIAL WOUNDS, SCALP/NECK/AXIL SIMPLE REPAIR, SUPERFICIAL WOUNDS, SCALP/NECK/AXIL SIMPLE REPAIR, SUPERFICIAL WOUNDS, SCALP/NECK/AXIL SIMPLE REPAIR, SUPERFICIAL WOUNDS, SCALP/NECK/AXIL SIMPLE REPAIR, SUPERFICIAL WOUNDS, SCALP/NECK/AXIL SZR TYPES + CURRENT SZR FREQ DOCD SIMPLE REPAIR, SUPERFICIAL WOUNDS, FACE/EARS/EYELI SIMPLE REPAIR, SUPERFICIAL WOUNDS, FACE/EARS/EYELI SIMPLE REPAIR, SUPERFICIAL WOUNDS, FACE/EARS/EYELI SIMPLE REPAIR, SUPERFICIAL WOUNDS, FACE/EARS/EYELI SIMPLE REPAIR, SUPERFICIAL WOUNDS, FACE/EARS/EYELI SIMPLE REPAIR, SUPERFICIAL WOUNDS, FACE/EARS/EYELI SIMPLE REPAIR, SUPERFICIAL WOUNDS, FACE/EARS/EYELI TREATMENT, SUPERFICIAL WOUND DEHISCENCE; SIMPLE CL TREATMENT, SUPERFICIAL WOUND DEHISCENCE; W/PACKING LAYER CLOSURE, WOUNDS, SCALP/AXILLAE/TRUNK/EXTREMI LAYER CLOSURE OF WOUNDS,SCALP,ETC 2.6 CM TO 7.5 CM LAYER CLOSURE, WOUNDS, SCALP/AXILLAE/TRUNK/EXTREMI LAYER CLOSURE, WOUNDS, SCALP/AXILLAE/TRUNK/EXTREMI LAYER CLOSURE, WOUNDS, SCALP/AXILLAE/TRUNK/EXTREMI LAYER CLOSURE, WOUNDS, SCALP/AXILLAE/TRUNK/EXTREMI LAYER CLOSURE, WOUNDS, NECK, HANDS, FEET AND/OR EX LAYER CLOSURE, WOUNDS, NECK, HANDS, FEET AND/OR EX LAYER CLOSURE, WOUNDS, NECK, HANDS, FEET AND/OR EX LAYER CLOSURE, WOUNDS, NECK, HANDS, FEET AND/OR EX LAYER CLOSURE, WOUNDS, NECK, HANDS, FEET AND/OR EX LAYER CLOSURE, WOUNDS, NECK, HANDS, FEET AND/OR EX LAYER CLOSURE, WOUNDS, FACE/EARS/EYELIDS/NOSE/LIPS LAYER CLOSURE, WOUNDS, FACE/EARS/EYELIDS/NOSE/LIPS LAYER CLOSURE, WOUNDS, FACE/EARS/EYELIDS/NOSE/LIPS LAYER CLOSURE, WOUNDS, FACE/EARS/EYELIDS/NOSE/LIPS 1/1/2009 1/1/2009 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y N N N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 12055 12056 12057 1205F 1220F 13100 13101 13102 13120 13121 13122 13131 13132 13133 13151 13152 13153 13160 14000 14001 1400F 14020 14021 14040 14041 14060 14061 14301 14302 14350 1450F 1451F 1460F 1461F 1490F 1491F 1493F 1494F 15002 15003 15004 15005 1500F 1501F 1502F 1503F 15040 1504F 15050 1505F 15100 15101 15110 15111 15115 15116 15120 15121 15130 15131 15135 15136 15150 15151 15152 15155 15156 15157 15200 15201 15220 15221 15240 15241 15260 15261 15271 15272 15273 15274 15275 15276 15277 15278 15570 15572 15574 15576 15600 15610 15620 LAYER CLOSURE, WOUNDS, FACE/EARS/EYELIDS/NOSE/LIPS LAYER CLOSURE, WOUNDS, FACE/EARS/EYELIDS/NOSE/LIPS LAYER CLOSURE, WOUNDS, FACE/EARS/EYELIDS/NOSE/LIPS ETIOLOGY OF EPILEPSY SYNDROME(S) RVWD DOCD PATIENT SCREENED FOR DEPRESSION REPAIR, COMPLEX, TRUNK; 1.1 TO 2.5 CM REPAIR, COMPLEX, TRUNK; 2.6 TO 7.5 CM REPAIR, COMPLEX TRUNK, EACH ADDL 5 CM OR LESS REPAIR, COMPLEX, SCALP, ARMS, AND/OR LEGS; 1.1 TO REPAIR, COMPLEX, SCALP, ARMS, AND/OR LEGS; 2.6 TO REPAIR, COMPLEX, SCALP, ETC EACH ADDL 5 CM OR LESS REPAIR, COMPLEX, FOREHEAD/CHEEKS/CHIN/MOUTH/NECK/A REPAIR, COMPLEX, FOREHEAD/CHEEKS/CHIN/MOUTH/NECK/A REPAIR, COMPLEX, FOREHEAD, EACH ADDL 5 CM OR LESS REPAIR, COMPLEX, EYELIDS, NOSE, EARS AND/OR LIPS; REPAIR, COMPLEX, EYELIDS, NOSE, EARS AND/OR LIPS; REPAIR, COMPLEX, EYELIDS, EACH ADDL 5 CM OR LESS SECONDARY CLOSURE, SURGICAL WOUND/DEHISCENCE, EXTE ADJACENT TISSUE TRANSFER/REARRANGEMENT, TRUNK; DEF ADJACENT TISSUE TRANSFER/REARRANGEMENT, TRUNK; DEF PRKNS DIAG RVIEWED ADJACENT TISSUE TRANSFER/REARRANGEMENT, SCALP, ARM ADJACENT TISSUE TRANSFER/REARRANGEMENT, SCALP, ARM ADJACENT TISSUE TRANSFER, FOREHEAD/CHEEKS/CHIN/MOU ADJACENT TISSUE TRANSFER, FOREHEAD/CHEEKS/CHIN/MOU ADJACENT TISSUE TRANSFER/REARRANGEMENT, EYELIDS/NO ADJACENT TISSUE TRANSFER/REARRANGEMENT, EYELIDS/NO ATT/R ANY AREA DEFECT 30.1-60SQCM ATT/R ANY AREA DEFECT EA ADDL 30SQCM OR PART FILLETED FINGER/TOE FLAP, W/PREPARATION, RECIPIENT SYMPTOMS IMPROVED/CONSIST W/TXMNT GOAL ASSESSMNT SYMPTOMS SHOW CLIN IMPRTNT DROP SINCE ASSESSMENT QUALIFYING CARD EVENT/DIAGNOSIS PRIOR 12 MONTHS NO QUAL CARD EVENT/DIAG IN PREVIOUS 12 MONTHS DEMENTIA SEVERITY CLASSIFIED MILD DEMENTIA SEVERITY CLASSIFIED MODERATE (DEM) DEMENTIA SEVERITY CLASSIFIED SEVERE COGNITION ASSESSED AND REVIEWED Wnd prep, ch/inf, trk/arm/lg Wnd prep, ch/inf addl 100 cm Wnd prep ch/inf, f/n/hf/g Wnd prep, f/n/hf/g, addl cm SYMP+SIGN DISTAL SYMM POLYNEUROPATHY REVWD+DOCD NOT INITIAL EVALUATION FOR CONDITION PT QUERIED RE PAIN W/FUNC USING RELIABLE INSTRM PT QUERIED RE SYMP RESPIRATORY INSUFFICIENCY HARVEST OF SKIN FOR TISSUE CULTURED SKIN, 100 CM O PATIENT HAS RESPIRATORY INSUFFICIENCY PINCH GRAFT, SINGLE/MULTIPLE, (EXCEPT ON FACE), 2 PATIENT DOES NOT HAVE RESPIRATORY INSUFFICIENCY SPLIT GRAFT, TRUNK/EXTREMITIES; 1ST 100 SQ CM/LESS SPLIT GRAFT, TRUNK/EXTREMITIES; ADDL 100 SQ CM/ 1P EPIDERMAL AUTOGRAFT, TRUNK, ARMS, LEGS, FIRST 100 EPIDERMAL AUTOGRAFT, TRUNK, ARMS, LEGS, EACH ADD'L EPIDERMAL AUTOGRAFT, FACE, SCALP, EYELIDS, MOUTH, EPIDERMAL AUTOGRAFT, FACE, SCALP, EYELIDS, MOUTH, SPLIT GRAFT, FACE/SCALP/MOUTH/HNDS/FEET/GENIT/MULT SPLIT GRAFT, FACE/SCALP/MOUTH/HNDS/FEET/GENIT/MULT DERMAL AUTOGRAFT, TRUNK , ARMS, LEGS, FIRST 100CM DERMAL AUTOGRAFT, TRUNK , ARMS, LEGS, FIRST EACH A DERMAL AUTOGRAFT, FACE, SCALP, EYELIDS, MOUTH, FIR DERMAL AUTOGRAFT, FACE, SCALP, EYELIDS, MOUTH, EAC CLTR SKIN AUTOGRAFT T/A/L 1ST 25 CM/< CLTR SKIN AGRFT T/A/L ADDL 1 CM-75 CM CLTR SKIN AGRFT T/A/L EA 100 CM/EA 1%BODY AREA CLTR SKIN AGRFT F/S/N/H/F/G/M/D GT 1ST 25CM/< CLTR SKIN AGRFT F/S/N/H/F/G/M/D GT ADDL 1-75CM CLTR SKIN AGRFT F/S/N/H/F/G/M/D GT EA 100 EA FULL THICKNESS GRAFT, FREE, W/DIRECT CLOSURE, DONO FULL THICKNESS GRAFT, FREE, W/DIRECT CLOSURE, DONO FULL THICKNESS GRAFT, FREE, W/CLOSURE DONOR SITE, FULL THICKNESS GRAFT, FREE, W/CLOSURE DONOR SITE, FULL THICKNESS GRAFT, FREE, W/CLOSURE DONOR, FACE/ FULL THICKNESS GRAFT, FREE, W/CLOSURE DONOR, FACE/ FULL THICKNESS GRAFT, FREE, W/CLOSURE DONOR SITE, FULL THICKNESS GRAFT, FREE, W/CLOSURE DONOR SITE, APP SKN SUB GRFT T/A/L AREA/<100SCM /<1ST 25 SCM APP SKN SUB GRFT T/A/L AREA/<100SCM EA ADL 25SCM APP SKN SUB GRFT T/A/L AREA/>100SCM 1ST 100SCM APP SKN SUB GRFT T/A/L AREA/>100SCM ADL 100SCM SUB GRFT F/S/N/H/F/G/M/D /<100SCM /<1ST 25 SCM SUB GRFT F/S/N/H/F/G/M/D /<100SCM EA ADL 25SCM SUB GRFT F/S/N/H/F/G/M/D />100SCM 1ST 100SCM SUB GRFT F/S/N/H/F/G/M/D />100SCM ADL 100SCM FORMATION, DIRECT/TUBED PEDICLE, W/WO TRANSFER; TR FORMATION, DIRECT/TUBED PEDICLE, W/WO TRANSFER; SC FORMATION, DIRECT/TUBED PEDICLE W/WO TRANSF; FACE/ FORMATION, DIRECT/TUBED PEDICLE, W/WO TRANSFER; EY DELAY, FLAP/SECTIONING, FLAP (DIVISION AND INSET); DELAY, FLAP/SECTIONING, FLAP (DIVISION AND INSET); DELAY, FLAP/SECTIONING, FLAP (DIVISION AND INSET); 1/1/2004 1/1/2004 1/1/2004 1/1/2010 10/1/2008 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 10/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2006 1/1/2013 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 15630 15650 15731 15732 15734 15736 15738 15740 15750 15756 15757 15758 15760 15770 15775 15776 15777 15780 15781 15782 15783 15786 15787 15788 15789 15792 15793 15819 15820 15821 15822 15823 15824 15825 15826 15828 15829 15830 15832 15833 15834 15835 15836 15837 15838 15839 15840 15841 15842 15845 15847 15850 15851 15852 15860 15876 15877 15878 15879 15920 15922 15931 15933 15934 15935 15936 15937 15940 15941 15944 15945 15946 15950 15951 15952 15953 15956 15958 15999 16000 16020 16025 16030 16035 16036 17000 17003 17004 17106 17107 17108 DELAY, FLAP/SECTIONING, FLAP (DIVISION AND INSET); TRANSFER, INTERMEDIATE, ANY PEDICLE FLAP, ANY LOCA Forehead flap w/vasc pedicle MUSCLE, MYOCUTANEOUS/FASCIOCUTANEOUS FLAP; HEAD AN MUSCLE, MYOCUTANEOUS/FASCIOCUTANEOUS FLAP; TRUNK MUSCLE, MYOCUTANEOUS/FASCIOCUTANEOUS FLAP; UPPER E MUSCLE, MYOCUTANEOUS/FASCIOCUTANEOUS FLAP; LOWER E FLAP ISLAND PEDICLE ANATOMIC NAMED AXIAL ARTERY FLAP; NEUROVASCULAR PEDICLE FREE MUSCLE/MYOCUTANEOUS FLAP W/MICROVASCULAR ANAS FREE SKIN FLAP W/MICROVASCULAR ANASTOMOSIS FREE FASCIAL FLAP W/MICROVASCULAR ANASTOMOSIS GRAFT; COMPOSITE, W/PRIMARY CLOSURE, DONOR AREA GRAFT; DERMA-FAT-FASCIA PUNCH GRAFT, HAIR TRANSPLANT; 1-15 PUNCH GRAFTS PUNCH GRAFT, HAIR TRANSPLANT; OVER 15 PUNCH GRAFTS IMPLNT BIO IMPLNT FOR SOFT TISSUE REINFORCEMENT DERMABRASION; TOTAL FACE DERMABRASION; SEGMENTAL, FACE DERMABRASION; REGIONAL, OTHER THAN FACE DERMABRASION; SUPERFICIAL, ANY SITE ABRASION; SINGLE LESION ABRASION; ADDL 4 LESIONS/LESS CHEMICAL PEEL, FACIAL; EPIDERMAL CHEMICAL PEEL, FACIAL; DERMAL CHEMICAL PEEL, NONFACIAL; EPIDERMAL CHEMICAL PEEL, NONFACIAL; DERMAL CERVICOPLASTY BLEPHAROPLASTY, LOWER EYELID BLEPHAROPLASTY, LOWER EYELID; W/EXTENSIVE HERNIATE BLEPHAROPLASTY, UPPER EYELID BLEPHAROPLASTY, UPPER EYELID; W/EXCESSIVE SKIN WEI RHYTIDECTOMY; FOREHEAD RHYTIDECTOMY; NECK W/PLATYSMAL TIGHTENING (PLATYSM RHYTIDECTOMY; GLABELLAR FROWN LINES RHYTIDECTOMY; CHEEK, CHIN, AND NECK RHYTIDECTOMY; SUPERFICIAL MUSCULOAPONEUROTIC SYSTE Exc skin abd EXCISION, EXCESSIVE SKIN AND SUBQ TISSUE (W/LIPECT EXCISION, EXCESSIVE SKIN AND SUBQ TISSUE (W/LIPECT EXCISION, EXCESSIVE SKIN AND SUBQ TISSUE (W/LIPECT EXCISION, EXCESSIVE SKIN AND SUBQ TISSUE (W/LIPECT EXCISION, EXCESSIVE SKIN AND SUBQ TISSUE (W/LIPECT EXCISION, EXCESSIVE SKIN AND SUBQ TISSUE (W/LIPECT EXCISION, EXCESSIVE SKIN AND SUBQ TISSUE (W/LIPECT EXCISION, EXCESSIVE SKIN AND SUBQ TISSUE (W/LIPECT GRAFT, FACIAL NERVE PARALYSIS; FREE FASCIA GRAFT ( GRAFT, FACIAL NERVE PARALYSIS; FREE MUSCLE GRAFT ( GRAFT, FACIAL NERVE PARALYSIS; FREE MUSCLE FLAP, M GRAFT, FACIAL NERVE PARALYSIS; REGIONAL MUSCLE TRA Exc skin abd add-on REMOVAL, SUTURES UNDER ANESTHESIA (OTHER THAN LOCA REMOVAL OF SUTURES UNDER ANESTHESIA(OTHER THAN LOC DRESSING CHANGE (FOR OTHER THAN BURNS) UNDER ANEST IV INJECTION, AGENT TO TEST VASCULAR FLOW IN FLAP/ SUCTION ASSISTED LIPECTOMY; HEAD AND NECK SUCTION ASSISTED LIPECTOMY; TRUNK SUCTION ASSISTED LIPECTOMY; UPPER EXTREMITY SUCTION ASSISTED LIPECTOMY; LOWER EXTREMITY EXCISION, COCCYGEAL PRESSURE ULCER, W/COCCYGECTOMY EXCISION, COCCYGEAL PRESSURE ULCER, W/COCCYGECTOMY EXCISION, SACRAL PRESSURE ULCER, W/PRIMARY SUTURE; EXCISION, SACRAL PRESSURE ULCER, W/PRIMARY SUTURE; EXCISION, SACRAL PRESSURE ULCER, W/SKIN FLAP CLOSU EXCISION, SACRAL PRESSURE ULCER, W/SKIN FLAP CLOSU EXCISION, SACRAL PRESSURE ULCER, PREP, MUSCLE/MYOC EXCISION, SACRAL PRESSURE ULCER, PREP, MUSCLE/MYOC EXCISION, ISCHIAL PRESSURE ULCER, W/PRIMARY SUTURE EXCISION, ISCHIAL PRESSURE ULCER, W/PRIMARY SUTURE EXCISION, ISCHIAL PRESSURE ULCER, W/SKIN FLAP CLOS EXCISION, ISCHIAL PRESSURE ULCER, W/SKIN FLAP CLOS EXCISION, ISCHIAL PRESSURE ULCER, W/OSTECTOMY, PRE EXCISION, TROCHANTERIC PRESSURE ULCER, W/PRIMARY S EXCISION, TROCHANTERIC PRESSURE ULCER, W/PRIMARY S EXCISION, TROCHANTERIC PRESSURE ULCER, W/SKIN FLAP EXCISION, TROCHANTERIC PRESSURE ULCER, W/SKIN FLAP EXCISION, TROCHANTERIC PRESSURE ULCER, PREP, MUSCL EXCISION, TROCHANTERIC PRESSURE ULCER, PREP, MUSCL UNLISTED PROC, EXCISION PRESSURE ULCER INITIAL TREATMENT, 1ST DEGREE BURN, WHEN NO MORE T DRESSINGS AND/OR DEBRIDEMENT, INITIAL/SUBSEQUENT; DRESSINGS AND/OR DEBRIDEMENT, INITIAL/SUBSEQUENT; DRESSINGS AND/OR DEBRIDEMENT, INITIAL/SUBSEQUENT; ESCHAROTOMY; INITIAL INCISION ESCHAROTOMY; ADDL INCISION DESTRUCTION, BENIGN/PREMALIG LESIONS, EXCEPT SKIN DESTRUCTION, BENIGN/PREMALIG LESIONS, EXCEPT SKIN DESTRUCTION, BENIGN/PREMALIG LESIONS, EXCEPT SKIN DESTRUCTION, CUTANEOUS VASCULAR PROLIFERATIVE LESI DESTRUCTION, CUTANEOUS VASCULAR PROLIFERATIVE LESI DESTRUCTION, CUTANEOUS VASCULAR PROLIFERATIVE LESI 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y N N N N N Y Y Y Y Y Y Y Y Y N N N N Y N N N N Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 17110 17111 17250 17260 17261 17262 17263 17264 17266 17270 17271 17272 17273 17274 17276 17280 17281 17282 17283 17284 17286 17311 17312 17313 17314 17315 17340 17360 17380 17999 19000 19001 19020 19030 19081 19082 19083 19084 19085 19086 19100 19101 19105 19110 19112 19120 19125 19126 19260 19271 19272 19281 19282 19283 19284 19285 19286 19287 19288 19296 19297 19298 19300 19301 19302 19303 19304 19305 19306 19307 19316 19318 19324 19325 19328 19330 19340 19342 19350 19355 19357 19361 19364 19366 19367 19368 19369 19370 19371 19380 19396 DESTRUCTION, FLAT WARTS, MOLLUSCUM CONTAGIOSUM/MIL DESTRUCTION, FLAT WARTS, MOLLUSCUM CONTAGIOSUM/MIL CHEMICAL CAUTERIZATION, GRANULATION TISSUE (PROUD DESTRUCTION, MALIGNANT LESION, TRUNK/ARMS/LEGS; LE DESTRUCTION, MALIGNANT LESION, TRUNK/ARMS/LEGS; LE DESTRUCTION, MALIGNANT LESION, TRUNK/ARMS/LEGS; LE DESTRUCTION, MALIGNANT LESION, TRUNK/ARMS/LEGS; LE DESTRUCTION, MALIGNANT LESION, TRUNK/ARMS/LEGS; LE DESTRUCTION, MALIGNANT LESION, TRUNK/ARMS/LEGS; LE DESTRUCTION, MALIGNANT LESION, SCALP/NECK/HANDS/FE DESTRUCTION, MALIGNANT LESION, SCALP/NECK/HANDS/FE DESTRUCTION, MALIGNANT LESION, SCALP/NECK/HANDS/FE DESTRUCTION, MALIGNANT LESION, SCALP/NECK/HANDS/FE DESTRUCTION, MALIGNANT LESION, SCALP/NECK/HANDS/FE DESTRUCTION, MALIGNANT LESION, SCALP/NECK/HANDS/FE DESTRUCTION, MALIGNANT LESION, FACE/EARS/EYELIDS/N DESTRUCTION, MALIGNANT LESION, FACE/EARS/EYELIDS/N DESTRUCTION, MALIGNANT LESION, FACE/EARS/EYELIDS/N DESTRUCTION, MALIGNANT LESION, FACE/EARS/EYELIDS/N DESTRUCTION, MALIGNANT LESION, FACE/EARS/EYELIDS/N DESTRUCTION, MALIGNANT LESION, FACE/EARS/EYELIDS/N Mohs, 1 stage, h/n/hf/g Mohs addl stage Mohs, 1 stage, t/a/l Mohs, addl stage, t/a/l Mohs surg, addl block CRYOTHERAPY (CO2 SLUSH, LIQUID N2) FOR ACNE CHEMICAL EXFOLIATION, ACNE ELECTROLYSIS EPILATION, EACH ONE HALF HOUR UNLISTED PROC, SKIN, MUCOUS MEMBRANE AND SUBQ TISS PUNCTURE ASPIRATION, CYST, BREAST; PUNCTURE ASPIRATION, CYST, BREAST; EACH ADDL CYST MASTOTOMY W/EXPLORATION/DRAINAGE, ABSCESS, DEEP INJECTION PROC ONLY, MAMMARY DUCTOGRAM/GALACTOGRAM BX BREAST W DEVICE 1ST LESION STEREOTACTIC GUIDE BX BREAST W DEVICE ADDL LESION STEREOTACT GUIDE BX BREAST W DEVICE 1ST LESION ULTRASOUND GUIDE BX BREAST W DEVICE ADDL LESION ULTRASOUND GUIDE BX BREAST W DEVICE 1ST LESION MAGNETIC RES GUIDE BX BREAST W DEVICE ADDL LESION MAGNET RES GUIDE BX, BREAST; PERCUT, NEEDLE CORE, NOT USING IMAGING BX, BREAST; OPEN INCISIONAL Cryosurg ablate fa, each NIPPLE EXPLORATION W/WO EXCISION SOLITARY/PAPILLOM EXCISION, LACTIFEROUS DUCT FISTULA EXCISION, BREAST LESION(S), OPEN, MALE/FEMALE; 1 O EXCISION, BREAST LESION, PREOP PLACEMENT, RADIOLOG EXCISION, BREAST LESION, PREOP PLACEMENT, RADIOLOG EXCISION, CHEST WALL TUMOR W/RIBS EXCISION, CHEST WALL TUMOR W/RIBS, W/RECONSTRUCT; EXCISION, CHEST WALL TUMOR W/RIBS, W/RECONSTRUCT; PERQ DEVICE PLACEMT BREAST LOC 1ST LES W GUIDNCE PERQ DEVICE PLACEMT BREAST LOC EA LES W GUIDNCE PERQ BREAST LOC DEVICE PLACEMT 1ST STRTCTC GUID PERQ BREAST LOC DEVICE PLACEMT EA LESION STRTCTC PERQ BREAST LOC DEVICE PLACEMT 1ST LESIO US IMAG PERQ BREAST LOC DEVICE PLACEMT EACH LES US IMAGE PERQ BREAST LOC DEVICE PLACEMT 1ST LESIO MR GUID PERQ BREAST LOC DEVICE PLACEMT ADD LESIO MR GUID PLACEMENT OF RADIOTHERAPY, FOLLOWING PARTIAL MASTE PLACEMENT OF RADIOTHERAPY CONCURRENT W PARTIAL MAS PLACEMENT OF RADIOTHERAPY, AT TIME OR SUBS TO PART Removal of breast tissue Partial mastectomy P-mastectomy w/ln removal Mast, simple, complete Mast, subq Mast, radical Mast, rad, urban type Mast, mod rad MASTOPEXY REDUCTION MAMMAPLASTY MAMMAPLASTY, AUGMENTATION; W/O PROSTHETIC IMPLANT MAMMAPLASTY, AUGMENTATION; W/PROSTHETIC IMPLANT REMOVAL, INTACT MAMMARY IMPLANT REMOVAL, MAMMARY IMPLANT MATL IMMEDIATE INSERTION, BREAST PROSTHESIS FOLLOWING M DELAYED INSERTION, BREAST PROSTHESIS FOLLOWING MAS NIPPLE/AREOLA RECONSTRUCTION CORRECTION, INVERTED NIPPLES BREAST RECONSTRUCTION W/TISSUE EXPANDER, IMMEDIATE BREAST RECONSTRUCTION W/LATISSIMUS DORSI FLAP, W/W BREAST RECONSTRUCTION W/FREE FLAP BREAST RECONSTRUCTION W/OTHER TECHNIQUE BREAST RECONSTRUCTION W/MYOCUTANEOUS (TRAM) FLAP, BREAST RECONSTRUCTION W/MYOCUTAN (TRAM) FLAP, SING BREAST RECONSTRUCTION W/MYOCUTANEOUS (TRAM) FLAP, OPEN PERIPROSTHETIC CAPSULOTOMY, BREAST PERIPROSTHETIC CAPSULECTOMY, BREAST REVISION, RECONSTRUCTED BREAST PREPARATION, MOULAGE, CUSTOM BREAST IMPLANT 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N Y N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 19499 20005 2000F 20100 20101 20102 20103 2010F 2014F 20150 2015F 2016F 2018F 2019F 20200 20205 20206 2020F 2021F 20220 20225 2022F 20240 20245 2024F 20250 20251 2026F 2027F 2028F 2029F 2030F 2031F 2040F 2044F 20500 20501 2050F 20520 20525 20526 20527 20550 20551 20552 20553 20555 20600 20604 20605 20606 2060F 20610 20611 20612 20615 20650 20660 20661 20662 20663 20664 20665 20670 20680 20690 20692 20693 20694 20696 20697 20802 20805 20808 20816 20822 20824 20827 20838 20900 20902 20910 20912 20920 20922 20924 20926 20930 20931 20936 20937 UNLISTED PROC, BREAST I&D SOFT TISSUE ABSCESS SUBFASC BLOOD PRESSURE MEASURED EXPLORATION, PENETRATING WOUND (SEP PROC); NECK EXPLORATION, PENETRATING WOUND (SEP PROC); CHEST EXPLORATION, PENETRATING WOUND (SEP PROC); ABDOMEN EXPLORATION OF PENETRATING WOUND, EXTREMITY Vital signs recorded Mental status assess EXCISION, EPIPHYSEAL BAR, W/WO AUTOGENOUS SOFT TIS ASTHMA IMPAIRMENT ASSESSED ASTHMA RISK ASSESSED Hydration status assess Dilated macul exam done BX, MUSCLE; SUPERFICIAL BX, MUSCLE; DEEP BX, MUSCLE, PERCUTANEOUS NEEDLE Dilated fundus eval done Dilat macul+exam done BX, BONE, TROCAR/NEEDLE; SUPERFICIAL BX, BONE, TROCAR/NEEDLE; DEEP Dil retina exam interp rev BX, BONE, EXCISIONAL; SUPERFICIAL BX, BONE, EXCISIONAL; DEEP 7 field photo interp doc rev BX, VERTEBRAL BODY, OPEN; THORACIC BX, VERTEBRAL BODY, OPEN; LUMBAR/CERVICAL Eye image valid to dx rev Optic nerve head eval done Foot exam performed Complete phys skin exam done H2O stat documented, normal H2O stat documented, dehydrated PHYS EXAM ON DATE OF INIT VST FOR LBP DONE DOC MNTL HLTH ASSESSB/4 INTRVN-BKSURG/EPID INJX INJECTION, SINUS TRACT; THERAPEUTIC (SEP PROC) INJECTION, SINUS TRACT; DX (SINOGRAM) WOUND CHAR SIZE ETC DOCD REMOVAL, FB IN MUSCLE/TENDON SHEATH; SIMPLE REMOVAL, FB IN MUSCLE/TENDON SHEATH; DEEP/COMPLICA INJECTION, THERAPEUTIC, CARPAL CANAL INJECTION ENZYME PALMAR FASCIAL CORD INJECTION(S); SINGLE TENDON SHEATH, LIGAMENT, APON INJECTION(S); SINGLE TENDON ORIGIN/INSERTION INJECTION(S); SINGLE/MULTIPLE TRIGGER POINT(S), 1INJECTION(S); SINGLE/MULTIPLE TRIGGER POINT(S), OV PLACEMENT OF NEEDLES OR CATHETERS INTO MUSCLE AND/ ARTHROCENTESIS ASPIR&/INJ SMALL JT/BURSA W/O US ARTHROCNT ASPIR&/INJ SMALL JT/BURSAW/US REC RPRT ARTHROCENTESIS ASPIR&/INJ INTERM JT/BURS W/O US ARTHROCENTESIS ASPIR&/INJ INTERM JT/BURS W/US PT INTRVWD BY EVAL CLINICIAN </DATE DIAG MDD ARTHROCENTESIS ASPIR&/INJ MAJOR JT/BURSA W/O US ARTHROCENTESIS ASPIR&/INJ MAJOR JT/BURSA W/US ASPIRATION AND/OR INJECTION, GANGLION CYST(S) ANY ASPIRATION AND INJECTION, TREATMENT, BONE CYST INSERTION, WIRE/PIN W/APPLICATION, SKELETAL TRACTI APPLICATION, CRANIAL TONGS, CALIPER/STEREOTACTIC F APPLICATION, HALO, W/REMOVAL; CRANIAL APPLICATION, HALO, W/REMOVAL; PELVIC APPLICATION, HALO, W/REMOVAL; FEMORAL APPL HALO 6/> PINS THIN SKULL OSTEOLOGY REMOVAL TONG/HALO APPLIED BY ANOTHER INDIVIDUAL REMOVAL, IMPLANT; SUPERFICIAL (SEP PROC) REMOVAL, IMPLANT; DEEP APPLICATION, UNIPLANE (PINS/WIRES IN ONE PLANE), U APPLICATION, MULTIPLANE, UNILAT, EXT FIXATION SYST ADJUSTMENT/REVISION, EXT FIXATION SYSTEM W/ANESTHE REMOVAL, UNDER ANESTHESIA, EXT FIXATION SYSTEM XTRNL FIXJ W/STEREOTACTIC ADJUSTMENT 1ST&SUBQ XTRNL FIXJ W/STRTCTC ADJUSTMENT EXCHANGE STRUT REPLANTATION, ARM (INCLUDES SURGICAL NECK, HUMERUS REPLANTATION, FOREARM (INCLUDES RADIUS AND ULNA TO REPLANTATION, HAND (INCLUDES HAND THROUGH METACARP REPLANTATION, DIGIT, W/O THUMB, (INC METACARPOPHAL REPLANTATION, DIGIT, W/O THUMB, (DISTAL TIP TO SUB REPLANTATION, THUMB (INCLUDES CARPOMETACARPAL JOIN REPLANTATION, THUMB (INCLUDES DISTAL TIP TO MP JOI REPLANTATION, FOOT, COMPLETE AMPUTATION BONE GRAFT, ANY DONOR AREA; MINOR/SMALL BONE GRAFT, ANY DONOR AREA; MAJOR/LARGE CARTILAGE GRAFT; COSTOCHONDRAL CARTILAGE GRAFT; NASAL SEPTUM FASCIA LATA GRAFT; STRIPPER FASCIA LATA GRAFT; INCISION AND AREA EXPOSURE, COM TENDON GRAFT, FROM A DISTANCE TISSUE GRAFTS, OTHER ALLOGRAFT FOR SPINE SURGERY ONLY MORSELIZED ALLOGRAFT FOR SPINE SURGERY ONLY STRUCTURAL AUTOGRAFT, SPINE SURGERY ONLY; LOCAL, SAME INCISIO AUTOGRAFT, SPINE SURGERY ONLY; MORSELIZED, SEP INC 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2012 1/1/2012 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2015 1/1/2004 1/1/2015 1/1/2010 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 20938 20950 20955 20956 20957 20962 20969 20970 20972 20973 20974 20975 20979 20982 20983 20985 20999 21010 21011 21012 21013 21014 21015 21016 21025 21026 21029 21030 21031 21032 21034 21040 21044 21045 21046 21047 21048 21049 21050 21060 21070 21073 21076 21077 21079 21080 21081 21082 21083 21084 21085 21086 21087 21088 21089 21100 21110 21116 21120 21121 21122 21123 21125 21127 21137 21138 21139 21141 21142 21143 21145 21146 21147 21150 21151 21154 21155 21159 21160 21172 21175 21179 21180 21181 21182 21183 21184 21188 21193 21194 21195 AUTOGRAFT, SPINE SURGERY ONLY; STRUCTURAL/BICORTIC MONITORING, INTERSTITIAL FLUID PRESSURE W/DEVICE I BONE GRAFT W/MICROVASCULAR ANASTOMOSIS; FIBULA BONE GRAFT W/MICROVASCULAR ANASTOMOSIS; ILIAC CRES BONE GRAFT W/MICROVASCULAR ANASTOMOSIS; METATARSAL BONE GRAFT W/MICROVASCULAR ANASTOMOSIS; OTHER THAN FREE OSTEOCUTANEOUS FLAP W/MICROVASC ANASTOMOSIS; FREE OSTEOCUTANEOUS FLAP W/MICROVASCULAR ANASTOMOS FREE OSTEOCUTANEOUS FLAP W/MICROVASCULAR ANASTOMOS FREE OSTEOCUTANEOUS FLAP W/MICROVASCULAR ANASTOMOS ELECTRICAL STIMULATION TO AID BONE HEALING; NONINV ELECTRICAL STIMULATION TO AID BONE HEALING; INVASI LOW INTENSITY ULTRASOUND STIMULATION TO AID BONE H ABLATION BONE TUMOR RF PERQ W/IMG GDN WHEN DONE ABLATJ BONE TUMOR CRYO PERQ W/IMG GDN WHEN PRFMD COMPUTER-ASSISTED SURGICAL NAVIGATIONAL PROCEDURE UNLISTED PROC, MUSCULOSKELETAL SYSTEM, GENERAL ARTHROTOMY, TEMPOROMANDIBULAR JOINT EXCISION TUMOR SOFT TISS FACE/SCALP SUBQ < 2CM EXCISION TUMOR SOFT TISS FACE/SCALP SUBQ 2+CM EXC TUMOR SOFT TISS FACE&SCALP SUBFASCIAL < 2CM EXC TUMOR SOFT TISS FACE&SCALP SUBFASCIAL 2+CM RAD RESECTION TUMOR SOFT TISS FACE/SCALP < 2CM RAD RESECTION TUMOR SOFT TISS FACE/SCALP 2 CM/> EXCISION, BONE; MANDIBLE EXCISION, BONE; FACIAL BONE(S) REMOVAL BY CONTOURING, BENIGN TUMOR, FACIAL BONE EXCISION, BENIGN TUMOR/CYST MAXILLA/ZYGOMA, ENUCLE EXCISION, TORUS MANDIBULARIS EXCISION, MAXILLARY TORUS PALATINUS EXCISION, MALIGNANT TUMOR, MAXILLA/ZYGOMA EXCISION, BENIGN TUMOR/CYST, MANDIBLE; ENUCLEATION EXCISION, MALIGNANT TUMOR, MANDIBLE EXCISION, MALIGNANT TUMOR, MANDIBLE; RADICAL RESEC EXCISION, BENIGN TUMOR/CYST, MANDIBLE; INTRA-ORAL EXCISION, BENIGN TUMOR/CYST, MANDIBLE; EXTRA-ORAL EXCISION, BENIGN TUMOR/CYST, MAXILLA; INTRA-ORAL O EXCISION, BENIGN TUMOR/CYST, MAXILLA; EXTRA-ORAL O CONDYLECTOMY, TEMPOROMANDIBULAR JOINT (SEP PROC) MENISCECTOMY, PARTIAL/COMPLETE, TEMPOROMANDIBULAR CORONOIDECTOMY (SEP PROC) MANIPULATION OF TEMPOROMANDIBULAR JOINT(S), THERAP IMPRESSION AND CUSTOM PREPARATION; SURGICAL OBTURA IMPRESSION AND CUSTOM PREPARATION; ORBITAL PROSTHE IMPRESSION AND CUSTOM PREPARATION; INTERIM OBTURAT IMPRESSION AND CUSTOM PREPARATION; DEFINITIVE OBTU IMPRESSION AND CUSTOM PREPARATION; MANDIBULAR RESE IMPRESSION AND CUSTOM PREPARATION; PALATAL AUGMENT IMPRESSION AND CUSTOM PREPARATION; PALATAL LIFT PR IMPRESSION AND CUSTOM PREPARATION; SPEECH AID PROS IMPRESSION AND CUSTOM PREPARATION; ORAL SURGICAL S IMPRESSION AND CUSTOM PREPARATION; AURICULAR PROST IMPRESSION AND CUSTOM PREPARATION; NASAL PROSTHESI IMPRESSION AND CUSTOM PREPARATION; FACIAL PROSTHES UNLISTED MAXILLOFACIAL PROSTHETIC PROC APPLICATION, HALO TYPE APPLIANCE, MAXILLOFACIAL FI APPLICATION, INTERDENTAL FIXATION DEVICE, NON-FX/D INJECTION PROC, TEMPOROMANDIBULAR JOINT ARTHROGRAP GENIOPLASTY; AUGMENTATION (AUTOGRAFT, ALLOGRAFT, P GENIOPLASTY; SLIDING OSTEOTOMY, SINGLE PIECE GENIOPLASTY; SLIDING OSTEOTOMIES, OVER 2 OSTEOTOMI GENIOPLASTY; SLIDING, AUGMENTATION W/INTERPOSITION AUGMENTATION, MANDIBULAR BODY/ANGLE; PROSTHETIC MA AUGMENTATION, MANDIBULAR BODY/ANGLE; W/BONE GRAFT/ REDUCTION FOREHEAD; CONTOURING ONLY REDUCTION FOREHEAD; CONTOURING/PROSTHESIS/BONE GRA REDUCTION FOREHEAD; CONTOURING AND SETBACK, ANTERI RECONSTRUCTION MIDFACE, LEFORT I; 1 PIECE, W/O BON RECONSTRUCTION MIDFACE, LEFORT I; 2 PIECES, W/O BO RECONSTRUCTION MIDFACE, LEFORT I; OVER 3 PIECES, W RECONSTRUCTION MIDFACE, LEFORT I; 1 PIECE, W/BONE RECONSTRUCTION MIDFACE, LEFORT I; 2 PIECES, W/BONE RECONSTRUCTION MIDFACE, LEFORT I; OVER 3 PIECES, W RECONSTRUCTION MIDFACE, LEFORT II; ANTERIOR INTRUS RECONSTRUCTION MIDFACE, LEFORT II; W/BONE GRAFTS RECONSTRUCTION MIDFACE, LEFORT III, W/BONE GRAFTS; RECONSTRUCTION MIDFACE, LEFORT III, W/BONE GRAFTS; RECONSTRUCTION MIDFACE, LEFORT III, (EXTRA/INTRACR RECONSTRUCTION MIDFACE, LEFORT III, (EXTRA/INTRACR RECONSTRUCTION SUPERIOR-LATERAL ORBITAL RIM AND LO RECONSTRUCTION, BIFRONTAL,SUPERIOR-LATERAL ORBITAL RECONSTRUCTION, MAJORITY, FOREHEAD AND SUPRAORBITA RECONSTRUCTION, MAJORITY, FOREHEAD AND SUPRAORBITA RECONSTRUCTION, CONTOURING, BENIGN TUMOR, CRANIAL RECONSTRUCTION, ORBIT/FOREHEAD/NASOETHMOID, FOLLOW RECONSTRUCTION, ORBIT/FOREHEAD/NASOETHMIOD, FOLLOW RECONSTRUCTION, ORBIT/FOREHEAD/NASOETHMOID, FOLLOW RECONSTRUCTION, MIDFACE, OSTEOTOMIES (NON-LEFORT T RECONSTRUCTION, MANDIBULAR RAMI, HORIZONTAL, VERTI RECONSTRUCTION, MANDIBULAR RAMI, HORIZONTAL, VERTI RECONSTRUCTION, MANDIBULAR RAMI AND/OR BODY, SAGIT 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2008 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 21196 21198 21199 21206 21208 21209 21210 21215 21230 21235 21240 21242 21243 21244 21245 21246 21247 21248 21249 21255 21256 21260 21261 21263 21267 21268 21270 21275 21280 21282 21295 21296 21299 21310 21315 21320 21325 21330 21335 21336 21337 21338 21339 21340 21343 21344 21345 21346 21347 21348 21355 21356 21360 21365 21366 21385 21386 21387 21390 21395 21400 21401 21406 21407 21408 21421 21422 21423 21431 21432 21433 21435 21436 21440 21445 21450 21451 21452 21453 21454 21461 21462 21465 21470 21480 21485 21490 21495 21497 21499 21501 RECONSTRUCTION, MANDIBULAR RAMI AND/OR BODY, SAGIT OSTEOTOMY, MANDIBLE, SEGMENTAL OSTEOTOMY, MANDIBLE, SEGMENTAL; W/GENIOGLOSSUS ADV OSTEOTOMY, MAXILLA, SEGMENTAL OSTEOPLASTY, FACIAL BONES; AUGMENTATION (AUTOGRAFT OSTEOPLASTY, FACIAL BONES; REDUCTION GRAFT, BONE; NASAL, MAXILLARY/MALAR AREAS (INCLUDE GRAFT, BONE; MANDIBLE (INCLUDES OBTAINING GRAFT) GRAFT; RIB CARTILAGE, AUTOGENOUS, FACE/CHIN/NOSE/E GRAFT; EAR CARTILAGE, AUTOGENOUS, NOSE/EAR (INCLUD ARTHROPLASTY, TEMPOROMANDIBULAR JOINT, W/WO AUTOGR ARTHROPLASTY, TEMPOROMANDIBULAR JOINT, W/ALLOGRAFT ARTHROPLASTY, TEMPOROMANDIBULAR JOINT, W/PROSTHETI RECONSTRUCTION, MANDIBLE, EXTRAORAL, W/TRANSOSTEAL RECONSTRUCTION, MANDIBLE/MAXILLA, SUBPERIOSTEAL IM RECONSTRUCTION, MANDIBLE/MAXILLA, SUBPERIOSTEAL IM RECONSTRUCTION, MANDIBULAR CONDYLE W/BONE AND CART RECONSTRUCTION, MANDIBLE/MAXILLA, ENDOSTEAL IMPLAN RECONSTRUCTION, MANDIBLE/MAXILLA, ENDOSTEAL IMPLAN RECONSTRUCTION, ZYGOMATIC ARCH/GLENOID FOSSA W/BON RECONSTRUCTION, ORBIT W/OSTEOTOMIES AND BONE GRAFT PERIORBITAL OSTEOTOMIES, ORBITAL HYPERTELORISM, W/ PERIORBITAL OSTEOTOMIES, ORBITAL HYPERTELORISM, W/ PERIORBITAL OSTEOTOMIES, ORBITAL HYPERTELORISM, W/ ORBITAL REPOSITIONING, PERIORBITAL OSTEOTOMIES, UN ORBITAL REPOSITIONING, PERIORBITAL OSTEOTOMIES, UN MALAR AUGMENTATION, PROSTHETIC MATL SECONDARY REVISION, ORBITOCRANIOFACIAL RECONSTRUCT MEDIAL CANTHOPEXY (SEP PROC) LATERAL CANTHOPEXY REDUCTION, MASSETER MUSCLE/BONE; EXTRAORAL APPROAC REDUCTION, MASSETER MUSCLE/BONE; INTRAORAL APPROAC UNLISTED CRANIOFACIAL AND MAXILLOFACIAL PROC CLOSED TREATMENT, NASAL BONE FX W/O MANIPULATION CLOSED TREATMENT, NASAL BONE FX; W/O STABILIZATION CLOSED TREATMENT, NASAL BONE FX; W/STABILIZATION OPEN TREATMENT, NASAL FX; UNCOMPLICATED OPEN TREATMENT, NASAL FX; COMPLICATED, W/INT AND/O OPEN TREATMENT, NASAL FX; W/CONCOMITANT OPEN TREAT OPEN TREATMENT, NASAL SEPTAL FX, W/WO STABILIZATIO CLOSED TREATMENT, NASAL SEPTAL FX, W/WO STABILIZAT OPEN TREATMENT, NASOETHMOID FX; W/O EXT FIXATION OPEN TREATMENT, NASOETHMOID FX; W/EXT FIXATION PERCUT TREATMENT, NASOETHMOID COMPLEX FX, W SPLINT OPEN TREATMENT, DEPRESSED FRONTAL SINUS FX OPEN TREATMENT, COMPLICATED FRONTAL SINUS FX CLOSED TREATMENT, NASOMAXILLARY COMPLEX FX (LEFORT OPEN TREATMENT, NASOMAXILLARY COMPLEX FX (LEFORT I OPEN TREATMENT, NASOMAXILLARY COMPLEX FX (LEFORT I OPEN TREATMENT, NASOMAXILLARY COMPLEX FX (LEFORT I PERCUT TREATMENT, FX, MALAR AREA, W/ZYGOMATIC ARCH OPEN TREATMENT OF DEPRESSED ZYGOMATIC ARCH FRACTUR OPEN TREATMENT, DEPRESSED MALAR FX, W/ZYGOMATIC AR OPEN TREATMENT, COMPLICATED FX, MALAR AREA, W/ZYGO OPEN TREATMENT, COMPLICATED FX, MALAR AREA, W/ZYGO OPEN TREATMENT, ORBITAL FLOOR BLOWOUT FX; TRANSANT OPEN TREATMENT, ORBITAL FLOOR BLOWOUT FX; PERIORBI OPEN TREATMENT, ORBITAL FLOOR BLOWOUT FX; COMBINED OPEN TREATMENT, ORBITAL FLOOR BLOWOUT FX; PERIORBI OPEN TREATMENT, ORBITAL FLOOR BLOWOUT FX; PERIORBI CLOSED TREATMENT, FX, ORBIT, EXCEPT BLOWOUT; W/O M CLOSED TREATMENT, FX, ORBIT, EXCEPT BLOWOUT; W/MAN OPEN TREATMENT, FX, ORBIT, EXCEPT BLOWOUT; W/O IMP OPEN TREATMENT, FX, ORBIT, EXCEPT BLOWOUT; W/IMPLA OPEN TREATMENT, FX, ORBIT, EXCEPT BLOWOUT; W/BONE CLOSED TREATMENT, PALATAL/MAXILLARY FX (LEFORT I T OPEN TREATMENT, PALATAL/MAXILLARY FX (LEFORT I TYP OPEN TREATMENT, PALATAL/MAXILLARY FX (LEFORT I TYP CLOSED TREATMENT, CRANIOFACIAL SEPARATION (LEFORT OPEN TREATMENT, CRANIOFACIAL SEPARATION (LEFORT II OPEN TREATMENT, CRANIOFACIAL SEPARATION (LEFORT II OPEN TREATMENT, CRANIOFACIAL SEPARATION (LEFORT II OPEN TREATMENT, CRANIOFACIAL SEPARATION (LEFORT II CLOSED TREATMENT, MANDIBULAR/MAXILLARY ALVEOLAR RI OPEN TREATMENT, MANDIBULAR/MAXILLARY ALVEOLAR RIDG CLOSED TREATMENT, MANDIBULAR FX; W/O MANIPULATION CLOSED TREATMENT, MANDIBULAR FX; W/MANIPULATION PERCUTANEOUS TREATMENT, MANDIBULAR FX, W/EXT FIXAT CLOSED TREATMENT, MANDIBULAR FX W/INTERDENTAL FIXA OPEN TREATMENT, MANDIBULAR FX W/EXT FIXATION OPEN TREATMENT, MANDIBULAR FX; W/O INTERDENTAL FIX OPEN TREATMENT, MANDIBULAR FX; W/INTERDENTAL FIXAT OPEN TREATMENT, MANDIBULAR CONDYLAR FX OPEN TREATMENT, MANDIBULAR FX, COMPLICATED, MULTIP CLOSED TREATMENT, TEMPOROMANDIBULAR DISLOCATION; I CLOSED TREATMENT, TEMPOROMANDIBULAR DISLOCATION; C OPEN TREATMENT, TEMPOROMANDIBULAR DISLOCATION OPEN TREATMENT, HYOID FX INTERDENTAL WIRING, CONDITION OTHER THAN FX UNLISTED MUSCULOSKELETAL PROC, HEAD INCISION AND DRAINAGE, DEEP ABSCESS/HEMATOMA, SOFT 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N Y N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 21502 21510 21550 21552 21554 21555 21556 21557 21558 21600 21610 21615 21616 21620 21627 21630 21632 21685 21700 21705 21720 21725 21740 21742 21743 21750 21805 21811 21812 21813 21820 21825 21899 21920 21925 21930 21931 21932 21933 21935 21936 22010 22015 22100 22101 22102 22103 22110 22112 22114 22116 22206 22207 22208 22210 22212 22214 22216 22220 22222 22224 22226 22305 22310 22315 22318 22319 22325 22326 22327 22328 22505 22510 22511 22512 22513 22514 22515 22526 22527 22532 22533 22534 22548 22551 22552 22554 22556 22558 22585 22586 INCISION AND DRAINAGE, DEEP ABSCESS/HEMATOMA, SOFT INCISION, DEEP, W/OPENING, BONE CORTEX, THORAX BX, SOFT TISSUE, NECK/THORAX EXC TUMOR SOFT TISSUE NECK/ANT THORAX SUBQ 3+CM EXC TUMOR SOFT TISSUE NECK/THORAX SUBFASC 5+CM EXCISION TUMOR, SOFT TISSUE, NECK/THORAX; SUBQ EXCISION TUMOR, SOFT TISSUE, NECK/THORAX; SUBFASC RAD RESECT TUMOR SOFT TISS NECK/ANT THORAX <5CM RAD RESECT TUMOR SOFT TISS NECK/ANT THORAX 5CM/> EXCISION, RIB, PARTIAL COSTOTRANSVERSECTOMY (SEP PROC) EXCISION 1ST AND/OR CERVICAL RIB EXCISION 1ST AND/OR CERVICAL RIB; W/SYMPATHECTOMY OSTECTOMY, STERNUM, PARTIAL STERNAL DEBRIDEMENT RADICAL RESECTION, STERNUM; RADICAL RESECTION, STERNUM; W/MEDIASTINAL LYMPHADE HYOID MYOTOMY AND SUSPENSION DIVISION, SCALENUS ANTICUS; W/O RESECTION, CERVICA DIVISION, SCALENUS ANTICUS; W/RESECTION, CERVICAL DIVISION, STERNOCLEIDOMASTOID, TORTICOLLIS, OPEN O DIVISION, STERNOCLEIDOMASTOID, TORTICOLLIS, OPEN O RECONSTRUCTIVE REPAIR, PECTUS EXCAVATUM/CARINATUM; RECONSTRUCTIVE REPAIR, PECTUS EXCAVATUM/CARINATUM; RECONSTRUCTIVE REPAIR, PECTUS EXCAVATUM/CARINATUM; CLOSURE, MEDIAN STERNOTOMY SEPARATION, W/WO DEBRID OPEN TREATMENT, RIB FX W/O FIXATION, EACH OPEN TX RIB FX W/FIXJ THORACOSCOPIC VIS 1-3 RIBS OPEN TX RIB FX W/FIXJ THORACOSCOPIC VIS 4-6 RIBS OPEN TX RIB FX W/FIXJ THORACOSCOPIC VIS 7+ RIBS CLOSED TREATMENT, STERNUM FX OPEN TREATMENT, STERNUM FX W/WO SKELETAL FIXATION UNLISTED PROC, NECK/THORAX BX, SOFT TISSUE, BACK/FLANK; SUPERFICIAL BX, SOFT TISSUE, BACK/FLANK; DEEP EXC, TUMOR, SOFT TISSUE, BACK/FLANK SUBQ EXCISION TUMOR SOFT TISSUE BACK/FLANK SUBQ 3+CM EXC TUMOR SOFT TISS BACK/FLANK SUBFASCIAL <5CM EXC TUMOR SOFT TISS BACK/FLANK SUBFASCIAL 5+CM RAD RESECTION TUMOR SOFT TISSUE BACK/FLANK <5CM RAD RESECTION TUMOR SOFT TISSUE BACK/FLANK 5CM/> I & D; OPEN OF DEEP ABSCESS; POSTERIOR SPINE; CERV I & D; OPEN OF DEEP ABSCESS; POSTERIOR SPINE; LUMB PARTIAL EXCISION, POSTERIOR VERTEBRAL COMPONENT, I PARTIAL EXCISION, POSTERIOR VERTEBRAL COMPONENT, I PARTIAL EXCISION, POSTERIOR VERTEBRAL COMPONENT, I PARTIAL EXCISION, POST VERTEBRAL COMPONENT, INTRIN PART EXCIS, VERTEBRAL BODY, BONY LESION, W/O SPINA PART EXCIS, VERTEBRAL BODY, BONY LESION, W/O SPINA PART EXCIS, VERTEBRAL BODY, BONY LESION, W/O SPINA PART EXCIS, VERTEBRAL BODY, BONY LESION, W/O SPINA OSTEOTOMY OF SPINE, POSTERIOR OR POSTEROLATERAL AP OSTEOTOMY OF SPINE, LUMBAR EACH ADDITIONAL VERTEBRAL SEGMENT (LIST SEPARATELY OSTEOTOMY, SPINE, POSTERIOR/POSTEROLATERAL APPROAC OSTEOTOMY, SPINE, POSTERIOR/POSTEROLATERAL APPROAC OSTEOTOMY, SPINE, POSTERIOR/POSTEROLATERAL APPROAC OSTEOTOMY, SPINE, POSTERIOR/POSTEROLATERAL APPROAC OSTEOTOMY, SPINE, W/DISKECTOMY, ANTERIOR APPROACH, OSTEOTOMY, SPINE, W/DISKECTOMY, ANTERIOR APPROACH, OSTEOTOMY, SPINE, W/DISKECTOMY, ANTERIOR APPROACH, OSTEOTOMY, SPINE, W/DISKECTOMY, ANTERIOR APPROACH, CLOSED TREATMENT, VERTEBRAL PROCESS FX(S) CLOSED TREATMENT, VERTEBRAL BODY FX(S), W/O MANIPU CLTX VRT FX&/DISLC CSTING/BRACING MNPJ/TRCJ OPEN TREAT AND/OR REDUCTION, ODONTOID FX(S) AND/OR OPEN TREAT AND/OR REDUCTION, ODONTOID FX(S) AND/OR OPEN TREAT AND/OR REDUCT, VERTBR FX(S) AND/OR DISL OPEN TREAT AND/OR REDUCT, VERTBR FX(S) AND/OR DISL OPEN TREAT AND/OR REDUCT, VERTBR FX(S) AND/OR DISL OPEN TREAT AND/OR REDUCT, VERTBR FX(S) AND/OR DISL MANIPULATION, SPINE, REQUIRING ANESTHESIA, ANY REG PERQ VERTEBROPLASTY UNI/BI INJX CERVICOTHORACIC PERQ VERTEBROPLASTY UNI/BI INJECTION LUMBOSACRAL VERTEBROPLASTY EACH ADDL CERVICOTHOR/LUMBOSACRAL PERQ VERT AGMNTJ CAVITY CRTJ UNI/BI CANNULATION PERQ VERT AGMNTJ CAVITY CRTJ UNI/BI CANNULJ LMBR PERQ VERT AGMNTJ CAVITY CRTJ UNI/BI CANNULJ EACH Idet, single level Idet, 1 or more levels LAT EXTRACAVITARY ARTHRODESIS, W/MIN DISKECTOMY; T LAT EXTRACAVITARY ARTHRODESIS, W/MIN DISKECTOMY; L LAT EXTRACAVITARY ARTHRODESIS, W/MIN DISKECTOMY; T ARTHRODESIS, ANTERIOR TRANSORAL/EXTRAORAL, ATLAS-A ARTHRD ANT INTERBODY DECOMPRESS CERVICAL BELW C2 ARTHRD ANT INTERDY CERVCL BELW C2 EA ADDL NTRSPC ARTHRODESIS, ANTERIOR INTERBODY, W/MININMAL DISKEC ARTHRODESIS, ANTERIOR INTERBODY, W/MININMAL DISKEC ARTHRODESIS, ANTERIOR INTERBODY, W/MININMAL DISKEC ARTHRODESIS, ANTERIOR INTERBODY, W/MININMAL DISKEC ARTHRODESIS PRESACRAL INTRBDY W/INSTRUMENT L5/S1 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2010 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 22590 22595 22600 22610 22612 22614 22630 22632 22633 22634 22800 22802 22804 22808 22810 22812 22818 22819 22830 22840 22841 22842 22843 22844 22845 22846 22847 22848 22849 22850 22851 22852 22855 22856 22857 22858 22861 22862 22864 22865 22899 22900 22901 22902 22903 22904 22905 22999 23000 23020 23030 23031 23035 23040 23044 23065 23066 23071 23073 23075 23076 23077 23078 23100 23101 23105 23106 23107 23120 23125 23130 23140 23145 23146 23150 23155 23156 23170 23172 23174 23180 23182 23184 23190 23195 23200 23210 23220 23330 23332 23333 ARTHRODESIS, POSTERIOR TECHNIQUE, CRANIOCERVICAL ARTHRODESIS, POSTERIOR TECHNIQUE, ATLAS-AXIS ARTHRODESIS, POSTERIOR/POSTEROLATERAL TECHNIQUE, S ARTHRODESIS POSTERIOR/POSTEROLATERAL THORACIC ARTHRODESIS POSTERIOR/POSTEROLATERAL LUMBAR ARTHRODESIS, POSTERIOR/POSTEROLATERAL TECHNIQUE, S ARTHRODESIS, POST INTERBODY W/LAMINECTOMY AND/OR D ARTHRODESIS, POST INTERBODY W/LAMINECT AND/OR DISK ARTHDSIS POST/POSTEROLATRL/POSTINTERBODY LUMBAR ARTHDSIS POST/POSTERLATRL/POSTINTRBDYADL SPC/SEG ARTHRODESIS, POSTERIOR, SPINAL DEFORMITY, W/WO CAS ARTHRODESIS, POSTERIOR, SPINAL DEFORMITY, W/WO CAS ARTHRODESIS, POSTERIOR, SPINAL DEFORMITY, W/WO CAS ARTHRODESIS, ANTERIOR, SPINAL DEFORMITY, W/WO CAST ARTHRODESIS, ANTERIOR, SPINAL DEFORMITY, W/WO CAST ARTHRODESIS, ANTERIOR, SPINAL DEFORMITY, W/WO CAST KYPHECTOMY, CIRCUMFERENTIAL EXPOSURE, SPINE; 1-2 V KYPHECTOMY, CIRCUMFERENTIAL EXPOSURE, SPINE; OVER EXPLORATION, SPINAL FUSION POST NON-SEGMENTAL INSTRUMENTATION INT SPINAL FIXATION, WIRING, SPINOUS PROCESSES POSTERIOR SEGMENTAL INSTRUMENTATION; 3-6 VERTEBRAL POSTERIOR SEGMENTAL INSTRUMENTATION; 7-12 VERTEBRA POSTERIOR SEGMENTAL INSTRUMENTATION; OVER 13 VERTE ANTERIOR INSTRUMENTATION; 2 TO 3 VERTEBRAL SEGMENT ANTERIOR INSTRUMENTATION; 4 TO 7 VERTEBRAL SEGMENT ANTERIOR INSTRUMENTATION;OVER 8 VERTEBRAL SEGMENT PELVIC FIXATION, NON-SACRUM REINSERTION, SPINAL FIXATION DEVICE REMOVAL, POSTERIOR NONSEGMENTAL INSTRUMENTATION APPLICATION INTERVERTEBRAL BIOMECHANICAL DEVICE REMOVAL, POSTERIOR SEGMENTAL INSTRUMENTATION REMOVAL, ANTERIOR INSTRUMENTATION TOT DISC ARTHRP ART DISC ANT APPRO 1 NTRSPC CRV Lumbar artif diskectomy TOT DISC ARTHRP ANT APPR DISC 2ND LEVEL CERVICAL REVJ RPLCMT DISC ARTHROPLASTY ANT 1 NTRSPC CRV Revise lumbar artif disc RMVL DISC ARTHROPLASTY ANT 1 INTERSPACE CERVICAL Remove lumb artif disc UNLISTED PROC, SPINE EXCISION, ABDOMINAL WALL TUMOR, SUBFASCIAL EXC TUMOR SOFT TISSUE ABDL WALL SUBFASCIAL 5+CM EXC TUMOR SOFT TISSUE ABDOMINAL WALL SUBQ <3CM EXC TUMOR SOFT TISSUE ABDOMINAL WALL SUBQ 3+CM RAD RESECTION TUMOR SOFT TISSUE ABDL WALL <5CM RAD RESECTION TUMOR SOFT TISSUE ABDL WALL 5 CM/> UNLISTED PROC, ABDOMEN, MUSCULOSKELETAL SYSTEM REMOVAL, SUBDELTOID CALCAREOUS DEPOSITS, OPEN CAPSULAR CONTRACTURE RELEASE INCISION AND DRAINAGE, SHOULDER AREA; DEEP ABSCESS INCISION AND DRAINAGE, SHOULDER AREA; INFECTED BUR INCISION, BONE CORTEX, SHOULDER AREA ARTHROTOMY, GLENOHUMERAL JOINT, W/EXPLORATION, DRA ARTHROTOMY, ACROMIOCLAVICULAR, STERNOCLAVICULAR JN BX, SOFT TISSUE, SHOULDER AREA; SUPERFICIAL BX, SOFT TISSUE, SHOULDER AREA; DEEP EXCISION TUMOR SOFT TISSUE SHOULDER SUBQ 3+CM EXC TUMOR SOFT TISSUE SHOULDER SUBFASCIAL 5+CM EXCISION, SOFT TISSUE TUMOR, SHOULDER AREA; SUBQ EXCISION, SOFT TISSUE TUMOR, SHOULDER AREA; SUBFA RAD RESECTION TUMOR SOFT TISSUE SHOULDER <5CM RAD RESECTION TUMOR SOFT TISSUE SHOULDER 5 CM/> ARTHROTOMY, GLENOHUMERAL JOINT, W/BX ARTHROTOMY, ACROMIO/STERNOCLAVICULAR JOINT, W/BX A ARTHROTOMY; GLENOHUMERAL JOINT, W/SYNOVECTOMY, W/W ARTHROTOMY; STERNOCLAVICULAR JOINT, W/SYNOVECTOMY, ARTHROTOMY, GLENOHUMERAL JOINT, W/EXPLORATION, W/W CLAVICULECTOMY; PARTIAL CLAVICULECTOMY; TOTAL ACROMIOPLASTY/ACROMIONECTOMY, PARTIAL, W/WO CORACO EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, CLAVIC EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, CLAVIC EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, CLAVIC EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, PROXIM EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, PROXIM EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, PROXIM SEQUESTRECTOMY, CLAVICLE SEQUESTRECTOMY, SCAPULA SEQUESTRECTOMY, HUMERAL HEAD TO SURGICAL NECK PARTIAL EXCISION, BONE, CLAVICLE PARTIAL EXCISION, BONE, SCAPULA PARTIAL EXCISION, BONE, PROXIMAL HUMERUS OSTECTOMY, SCAPULA, PARTIAL RESECTION, HUMERAL HEAD RADICAL RESECTION, TUMOR; CLAVICLE RADICAL RESECTION, TUMOR; SCAPULA RADICAL RESECTION, BONE TUMOR, PROXIMAL HUMERUS REMOVAL, FB, SHOULDER; SUBQ REMOVAL, FB, SHOULDER; COMPLICATED REMOVAL SHOULDER FOREIGN BODY DEEP SUBFASCIAL/IM 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2007 1/1/2015 1/1/2009 1/1/2007 1/1/2009 1/1/2007 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 23334 23335 23350 23395 23397 23400 23405 23406 23410 23412 23415 23420 23430 23440 23450 23455 23460 23462 23465 23466 23470 23472 23473 23474 23480 23485 23490 23491 23500 23505 23515 23520 23525 23530 23532 23540 23545 23550 23552 23570 23575 23585 23600 23605 23615 23616 23620 23625 23630 23650 23655 23660 23665 23670 23675 23680 23700 23800 23802 23900 23920 23921 23929 23930 23931 23935 24000 24006 24065 24066 24071 24073 24075 24076 24077 24079 24100 24101 24102 24105 24110 24115 24116 24120 24125 24126 24130 24134 24136 24138 24140 PROSTHESIS REMOVAL HUMERAL/GLENOID COMPONENT PROSTHESIS REMOVAL HUMERAL AND GLENOID COMPONENT INJECTION PROC, SHOULDER ARTHROGRAPHY/ENHANCED CT/ MUSCLE TRANSFER, ANY TYPE, SHOULDER/UPPER ARM; SIN MUSCLE TRANSFER, ANY TYPE, SHOULDER/UPPER ARM; MUL SCAPULOPEXY TENOTOMY, SHOULDER AREA; SINGLE TENDON TENOTOMY, SHOULDER AREA; MULTIPLE TENDONS THROUGH REPAIR, RUPTURED MUSCULOTENDINOUS CUFF, OPEN; ACUT REPAIR, RUPTURED MUSCULOTENDINOUS CUFF; CHRONIC CORACOACROMIAL LIGAMENT RELEASE, W/WO ACROMIOPLAST RECONSTRUCTION, COMPLETE SHOULDER (ROTATOR) CUFF A TENODESIS, LONG TENDON, BICEPS RESECTION/TRANSPLANTATION, LONG TENDON, BICEPS CAPSULORRHAPHY, ANTERIOR; PUTTI-PLATT PROC/MAGNUSO CAPSULORRHAPHY, ANTERIOR; W/LABRAL REPAIR CAPSULORRHAPHY, ANTERIOR, ANY TYPE; W/BONE BLOCK CAPSULORRHAPHY, ANTERIOR, ANY TYPE; W/CORACOID PRO CAPSULORRHAPHY, GLENOHUMERAL JOINT, POSTERIOR, W/W CAPSULORRHAPHY, GLENOHUMERAL JOINT, ANY TYPE MULTI ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER REVIS SHOULDER ARTHRPLSTY HUMERAL/GLENOID COMPNT REVIS SHOULDER ARTHRPLSTY HUMERAL&GLENOID COMPNT OSTEOTOMY, CLAVICLE, W/WO INT FIXATION; OSTEOTOMY, CLAVICLE, W/WO INT FIXATION; W/BONE GRA PROPHYLACTIC TREATMENT W/WO METHYLMETHACRYLATE; CL PROPHYLACTIC TREATMENT W/WO METHYLMETHACRYLATE; PR CLOSED TREATMENT, CLAVICULAR FX; W/O MANIPULATION CLOSED TREATMENT, CLAVICULAR FX; W/MANIPULATION OPEN TREATMENT, CLAVICULAR FX, W/WO INT/EXT FIXATI CLOSED TREATMENT, STERNOCLAVICULAR DISLOCATION; W/ CLOSED TREATMENT, STERNOCLAVICULAR DISLOCATION; W/ OPEN TREATMENT, STERNOCLAVICULAR DISLOCATION, ACUT OPEN TREATMENT, STERNOCLAVICULAR DISLOCATION, ACUT CLOSED TREATMENT, ACROMIOCLAVICULAR DISLOCATION; W CLOSED TREATMENT, ACROMIOCLAVICULAR DISLOCATION; W OPEN TREATMENT, ACROMIOCLAVICULAR DISLOCATION, ACU OPEN TREATMENT, ACROMIOCLAVICULAR DISLOCATION, ACU CLOSED TREATMENT, SCAPULAR FX; W/O MANIPULATION CLOSED TREATMENT, SCAPULAR FX; W/MANIPULATION, W/W OPEN TREATMENT, SCAPULAR FX, W/WO INTERNAL FIXATIO CLOSED TREATMENT, PROXIMAL HUMERAL FX; W/O MANIPUL CLOSED TREATMENT, PROXIMAL HUMERAL FX; W/MANIPULAT OPEN TREATMENT, PROXIMAL HUMERAL FX, W/WO INT/EXT OPEN TREATMENT, PROXIMAL HUMERAL FX, W/WO INT/EXT CLOSED TREATMENT, GREATER HUMERAL TUBEROSITY FX; W CLOSED TREATMENT, GREATER HUMERAL TUBEROSITY FX; W OPEN TREATMENT, GREATER HUMERAL TUBEROSITY FX W/WO CLOSED TREATMENT, SHOULDER DISLOCATION, W/MANIPULA CLOSED TREATMENT, SHOULDER DISLOCATION, W/MANIPULA OPEN TREATMENT, ACUTE SHOULDER DISLOCATION CLOSED TREATMENT, SHOULDER DISLOCATION, W/FX, GREA OPEN TREATMENT, SHOULDER DISLOCATION, W/FX, GREATE CLOSED TREATMENT, SHOULDER DISLOCATION, W/SURGICAL OPEN TREATMENT, SHOULDER DISLOCATION, W/SURGICAL/A MANIPULATION W/ANESTHESIA, SHOULDER JOINT, W/APPLI ARTHRODESIS, GLENOHUMERAL JOINT ARTHRODESIS, GLENOHUMERAL JOINT; W/AUTOGENOUS GRAF INTERTHORACOSCAPULAR AMPUTATION (FOREQUARTER) DISARTICULATION, SHOULDER DISARTICULATION, SHOULDER; SECONDARY CLOSURE/SCAR UNLISTED PROC, SHOULDER INCISION AND DRAINAGE, UPPER ARM/ELBOW AREA; DEEP INCISION AND DRAINAGE, UPPER ARM/ELBOW AREA; BURSA INCISION, DEEP, W/OPENING, BONE CORTEX, HUMERUS/EL ARTHROTOMY, ELBOW, W/EXPLORATION, DRAINAGE/REMOVAL ARTHROTOMY, ELBOW, W/CAPSULAR EXCISION, CAPSULAR R BX, SOFT TISSUE, UPPER ARM/ELBOW AREA; SUPERFICIAL BX, SOFT TISSUE, UPPER ARM/ELBOW AREA; DEEP (SUBFA EXC TUMOR SOFT TISSUE UPPER ARM/ELBOW SUBQ 3+CM EXC TUMOR SOFT TISS UPPER ARM/ELBW SUBFASC 5+CM EXC, TUMOR, SOFT TISSUE, UPPER ARM/ELBOW AREA SUBQ EXC, TUMOR, SOFT TISSUE, UPPER ARM/ELBOW SUBFASC RAD RESECT TUMOR SOFT TISS UPPER ARM/ELBOW <5CM RAD RESECT TUMOR SOFT TISS UPPER ARM/ELBOW 5CM/> ARTHROTOMY, ELBOW; W/SYNOVIAL BX ONLY ARTHROTOMY, ELBOW; W/JOINT EXPLORATION, W/WO BX, W ARTHROTOMY, ELBOW; W/SYNOVECTOMY EXCISION, OLECRANON BURSA EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, HUMERU EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, HUMERU EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, HUMERU EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, HEAD/N EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, HEAD/N EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, HEAD/N EXCISION, RADIAL HEAD SEQUESTRECTOMY, SHAFT/DISTAL HUMERUS SEQUESTRECTOMY, RADIAL HEAD/NECK SEQUESTRECTOMY, OLECRANON PROCESS PARTIAL EXCISION, BONE, HUMERUS 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 24145 24147 24149 24150 24152 24155 24160 24164 24200 24201 24220 24300 24301 24305 24310 24320 24330 24331 24332 24340 24341 24342 24343 24344 24345 24346 24357 24358 24359 24360 24361 24362 24363 24365 24366 24370 24371 24400 24410 24420 24430 24435 24470 24495 24498 24500 24505 24515 24516 24530 24535 24538 24545 24546 24560 24565 24566 24575 24576 24577 24579 24582 24586 24587 24600 24605 24615 24620 24635 24640 24650 24655 24665 24666 24670 24675 24685 24800 24802 24900 24920 24925 24930 24931 24935 24940 24999 25000 25001 25020 25023 PARTIAL EXCISION, BONE, RADIAL HEAD/NECK PARTIAL EXCISION, BONE, OLECRANON PROCESS RADICAL RESECTION, CAPSULE/SOFT TISSUE/HETEROTOPIC RADICAL RESECTION, TUMOR, SHAFT/DISTAL HUMERUS RADICAL RESECTION, TUMOR, RADIAL HEAD/NECK RESECTION, ELBOW JOINT (ARTHRECTOMY) PROSTHESIS REMOVAL HUMERAL AND ULNAR COMPONENTS PROSTHESIS REMOVAL RADIAL HEAD REMOVAL, FB, UPPER ARM/ELBOW AREA; SUBQ REMOVAL, FB, UPPER ARM/ELBOW AREA; DEEP (SUBFASCIA INJECTION PROC, ELBOW ARTHROGRAPHY MANIPULATION, ELBOW, UNDER ANESTHESIA MUSCLE/TENDON TRANSFER, ANY TYPE, UPPER ARM/ELBOW, TENDON LENGTHENING, UPPER ARM/ELBOW, EACH TENDON TENOTOMY, OPEN, ELBOW TO SHOULDER, EACH TENDON TENOPLASTY, W/MUSCLE TRANSFER, W/WO FREE GRAFT, EL FLEXOR-PLASTY, ELBOW FLEXOR-PLASTY, ELBOW; W/EXTENSOR ADVANCEMENT TENOLYSIS, TRICEPS TENODESIS, BICEPS TENDON AT ELBOW (SEP PROC) REPAIR, TENDON/MUSCLE, UPPER ARM/ELBOW, EACH TENDO REINSERTION, RUPTURED BICEPS/TRICEPS TENDON, DISTA REPAIR LATERAL COLLATERAL LIGAMENT, ELBOW, W/LOCAL RECONSTRUCTION LATERAL COLLATERAL LIGAMENT, ELBOW, REPAIR MEDIAL COLLATERAL LIGAMENT, ELBOW, W/LOCAL RECONSTRUCTION MEDIAL COLLATERAL LIGAMENT, ELBOW, TENOTOMY, ELBOW, LATERAL OR MEDIAL EG, EPICONDYLIT DEBRIDEMENT, SOFT TISSUE AND/OR BONE, OPEN DEBRIDEMENT, SOFT TISSUE AND/OR BONE, OPEN WITH TE ARTHROPLASTY, ELBOW; W/MEMBRANE ARTHROPLASTY, ELBOW; W/DISTAL HUMERAL PROSTHETIC R ARTHROPLASTY, ELBOW; W/IMPLANT AND FASCIA LATA LIG ARTHROPLASTY, ELBOW; W/DISTAL HUMERUS/PROXIMAL ULN ARTHROPLASTY, RADIAL HEAD ARTHROPLASTY, RADIAL HEAD; W/IMPLANT REVIS ELBOW ARTHRPLSTY HUMERAL/ULNA COMPNT REVIS ELBOW ARTHRPLSTY HUMERAL&ULNA COMPNT OSTEOTOMY, HUMERUS, W/WO INT FIXATION MULTIPLE OSTEOTOMIES W/REALIGNMENT ON INTRAMEDULLA OSTEOPLASTY, HUMERUS (EXCLUDING 64876) REPAIR, NONUNION/MALUNION, HUMERUS; W/O GRAFT REPAIR, NONUNION/MALUNION, HUMERUS; W/ILIAC/OTHER HEMIEPIPHYSEAL ARREST DECOMPRESSION FASCIOTOMY, FOREARM, W/BRACHIAL ARTE PROPHYLACTIC TREATMENT, W/WO METHYLMETHACRYLATE, H CLOSED TREATMENT, HUMERAL SHAFT FX; W/O MANIPULATI CLOSED TREATMENT, HUMERAL SHAFT FX; W/MANIPULATION OPEN TREATMENT, HUMERAL SHAFT FX W/PLATE/SCREWS, W TREATMENT, HUMERAL SHAFT FX, W/INSERTION, INTRAMED CLOSED TREATMENT, SUPRACONDYLAR/TRANSCONDYLAR HUME CLOSED TREATMENT, SUPRACONDYLAR/TRANSCONDYLAR HUME PERCUTANEOUS SKELETAL FIXATION, SUPRACONDYLAR/TRAN OPEN TREATMENT, HUMERAL SUPRACONDYLAR/TRANSCONDYLA OPEN TREATMENT, HUMERAL SUPRACONDYLAR/TRANSCONDYLA CLOSED TREATMENT, HUMERAL EPICONDYLAR FX, MEDIAL/L CLOSED TREATMENT, HUMERAL EPICONDYLAR FX, MEDIAL/L PERCUTANEOUS SKELETAL FIXATION, HUMERAL EPICONDYLA OPEN TREATMENT, HUMERAL EPICONDYLAR FX, MEDIAL/LAT CLOSED TREATMENT, HUMERAL CONDYLAR FX, MEDIAL/LATE CLOSED TREATMENT, HUMERAL CONDYLAR FX, MEDIAL/LATE OPEN TREATMENT, HUMERAL CONDYLAR FX, MEDIAL/LATERA PERCUTANEOUS SKELETAL FIXATION, HUMERAL CONDYLAR F OPEN TREATMENT, PERIARTICULAR FX/DISLOCATION, ELBO OPEN TREATMENT, PERIARTICULAR FX/DISLOCATION, ELBO TREATMENT, CLOSED ELBOW DISLOCATION; W/O ANESTHESI TREATMENT, CLOSED ELBOW DISLOCATION; REQUIRING ANE OPEN TREATMENT, ACUTE/CHRONIC ELBOW DISLOCATION CLOSED TREATMENT, MONTEGGIA TYPE, FX DISLOCATION, OPEN TREATMENT, MONTEGGIA TYPE, FX DISLOCATION, EL CLOSED TREATMENT, RADIAL HEAD SUBLUXATION IN CHILD CLOSED TREATMENT, RADIAL HEAD/NECK FX; W/O MANIPUL CLOSED TREATMENT, RADIAL HEAD/NECK FX; W/MANIPULAT OPEN TREATMENT, RADIAL HEAD/NECK FX W/WO INT FIXAT OPEN TREATMENT, RADIAL HEAD/NECK FX W/WO INT FIXAT CLOSED TREATMENT, ULNAR FX, PROXIMAL END (OLECRANO CLOSED TREATMENT, ULNAR FX, PROXIMAL END (OLECRANO OPEN TREATMENT, ULNAR FX, PROXIMAL END (OLECRANON ARTHRODESIS, ELBOW JOINT; LOCAL ARTHRODESIS, ELBOW JOINT; W/AUTOGENOUS GRAFT (INCL AMPUTATION, ARM THROUGH HUMERUS; W/PRIMARY CLOSURE AMPUTATION, ARM THROUGH HUMERUS; OPEN, CIRCULAR (G AMPUTATION, ARM THROUGH HUMERUS; SECONDARY CLOSURE AMPUTATION, ARM THROUGH HUMERUS; RE-AMPUTATION AMPUTATION, ARM THROUGH HUMERUS; W/IMPLANT STUMP ELONGATION, UPPER EXTREMITY CINEPLASTY, UPPER EXTREMITY, COMPLETE PROC UNLISTED PROC, HUMERUS/ELBOW INCISION, EXTENSOR TENDON SHEATH, WRIST INCISION, FLEXOR TENDON SHEATH, WRIST DECOMPRES FASCIOTOMY, FOREARM AND/OR WRIST, FLEXOR DECOMPRES FASCIOTOMY, FOREARM AND/OR WRIST, FLEXOR 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 25024 25025 25028 25031 25035 25040 25065 25066 25071 25073 25075 25076 25077 25078 25085 25100 25101 25105 25107 25109 25110 25111 25112 25115 25116 25118 25119 25120 25125 25126 25130 25135 25136 25145 25150 25151 25170 25210 25215 25230 25240 25246 25248 25250 25251 25259 25260 25263 25265 25270 25272 25274 25275 25280 25290 25295 25300 25301 25310 25312 25315 25316 25320 25332 25335 25337 25350 25355 25360 25365 25370 25375 25390 25391 25392 25393 25394 25400 25405 25415 25420 25425 25426 25430 25431 25440 25441 25442 25443 25444 25445 DECOMPRES FASCIOTOMY, FOREARM AND/OR WRIST, FLEXOR DECOMPRES FASCIOTOMY, FOREARM AND/OR WRIST, FLEXOR INCISION AND DRAINAGE, FOREARM AND/OR WRIST; DEEP INCISION AND DRAINAGE, FOREARM AND/OR WRIST; BURSA INCISION, DEEP, BONE CORTEX, FOREARM AND/OR WRIST ARTHROTOMY, RADIOCARPAL/MIDCARPAL JOINT, W/EXPLORE BX, SOFT TISSUE, FOREARM AND/OR WRIST; SUPERFICIAL BX, SOFT TISSUE, FOREARM AND/OR WRIST; DEEP (SUBFA EXC TUMOR SOFT TISS FOREARM AND/WRIST SUBQ 3+CM EXC TUMOR SFT TISS FOREARM&//WRIST SUBFASC 3+CM EXC, TUMOR, SOFT TISSUE FOREARM AND/OR WRIST SUBQ EXC, TUMOR, SFT TISSUE FOREARM AND/OR WRIST SUBFAS RAD RESECT TUMOR SOFT TISS FOREARM&/WRIST <3 CM RAD RESCJ TUM SOFT TISSUE FOREARM&/WRIST 3 CM/> CAPSULOTOMY, WRIST ARTHROTOMY, WRIST JOINT; W/BX ARTHROTOMY, WRIST JOINT; W/JOINT EXPLORATION, W/WO ARTHROTOMY, WRIST JOINT; W/SYNOVECTOMY ARTHROTOMY, DISTAL RADIOULNAR JOINT W/REPAIR, TRIA Excise tendon forearm/wrist EXCISION, LESION, TENDON SHEATH, FOREARM AND/OR WR EXCISION, GANGLION, WRIST (DORSAL/VOLAR); PRIMARY EXCISION, GANGLION, WRIST (DORSAL/VOLAR); RECURREN RADICAL EXCISION, BURSA, SYNOVIA, WRIST/FOREARM TE RADICAL EXCISION, BURSA, SYNOVIA, WRIST/FOREARM TE SYNOVECTOMY, EXTENSOR TENDON SHEATH, WRIST, SINGLE SYNOVECTOMY, EXTENSOR TENDON SHEATH, WRIST, SINGLE EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, RADIUS EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, RADIUS EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, RADIUS EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, CARPAL EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, CARPAL EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, CARPAL SEQUESTRECTOMY, FOREARM AND/OR WRIST PARTIAL EXCISION, BONE; ULNA PARTIAL EXCISION, BONE; RADIUS RADICAL RESECTION, TUMOR, RADIUS/ULNA CARPECTOMY; ONE BONE CARPECTOMY; ALL BONES, PROXIMAL ROW RADIAL STYLOIDECTOMY (SEP PROC) EXCISION DISTAL ULNA PARTIAL/COMPLETE INJECTION PROC, WRIST ARTHROGRAPHY EXPLORATION W/REMOVAL, DEEP FB, FOREARM/WRIST REMOVAL, WRIST PROSTHESIS; (SEP PROC) REMOVAL, WRIST PROSTHESIS; COMPLICATED, W/TOTAL WR MANIPULATION, WRIST, UNDER ANESTHESIA REPAIR, TENDON/MUSCLE, FLEXOR, FOREARM AND/OR WRIS REPAIR, TENDON/MUSCLE, FLEXOR, FOREARM AND/OR WRIS REPAIR, TENDON/MUSCLE, FLEXOR, FOREARM AND/OR WRIS REPAIR, TENDON/MUSCLE, EXTENSOR, FOREARM AND/OR WR REPAIR, TENDON/MUSCLE, EXTENSOR, FOREARM AND/OR WR REPAIR, TENDON/MUSCLE, EXTENSOR, FOREARM AND/OR WR REPAIR, TENDON SHEATH, EXTENSOR, FOREARM AND/OR WR LENGTHENING/SHORTENING, FLEXOR/EXTENSOR TENDON, FO TENOTOMY, OPEN, FLEXOR/EXTENSOR TENDON, FOREARM AN TENOLYSIS, FLEXOR/EXTENSOR TENDON, FOREARM AND/OR TENODESIS AT WRIST; FLEXORS, FINGERS TENODESIS AT WRIST; EXTENSORS, FINGERS TENDON TRANSPLANTATION/TRANSFER, FLEXOR/EXTENSOR, TENDON TRANSPLANTATION/TRANSF, FLEXOR/EXTENS, FORE FLEXOR ORIGIN SLIDE, FOREARM AND/OR WRIST FLEXOR ORIGIN SLIDE, FOREARM AND/OR WRIST; W/TENDO CAPSULORRHAPHY/RECONSTRUCTION, WRIST, OPEN, W/SYNO ARTHROPLASTY, WRIST, W/WO INTERPOSITION/INT/EXT FI CENTRALIZATION, WRIST ON ULNA RECONSTRUCTION, STABILIZATION, DISTAL ULNA/RADIOUL OSTEOTOMY, RADIUS; DISTAL THIRD OSTEOTOMY, RADIUS; MIDDLE/PROXIMAL THIRD OSTEOTOMY; ULNA OSTEOTOMY; RADIUS AND ULNA MULTIPLE OSTEOTOMIES, W/REALIGNMENT ON INTRAMEDULL MULTIPLE OSTEOTOMIES, W/REALIGNMENT ON INTRAMEDULL OSTEOPLASTY, RADIUS/ULNA; SHORTENING OSTEOPLASTY, RADIUS/ULNA; LENGTHENING W/AUTOGRAFT OSTEOPLASTY, RADIUS AND ULNA; SHORTENING (EXCLUDIN OSTEOPLASTY, RADIUS AND ULNA; LENGTHENING W/AUTOGR OSTEOPLASTY, CARPAL BONE, SHORTENING REPAIR, NONUNION/MALUNION, RADIUS/ULNA; W/O GRAFT REPAIR, NONUNION/MALUNION, RADIUS/ULNA; W/AUTOGRAF REPAIR, NONUNION/MALUNION, RADIUS AND ULNA; W/O GR REPAIR, NONUNION/MALUNION, RADIUS AND ULNA; W/AUTO REPAIR, DEFECT W/AUTOGRAFT; RADIUS/ULNA REPAIR, DEFECT W/AUTOGRAFT; RADIUS AND ULNA INSERTION, VASCULAR PEDICLE, CARPAL BONE (HARII PR REPAIR, NONUNION, CARPAL BONE (EXCL CARPAL SCAPHOI REPAIR, NONUNION, SCAPHOID CARPAL BONE, W/WO RADIA ARTHROPLASTY W/PROSTHETIC REPLACEMENT; DISTAL RADI ARTHROPLASTY W/PROSTHETIC REPLACEMENT; DISTAL ULNA ARTHROPLASTY W/PROSTHETIC REPLACEMENT; SCAPHOID CA ARTHROPLASTY W/PROSTHETIC REPLACEMENT; LUNATE ARTHROPLASTY W/PROSTHETIC REPLACEMENT; TRAPEZIUM 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 25446 25447 25449 25450 25455 25490 25491 25492 25500 25505 25515 25520 25525 25526 25530 25535 25545 25560 25565 25574 25575 25600 25605 25606 25607 25608 25609 25622 25624 25628 25630 25635 25645 25650 25651 25652 25660 25670 25671 25675 25676 25680 25685 25690 25695 25800 25805 25810 25820 25825 25830 25900 25905 25907 25909 25915 25920 25922 25924 25927 25929 25931 25999 26010 26011 26020 26025 26030 26034 26035 26037 26040 26045 26055 26060 26070 26075 26080 26100 26105 26110 26111 26113 26115 26116 26117 26118 26121 26123 26125 26130 ARTHROPLASTY W/PROSTHETIC REPLACEMENT; DISTAL RADI ARTHROPLASTY, INTERPOSITION, INTERCARPAL/CARPOMETA REVISION, ARTHROPLASTY, W/REMOVAL, IMPLANT, WRIST EPIPHYSEAL ARREST, EPIPHYSIODESIS/STAPLING; DISTAL EPIPHYSEAL ARREST, EPIPHYSIODESIS/STAPLING; DISTAL PROPHYLACTIC TREATMENT (NAIL/PIN/PLATE/WIRE) W/WO PROPHYLACTIC TREATMENT (NAIL/PIN/PLATE/WIRE) W/WO PROPHYLACTIC TREATMENT (NAIL/PIN/PLATE/WIRE) W/WO CLOSED TREATMENT, RADIAL SHAFT FX; W/O MANIPULATIO CLOSED TREATMENT, RADIAL SHAFT FX; W/MANIPULATION OPEN TREATMENT, RADIAL SHAFT FX, W/WO INT/EXT FIXA CLOSED TREATMENT, RADIAL SHAFT FX, AND DISLOCATION OPEN TREATMENT, RADIAL SHAFT FX, W/FIXATION AND CL OPEN TX, RADIAL SHAFT FX AND DISTAL RADIOULNAR JT, CLOSED TREATMENT, ULNAR SHAFT FX; W/O MANIPULATION CLOSED TREATMENT, ULNAR SHAFT FX; W/MANIPULATION OPEN TREATMENT, ULNAR SHAFT FX, W/WO INT/EXT FIXAT CLOSED TREATMENT, RADIAL AND ULNAR SHAFT FXS; W/O CLOSED TREATMENT, RADIAL AND ULNAR SHAFT FXS; W/MA OPEN TREATMENT, RADIAL AND ULNAR SHAFT FXS, W/INT/ OPEN TREATMENT, RADIAL AND ULNAR SHAFT FXS, W/INT/ CLOSED TREATMENT, DISTAL RADIAL FX/EPIPHYSEAL SEPA CLOSED TREATMENT, DISTAL RADIAL FX/EPIPHYSEAL SEPA Treat fx distal radial Treat fx rad extra-articul Treat fx rad intra-articul Treat fx radial 3+ frag CLOSED TREATMENT, CARPAL SCAPHOID (NAVICULAR) FX; CLOSED TREATMENT, CARPAL SCAPHOID (NAVICULAR) FX; OPEN TREATMENT, CARPAL SCAPHOID (NAVICULAR) FX, W/ CLOSED TREATMENT, CARPAL BONE FX (EXCLUDING CARPAL CLOSED TREATMENT, CARPAL BONE FX (EXCLUDING CARPAL OPEN TREATMENT, CARPAL BONE FX (EXCLUDING CARPAL S CLOSED TREATMENT, ULNAR STYLOID FX PERCUTANEOUS SKELETAL FIXATION, ULNAR STYLOID FRAC OPEN TREATMENT, ULNAR STYLOID FRACTURE CLOSED TREATMENT, RADIOCARPAL/INTERCARPAL DISLOCAT OPEN TREATMENT, RADIOCARPAL/INTERCARPAL DISLOCATIO PERCUTANEOUS SKELETAL FIXATION, DISTAL RADIOULNAR CLOSED TREATMENT, DISTAL RADIOULNAR DISLOCATION W/ OPEN TREATMENT, DISTAL RADIOULNAR DISLOCATION, ACU CLOSED TREATMENT, TRANS-SCAPHOPERILUNAR TYPE FX DI OPEN TREATMENT, TRANS-SCAPHOPERILUNAR TYPE FX DISL CLOSED TREATMENT, LUNATE DISLOCATION, W/MANIPULATI OPEN TREATMENT, LUNATE DISLOCATION ARTHRODESIS, WRIST; COMPLETE W/O BONE GRAFT ARTHRODESIS, WRIST; W/SLIDING GRAFT ARTHRODESIS, WRIST; W/ILIAC/OTHER AUTOGRAFT (INCLU ARTHRODESIS, WRIST; LIMITED, W/O BONE GRAFT ARTHRODESIS, WRIST; W/AUTOGRAFT (INCLUDES OBTAININ ARTHRODESIS, DISTAL RADIOULNAR JOINT AND SEGMENTAL AMPUTATION, FOREARM, THROUGH RADIUS AND ULNA AMPUTATION, FOREARM, THROUGH RADIUS AND ULNA; OPEN AMPUTATION, FOREARM, THROUGH RADIUS AND ULNA; SECO AMPUTATION, FOREARM, THROUGH RADIUS AND ULNA; RE-A KRUKENBERG PROC DISARTICULATION THROUGH WRIST DISARTICULATION THROUGH WRIST; SECONDARY CLOSURE/S DISARTICULATION THROUGH WRIST; RE-AMPUTATION TRANSMETACARPAL AMPUTATION TRANSMETACARPAL AMPUTATION; SECONDARY CLOSURE/SCAR TRANSMETACARPAL AMPUTATION; RE-AMPUTATION UNLISTED PROC, FOREARM/WRIST DRAINAGE, FINGER ABSCESS; SIMPLE DRAINAGE, FINGER ABSCESS; COMPLICATED DRAINAGE, TENDON SHEATH, DIGIT AND/OR PALM, EACH DRAINAGE, PALMAR BURSA; SINGLE, BURSA DRAINAGE, PALMAR BURSA; MULTIPLE BURSA INCISION, BONE CORTEX, HAND/FINGER DECOMPRESSION FINGERS AND/OR HAND, INJECTION INJUR DECOMPRESSIVE FASCIOTOMY, HAND (EXCLUDES 26035) FASCIOTOMY, PALMAR; PERCUTANEOUS FASCIOTOMY, PALMAR; OPEN, PARTIAL TENDON SHEATH INCISION TENOTOMY, PERCUTANEOUS, SINGLE, EACH DIGIT ARTHROTOMY, EXPLORATION/DRAINAGE/REMOVAL, LOOSE/FB ARTHROTOMY, EXPLORATION/DRAINAGE/REMOVAL, LOOSE/FB ARTHROTOMY, EXPLORATION/DRAINAGE/REMOVAL, LOOSE/FB ARTHROTOMY W/BX; CARPOMETACARPAL JOINT, EACH ARTHROTOMY W/BX; METACARPOPHALANGEAL JOINT, EACH ARTHROTOMY W/BX; INTERPHALANGEAL JOINT, EACH EX TUM/VASC MALF SFT TISS HAND/FNGR SUBQ 1.5+CM EX TUM/VASC MAL SFT TIS HAND/FNGR SUBFSC 1.5+CM EXC, TUMOR/VASCLAR MALFORMATION, SOFT TISS SUBQ EXC, TUMOR/VASCLAR MALFORMATION, SOFT TISS SUBFASC RAD RESECT TUMOR SOFT TISSUE HAND/FINGER <3CM RAD RESCJ TUM SOFT TISSUE HAND/FINGER 3 CM/> FASCIECTOMY, PALM ONLY W/WO Z-PLASTY/LOCAL TISSUE FASCIECTOMY, PARTIAL PALMAR W/RELEASE, SINGLE DIGI FASCIECTOM, PARTL PALMAR W/RELEASE, SNGL DIGIT, W/ SYNOVECTOMY, CARPOMETACARPAL JOINT 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 26135 26140 26145 26160 26170 26180 26185 26200 26205 26210 26215 26230 26235 26236 26250 26260 26262 26320 26340 26341 26350 26352 26356 26357 26358 26370 26372 26373 26390 26392 26410 26412 26415 26416 26418 26420 26426 26428 26432 26433 26434 26437 26440 26442 26445 26449 26450 26455 26460 26471 26474 26476 26477 26478 26479 26480 26483 26485 26489 26490 26492 26494 26496 26497 26498 26499 26500 26502 26508 26510 26516 26517 26518 26520 26525 26530 26531 26535 26536 26540 26541 26542 26545 26546 26548 26550 26551 26553 26554 26555 26556 SYNOVECTOMY, METACARPOPHALANGEAL JOINT, W/INTRINSI SYNOVECTOMY, PROXIMAL INTERPHALANGEAL JOINT, W/EXT SYNOVECTOMY, TENDON SHEATH, RADICAL, FLEXOR TENDON EXCISION, LESION, TENDON SHEATH/JOINT CAPSULE, HAN EXCISION, TENDON, PALM, FLEXOR, SINGLE (SEP PROC), EXCISION, TENDON, FINGER, FLEXOR (SEP PROC), EACH SESAMOIDECTOMY, THUMB/FINGER (SEP PROC) EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, METACA EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, METACA EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, PHALAN EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, PHALAN PARTIAL EXCISION, BONE; METACARPAL PARTIAL EXCISION, BONE; PROXIMAL/MIDDLE PHALANX, F PARTIAL EXCISION, BONE; DISTAL PHALANX, FINGER RADICAL RESECTION TUMOR METACARPAL RAD RESECTION TUMOR PROX/MIDDLE PHALANX FINGER RADICAL RESECTION TUMOR DISTAL PHALANX FINGER REMOVAL, IMPLANT, FINGER/HAND MANIPULATION, FINGER JOINT, UNDER ANESTHESIA, EACH MANIPLATN PALAR FASCIAL CRD POST INJ SINGLE CORD FLEXOR TENDON REPAIR/ADVANCE, NOT IN ZONE 2 (NO MA FLEXOR TENDON, REPAIR/ADVANCE, NOT IN (NO MANS LAN FLEXOR TENDON, REPAIR/ADVANCE, IN ZONE 2 (NO MANS FLEXOR TENDON, REPAIR/ADVANCE, IN ZONE 2 (NO MANS FLEXOR TENDON, REPAIR/ADVANCE, IN ZONE 2 (NO MANS PROFUNDUS TENDON REPAIR/ADVANCE, W/INTACT SUPERFIC PROFUNDUS TENDON REPAIR/ADVANCE, W/INTACT SUPERFIC PROFUNDUS TENDON REPAIR/ADVANCE, W/INTACT SUPERFIC FLEXOR TENDON EXCISION, IMPLANTATION SYNTHETIC ROD REMOVAL, SYNTHETIC ROD AND INSERTION, FLEXOR TENDO REPAIR, EXTENSOR TENDON, HAND, PRIMARY/SECONDARY; REPAIR, EXTENSOR TENDON, HAND, PRIMARY/SECONDARY; EXTENSOR TENDON EXCISION, IMPLANTATION SYNTHETIC R REMOVAL, SYNTHETIC ROD AND INSERTION, EXTENSOR TEN REPAIR, EXTENSOR TENDON, FINGER, PRIMARY/SECONDARY REPAIR, EXTENSOR TENDON, FINGER, PRIMARY/SECONDARY REPAIR, EXTENSOR TENDON, CENTRAL SLIP, SECONDARY; REPAIR, EXTENSOR TENDON, CENTRAL SLIP, SECONDARY; CLOSED TREATMENT, EXTENSOR TENDON, DISTAL INSERTIO REPAIR, EXTENSOR TENDON, DISTAL INSERTION, PRIMARY REPAIR, EXTENSOR TENDON, DISTAL INSERTION, PRIMARY REALIGNMENT, EXTENSOR TENDON, HAND, EACH TENDON TENOLYSIS, FLEXOR TENDON; PALM/FINGER, EACH TENDON TENOLYSIS, FLEXOR TENDON; PALM AND FINGER, EACH TE TENOLYSIS, EXTENSOR TENDON, HAND/FINGER; EACH TEND TENOLYSIS, COMPLEX, EXTENSOR TENDON, FINGER, W/FOR TENOTOMY, FLEXOR, PALM, OPEN, EACH TENDON TENOTOMY, FLEXOR, FINGER, OPEN, EACH TENDON TENOTOMY, EXTENSOR, HAND/FINGER, OPEN, EACH TENDON TENODESIS; PROXIMAL INTERPHALANGEAL JOINT, EACH JO TENODESIS; DISTAL JOINT, EACH JOINT LENGTHENING, TENDON, EXTENSOR, HAND/FINGER, EACH T SHORTENING, TENDON, EXTENSOR, HAND/FINGER, EACH TE LENGTHENING, TENDON, FLEXOR, HAND/FINGER, EACH TEN SHORTENING, TENDON, FLEXOR, HAND/FINGER, EACH TEND TENDON TRANSFER/TRANSPLANT, CARPOMETACARPAL/DORSUM TENDON TRANSFER/TRANSPLANT, CARPOMETACARPAL/DORSUM TENDON TRANSFER/TRANSPLANT, PALMAR; W/O FREE TENDO TENDON TRANSFER/TRANSPLANT, PALMAR; W/FREE TENDON OPPONENSPLASTY; SUPERFICIALIS TENDON TRANSFER TYPE OPPONENSPLASTY; TENDON TRANSFER W/GRAFT (INCLUDES OPPONENSPLASTY; HYPOTHENAR MUSCLE TRANSFER OPPONENSPLASTY; OTHER METHODS TRANSFER, TENDON TO RESTORE INTRINSIC FUNCTION; RI TRANSFER, TENDON TO RESTORE INTRINSIC FUNCTION; AL CORRECTION CLAW FINGER, OTHER METHODS RECONSTRUCTION, TENDON PULLEY, EACH TENDON; W/LOCA RECONSTRUCTION, TENDON PULLEY, EACH TENDON; W/TEND RELEASE, THENAR MUSCLE(S) CROSS INTRINSIC TRANSFER, EACH TENDON CAPSULODESIS, METACARPOPHALANGEAL JOINT; SINGLE DI CAPSULODESIS, METACARPOPHALANGEAL JOINT; TWO DIGIT CAPSULODESIS, METACARPOPHALANGEAL JOINT; THREE/FOU CAPSULECTOMY/CAPSULOTOMY; METACARPOPHALANGEAL JOIN CAPSULECTOMY/CAPSULOTOMY; INTERPHALANGEAL JOINT, E ARTHROPLASTY, METACARPOPHALANGEAL JOINT; EACH JOIN ARTHROPLASTY, METACARPOPHALANGEAL JOINT; W/PROSTHE ARTHROPLASTY, INTERPHALANGEAL JOINT; EACH JOINT ARTHROPLASTY, INTERPHALANGEAL JOINT; W/PROSTHETIC REPAIR, COLLATERAL LIGAMENT, METACARPOPHALANGEAL/I RECONSTRUCTION, COLLATERAL LIGAMENT, METACARPOPHAL PRIMARY REPAIR, COLLATERAL LIGAMENT, METACARPOPHAL RECONSTRUCTION, COLLATERAL LIGAMENT, INTERPHALANGE REPAIR, NONUNION, METACARPAL/PHALANX, (INCLUDES OB REPAIR AND RECONSTRUCTION, FINGER, VOLAR PLATE, IN POLLICIZATION, DIGIT TRANSFER, TOE-TO-HAND W/MICROVASCULAR ANASTOMOSIS; TRANSFER, TOE-TO-HAND W/MICROVASCULAR ANASTOMOSIS; TRANSFER, TOE-TO-HAND W/MICROVASCULAR ANASTOMOSIS; TRANSFER, FINGER TO ANOTHER POSITION W/O MICROVASC TRANSFER, FREE TOE JOINT, W/MICROVASCULAR ANASTOMO 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 26560 26561 26562 26565 26567 26568 26580 26587 26590 26591 26593 26596 26600 26605 26607 26608 26615 26641 26645 26650 26665 26670 26675 26676 26685 26686 26700 26705 26706 26715 26720 26725 26727 26735 26740 26742 26746 26750 26755 26756 26765 26770 26775 26776 26785 26820 26841 26842 26843 26844 26850 26852 26860 26861 26862 26863 26910 26951 26952 26989 26990 26991 26992 27000 27001 27003 27005 27006 27025 27027 27030 27033 27035 27036 27040 27041 27043 27045 27047 27048 27049 27050 27052 27054 27057 27059 27060 27062 27065 27066 27067 REPAIR, SYNDACTYLY (WEB FINGER) EACH WEB SPACE; W/ REPAIR, SYNDACTYLY (WEB FINGER) EACH WEB SPACE; W/ REPAIR, SYNDACTYLY (WEB FINGER) EACH WEB SPACE; CO OSTEOTOMY; METACARPAL, EACH OSTEOTOMY; PHALANX, FINGER, EACH OSTEOPLASTY, LENGTHENING, METACARPAL/PHALANX REPAIR CLEFT HAND RECONSTRUCTION, POLYDACTYLOUS DIGIT, SOFT TISSUE A REPAIR MACRODACTYLIA, EACH DIGIT REPAIR, INTRINSIC MUSCLES, HAND, EACH MUSCLE RELEASE, INTRINSIC MUSCLES, HAND, EACH MUSCLE EXCISION, CONSTRICTING RING, FINGER, W/MULTIPLE ZCLOSED TREATMENT, METACARPAL FX, SINGLE; W/O MANIP CLOSED TREATMENT, METACARPAL FX, SINGLE; W/MANIPUL CLOSED TREATMENT, METACARPAL FX, W/MANIPULATION, W PERCUTANEOUS SKELETAL FIXATION, METACARPAL FX, EAC OPEN TREATMENT, METACARPAL FX, SINGLE, W/WO INT/EX CLOSED TREATMENT, CARPOMETACARPAL DISLOCATION, THU CLOSED TREATMENT, CARPOMETACARPAL FX/DISLOCATION, PERCUTANEOUS SKELETAL FIXATION, CARPOMETACARPAL FX OPEN TREATMENT, CARPOMETACARPAL FX DISLOCATION, TH CLOSED TX, CARPOMETACARPAL DISLOCATION, NON-THUMB CLOSED TREATMENT, CARPOMETACARPAL DISLOCATION, NON PERCUTANEOUS SKELETAL FIXATION, CARPOMETACARPAL DI OPEN TREATMENT, CARPOMETACARPAL DISLOCATION, NON-T OPEN TREATMENT, CARPOMETACARPAL DISLOCATION, NON-T CLOSED TREATMENT, METACARPOPHALANGEAL DISLOCATION, CLOSED TREATMENT, METACARPOPHALANGEAL DISLOCATION, PERCUTANEOUS SKELETAL FIXATION, METACARPOPHALANGEA OPEN TREATMENT, METACARPOPHALANGEAL DISLOCATION, S CLOSED TREATMENT, PHALANGEAL SHAFT FX, PROXIMAL/MI CLOSED TREATMENT, PHALANGEAL SHAFT FX, PROXIMAL/MI PERCUTAN SKELETAL FIXATN, UNSTABLE PHALANGL SHAFT OPEN TREATMENT, PHALANGEAL SHAFT FX, PROXIMAL/MIDD CLOSED TREATMENT, ARTICULAR FX, MCP/IP JOINT; W/O CLOSED TREATMENT, ARTICULAR FX, MCP/IP JOINT; W/MA OPEN TREATMENT, ARTICULAR FX, INVOLVING MCP/IP JOI CLOSED TREATMENT, DISTAL PHALANGEAL FX, FINGER/THU CLOSED TREATMENT, DISTAL PHALANGEAL FX, FINGER/THU PERCUTANEOUS SKELETAL FIXATION, DISTAL PHALANGEAL OPEN TREATMENT, DISTAL PHALANGEAL FX, FINGER/THUMB CLOSED TREATMENT, IP JOINT DISLOCATION, SINGLE, W/ CLSD TRTMT INTERPHALANGEL JT DISLOCATION, REQ ANES PERCUTANEOUS SKELETAL FIXATION, IP JOINT DISLOCATI OPEN TREATMENT, IP JOINT DISLOCATION, W/WO INT/EXT FUSION IN OPPOSITION, THUMB, W/AUTOGENOUS GRAFT (I ARTHRODESIS, CARPOMETACARPAL JOINT, THUMB, W/WO IN ARTHRODESIS, CARPOMETACARPAL JOINT, THUMB, W/WO IN ARTHRODESIS, CARPOMETACARPAL JOINT, DIGIT, OTHER T ARTHRODESIS, CARPOMETACARPAL JOINT, DIGIT, OTHER T ARTHRODESIS, METACARPOPHALANGEAL JOINT, W/WO INT F ARTHRODESIS, METACARPOPHALANGEAL JOINT W/WO INT FI ARTHRODESIS, INTERPHALANGEAL JOINT, W/WO INT FIXAT ARTHRODESIS, INTERPHALANGEAL JOINT, W/WO INT FIXAT ARTHRODESIS, INTERPHALANGEAL JOINT, W/WO INT FIXAT ARTHRODESIS, INTERPHALANGEAL JOINT, W/WO INT FIXAT AMPUTATION, METACARPAL, W/FINGER/THUMB, SINGLE, W/ AMPUTATION, FINGER/THUMB, PRIMARY/SECOND, ANY JNT/ AMPUTATION, FINGER/THUMB, PRIMARY/SECOND, ANY JNT/ UNLISTED PROC, HANDS/FINGERS INCISION AND DRAINAGE, PELVIS/HIP JOINT AREA; DEEP INCISION AND DRAINAGE, PELVIS/HIP JOINT AREA; INFE INCISION, BONE CORTEX, PELVIS AND/OR HIP JOINT TENOTOMY, ADDUCTOR, HIP, PERCUTANEOUS (SEP PROC) TENOTOMY, ADDUCTOR, HIP, OPEN TENOTOMY, ADDUCTOR, SUBQ, OPEN, W/OBTURATOR NEUREC TENOTOMY, HIP FLEXOR(S), OPEN (SEP PROC) TENOTOMY, ABDUCTORS AND/OR EXTENSOR(S), HIP, OPEN FASCIOTOMY, HIP/THIGH, ANY TYPE DECOMPRESSION FASCIOTOMY PELVIC COMPARTMENT UNI ARTHROTOMY, HIP, W/DRAINAGE ARTHROTOMY, HIP, W/EXPLORATION/REMOVAL, LOOSE/FB HIP JOINT DENERVATION, INTRAARTICULAR BRANCHES, SC CAPSULECTOMY/CAPSULOTOMY, HIP, W/HIP FLEXOR MUSCLE BX, SOFT TISSUE, PELVIS AND HIP AREA; SUPERFICIAL BX, SOFT TISSUE, PELVIS AND HIP AREA; DEEP, SUBFAS EXCISION TUMOR SOFT TISSUE PELVIS&HIP SUBQ 3+CM EXC TUMOR SOFT TISSUE PELVIS & HIP SUBFASC 5+CM EXCISION, TUMOR, PELVIS AND HIP AREA; SUBQ TISSUE EXCISION, TUMOR, PELVIS AND HIP AREA; DEEP, SUBFAS RAD RESECT TUMOR SOFT TISSUE PELVIS & HIP <5 CM ARTHROTOMY, W/BX; SACROILIAC JOINT ARTHROTOMY, W/BX; HIP JOINT ARTHROTOMY, W/SYNOVECTOMY, HIP JOINT DCMPRN FASCIOTOMY PELVIC CMPRT DBRDMT MUSCLE UNI RAD RESECTION TUMOR SOFT TISS PELVIS&HIP 5 CM/> EXCISION; ISCHIAL BURSA EXCISION; TROCHANTERIC BURSA/CALCIFICATION EXCISION BONE CYST/B9 TUMOR SUPERFICIAL EXCISION BONE CYST/B9 TUMOR DEEP EXC B1 CST/B9 TUM W/AGRFT REQ SEP INC 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 27070 27071 27075 27076 27077 27078 27080 27086 27087 27090 27091 27093 27095 27096 27097 27098 27100 27105 27110 27111 27120 27122 27125 27130 27132 27134 27137 27138 27140 27146 27147 27151 27156 27158 27161 27165 27170 27175 27176 27177 27178 27179 27181 27185 27187 27193 27194 27200 27202 27215 27216 27217 27218 27220 27222 27226 27227 27228 27230 27232 27235 27236 27238 27240 27244 27245 27246 27248 27250 27252 27253 27254 27256 27257 27258 27259 27265 27266 27267 27268 27269 27275 27279 27280 27282 27284 27286 27290 27295 27299 27301 PARTIAL EXCISION SUPERFICIAL PELVIS PARTIAL EXCISION DEEP PELVIS RAD RESCT TUMOR WING OF ILIUM 1 PUBIC/ISCHIAL RAD RESCT TUMOR ILIUM ACETABULUM BOTH PUBIC RADICAL RESCTION TUMOR INNOMINATE BONE TOTAL RAD RESCT TUMOR ISCHIAL TUBEROSITY&GRT TRCHNTR COCCYGECTOMY, PRIMARY REMOVAL, FB, PELVIS/HIP; SUBQ TISSUE REMOVAL, FB, PELVIS/HIP; DEEP (SUBFASCIAL/IM) REMOVAL, HIP PROSTHESIS; (SEP PROC) REMOVAL, HIP PROSTHESIS; COMPLICATED, W/TOTAL HIP INJECTION PROC, HIP ARTHROGRAPHY; W/O ANESTHESIA INJECTION PROC, HIP ARTHROGRAPHY; W/ANESTHESIA INJECT SI JOINT ARTHRGRPHY&/ANES/STEROID W/IMAGE RELEASE/RECESSION, HAMSTRING, PROXIMAL TRANSFER, ADDUCTOR TO ISCHIUM TRANSFER EXT OBLIQUE MUSCLE TO GREATER TROCHANTER TRANSFER PARASPINAL MUSCLE TO HIP (INCLUDES FASCIA TRANSFER ILIOPSOAS; TO GREATER TROCHANTER, FEMUR TRANSFER ILIOPSOAS; TO FEMORAL NECK ACETABULOPLASTY; ACETABULOPLASTY; RESECTION, FEMORAL HEAD HEMIARTHROPLASTY, HIP, PARTIAL ARTHROPLASTY, ACETABULAR/PROXIMAL FEMORAL PROSTHET CONVERSION, PREVIOUS HIP SURGERY TO TOTAL HIP ARTH REVISION, TOTAL HIP ARTHROPLASTY; BOTH COMPONENTS, REVISION, TOTAL HIP ARTHROPLASTY; ACETABULAR COMPO REVISION, TOTAL HIP ARTHROPLASTY; FEMORAL COMPONEN OSTEOTOMY AND TRANSFER, GREATER TROCHANTER, FEMUR OSTEOTOMY, ILIAC, ACETABULAR/INNOMINATE BONE OSTEOTOMY, ILIAC, ACETABULAR/INNOMINATE BONE; W/OP OSTEOTOMY, ILIAC, ACETABULAR/INNOMINATE BONE; W/FE OSTEOTOMY, ILIAC, ACETABULAR/INNOMINATE BONE; W/FE OSTEOTOMY, PELVIS, BILAT OSTEOTOMY, FEMORAL NECK (SEP PROC) OSTEOTOMY, INTERTROCHANTERIC/SUBTROCHANTERIC W/INT BONE GRAFT, FEMORAL HEAD, NECK, INTERTROCHANTERIC/ TREATMENT, SLIPPED FEMORAL EPIPHYSIS; TRACTION, W/ TREATMENT, SLIPPED FEMORAL EPIPHYSIS; SINGLE/MULTI OPEN TREATMENT, SLIPPED FEMORAL EPIPHYSIS; SINGLE/ OPEN TREATMENT, SLIPPED FEMORAL EPIPHYSIS; CLOSED OPEN TREATMENT, SLIPPED FEMORAL EPIPHYSIS; OSTEOPL OPEN TREATMENT, SLIPPED FEMORAL EPIPHYSIS; OSTEOTO EPIPHYSEAL ARREST, EPIPHYSIODESIS/STAPLING, GREATE PROPHYLACTIC TREATMENT (NAIL/PIN/PLATE/WIRE) W/WO CLOSED TREATMENT, PELVIC RING FX/DISLOCATION/DIAST CLOSED TREATMENT, PELVIC RING FX/DISLOCATION/DIAST CLOSED TREATMENT, COCCYGEAL FX OPEN TREATMENT, COCCYGEAL FX OPEN TREATMENT, ILIAC SPINE, TUBEROSITY AVULSION/I PERCUTANEOUS SKELETAL FIXATION, POSTERIOR PELVIC R OPEN TREATMENT, ANTERIOR RING FX AND/OR DISLOCATIO OPEN TREATMENT, POSTERIOR RING FX AND/OR DISLOCATI CLOSED TREATMENT, ACETABULUM (HIP SOCKET) FX(S); W CLOSED TREATMENT, ACETABULUM (HIP SOCKET) FX(S); W OPEN TREATMENT, POSTERIOR/ANTERIOR ACETABULAR WALL OPEN TREATMENT, ACETABULAR FX INVOLVING ANTERIOR/P OPEN TREATMENT, ACETABULAR FX INVOLVING ANTERIOR/P CLOSED TREATMENT, FEMORAL FX, PROXIMAL END, NECK; CLOSED TREATMENT, FEMORAL FX, PROXIMAL END, NECK; PERCUTANEOUS SKELETAL FIXATION, FEMORAL FX, PROXIM OPEN TREATMENT, FEMORAL FX, PROXIMAL END, NECK, IN CLOSED TREATMENT, INTER/PER/SUBTROCHANTERIC FEMORA CLOSED TREATMENT, INTER/PER/SUBTROCHANTERIC FEMORA TREATMENT, INTER/PER/SUBTROCHANTERIC FEMORAL FX; W OPEN TREATMENT, INTER/PER/SUBTROCHANTERIC FEMORAL CLOSED TREATMENT, GREATER TROCHANTERIC FX, W/O MAN OPEN TREATMENT, GREATER TROCHANTERIC FX, W/WO INT/ CLOSED TREATMENT, HIP DISLOCATION, TRAUMATIC; W/O CLOSED TREATMENT, HIP DISLOCATION, TRAUMATIC; REQU OPEN TREATMENT, HIP DISLOCATION, TRAUMATIC, W/O IN OPEN TREATMENT, HIP DISLOCATION, TRAUMATIC, W/ACET TREATMENT, SPONTANEOUS HIP DISLOCATION, ABDUCTION, TREATMENT, SPONTANEOUS HIP DISLOCATION, ABDUCTION, OPEN TREATMENT, SPONTANEOUS HIP DISLOCATION, REPLA OPEN TREATMENT, SPONTANEOUS HIP DISLOCATION, REPLA CLOSED TREATMENT, POST HIP ARTHROPLASTY DISLOCATIO CLOSED TREATMENT, POST HIP ARTHROPLASTY DISLOCATIO CLOSED TREATMENT OF FEMORAL FRACTURE, PROXIMAL END CLOSED TRMT OF FEMORAL FRACTURE W/MANIPULATION OPEN TRMT OF FEMORAL FX, PROXIMAL END,HEAD,INCLUDE MANIPULATION, HIP JOINT, REQUIRING GENERAL ANESTHE ARTHRODESIS SACROILIAC JOINT PERCUTANEOUS ARTHRODESIS SACROILIAC JOINT W/OBTAINING GRAFT ARTHRODESIS, SYMPHYSIS PUBIS (INCLUDING OBTAINING ARTHRODESIS, HIP JOINT (INCLUDING OBTAINING GRAFT) ARTHRODESIS, HIP JOINT (INCLUDING OBTAINING GRAFT) INTERPELVIABDOMINAL AMPUTATION (HINDQUARTER AMPUTA DISARTICULATION, HIP UNLISTED PROC, PELVIS/HIP JOINT INCISION AND DRAINAGE, DEEP ABSCESS, BURSA/HEMATOM 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 27303 27305 27306 27307 27310 27323 27324 27325 27326 27327 27328 27329 27330 27331 27332 27333 27334 27335 27337 27339 27340 27345 27347 27350 27355 27356 27357 27358 27360 27364 27365 27370 27372 27380 27381 27385 27386 27390 27391 27392 27393 27394 27395 27396 27397 27400 27403 27405 27407 27409 27412 27415 27416 27418 27420 27422 27424 27425 27427 27428 27429 27430 27435 27437 27438 27440 27441 27442 27443 27445 27446 27447 27448 27450 27454 27455 27457 27465 27466 27468 27470 27472 27475 27477 27479 27485 27486 27487 27488 27495 27496 INCISION, DEEP, W/OPENING, BONE CORTEX, FEMUR/KNEE FASCIOTOMY, ILIOTIBIAL (TENOTOMY), OPEN TENOTOMY, PERCUTANEOUS, ADDUCTOR/HAMSTRING; SINGLE TENOTOMY, PERCUTANEOUS, ADDUCTOR/HAMSTRING; MULTIP ARTHROTOMY, KNEE, W/EXPLORATION, DRAINAGE/REMOVAL, BX, SOFT TISSUE, THIGH/KNEE AREA; SUPERFICIAL BX, SOFT TISSUE, THIGH/KNEE AREA; DEEP (SUBFASCIAL Neurectomy, hamstring Neurectomy, popliteal EXCISION, TUMOR, THIGH/KNEE AREA; SUBQ EXCISION, TUMOR, THIGH/KNEE AREA; DEEP/SUBFASCIAL/ RAD RESECT TUMOR SOFT TISSUE THIGH/KNEE <5CM ARTHROTOMY, KNEE; W/SYNOVIAL BX ONLY ARTHROTOMY, KNEE; W/JOINT EXPLORATION, BX/REMOVAL, ARTHROTOMY, W/EXCISION, SEMILUNAR CARTILAGE (MENIS ARTHROTOMY, W/EXCISION, SEMILUNAR CARTILAGE (MENIS ARTHROTOMY, W/SYNOVECTOMY KNEE; ANTERIOR/POSTERIOR ARTHROTOMY, W/SYNOVECTOMY KNEE; ANTERIOR AND POSTE EXCISON TUMOR SOFT TISSUE THIGH/KNEE SUBQ 3+CM EXC TUMOR SOFT TISSUE THIGH/KNEE SUBFASC 5+CM EXCISION, PREPATELLAR BURSA EXCISION, SYNOVIAL CYST, POPLITEAL SPACE EXCISION, LESION, MENISCUS/CAPSULE, KNEE PATELLECTOMY/HEMIPATELLECTOMY EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, FEMUR EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, FEMUR; EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, FEMUR; EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, FEMUR; PARTIAL EXCISION, BONE, FEMUR, PROXIMAL TIBIA AND/ RAD RESECTION TUMOR SOFT TIS THIGH/KNEE 5 CM/> RADICAL RESECTION, TUMOR, BONE, FEMUR/KNEE INJECTION KNEE ARTHROGRAPHY REMOVAL, FB, DEEP, THIGH REGION/KNEE AREA SUTURE, INFRAPATELLAR TENDON; PRIMARY SUTURE, INFRAPATELLAR TENDON; SECONDARY RECONSTRUC SUTURE, QUADRICEPS/HAMSTRING MUSCLE RUPTURE; PRIMA SUTURE, QUADRICEPS/HAMSTRING MUSCLE RUPTURE; SECON TENOTOMY, OPEN, HAMSTRING, KNEE TO HIP; SINGLE TEN TENOTOMY, OPEN, HAMSTRING, KNEE TO HIP; MULTIPLE T TENOTOMY, OPEN, HAMSTRING, KNEE TO HIP; MULTIPLE T LENGTHENING, HAMSTRING TENDON; SINGLE TENDON LENGTHENING, HAMSTRING TENDON; MULTIPLE TENDONS, O LENGTHENING, HAMSTRING TENDON; MULTIPLE TENDONS, B TRANSPLANT, HAMSTRING TENDON TO PATELLA; SINGLE TE TRANSPLANT, HAMSTRING TENDON TO PATELLA; MULTIPLE TRANSFER, TENDON/MUSCLE, HAMSTRINGS TO FEMUR ARTHROTOMY W/MENISCUS REPAIR, KNEE REPAIR, PRIMARY, TORN LIGAMENT AND/OR CAPSULE, KNE REPAIR, PRIMARY, TORN LIGAMENT AND/OR CAPSULE, KNE REPAIR, PRIMARY, TORN LIGAMENT AND/OR CAPSULE, KNE AUTOLOGOUS CHONDROCYTE IMPLANTATION, KNEE OSTEOCHONDRAL ALLOGRAFT, KNEE, OPEN OSTEOCHONDRAL AUTOGRAFT(S), KNEE, OPEN (EG, MOSAIC ANTERIOR TIBIAL TUBERCLEPLASTY RECONSTRUCTION, DISLOCATING PATELLA RECONSTRUCTION, DISLOCATING PATELLA; W/EXTENSOR RE RECONSTRUCTION, DISLOCATING PATELLA; W/PATELLECTOM LATERAL RETINACULAR RELEASE, OPEN LIGAMENTOUS RECONSTRUCTION (AUGMENTATION), KNEE; E LIGAMENTOUS RECONSTRUCTION (AUGMENTATION), KNEE; I LIGAMENTOUS RECONSTRUCTION (AUGMENTATION), KNEE; I QUADRICEPSPLASTY CAPSULOTOMY, POSTERIOR CAPSULAR RELEASE, KNEE ARTHROPLASTY, PATELLA; W/O PROSTHESIS ARTHROPLASTY, PATELLA; W/PROSTHESIS ARTHROPLASTY, KNEE, TIBIAL PLATEAU ARTHROPLASTY, KNEE, TIBIAL PLATEAU; W/DEBRIDEMENT ARTHROPLASTY, FEMORAL CONDYLES/TIBIAL PLATEAU(S), ARTHROPLASTY, FEMORAL CONDYLES/TIBIAL PLATEAU(S), ARTHROPLASTY, KNEE, HINGE PROSTHESIS ARTHROPLASTY, KNEE, CONDYLE AND PLATEAU; MEDIAL/LA ARTHROPLASTY, KNEE, CONDYLE AND PLATEAU; MEDIAL AN OSTEOTOMY, FEMUR, SHAFT/SUPRACONDYLAR; W/O FIXATIO OSTEOTOMY, FEMUR, SHAFT/SUPRACONDYLAR; W/FIXATION OSTEOTOMY, MULTIPLE, FEMORAL SHAFT, W/REALIGNMENT OSTEOTOMY, PROXIMAL TIBIA W/FIBULAR EXCISION/OSTEO OSTEOTOMY, PROXIMAL TIBIA W/FIBULAR EXCISION/OSTEO OSTEOPLASTY, FEMUR; SHORTENING (EXCLUDING 64876) OSTEOPLASTY, FEMUR; LENGTHENING OSTEOPLASTY, FEMUR; COMBINED, LENGTHENING AND SHOR REPAIR, NONUNION/MALUNION, FEMUR, DISTAL TO HEAD/N REPAIR, NONUNION/MALUNION, FEMUR, DISTAL TO HEAD/N ARREST, EPIPHYSEAL, ANY METHOD; DISTAL FEMUR ARREST, EPIPHYSEAL, ANY METHOD; TIBIA AND FIBULA, ARREST, EPIPHYSEAL; COMBINED DISTAL FEMUR/PROXIMAL ARREST, HEMIEPIPHYSEAL, DISTAL FEMUR/PROXIMAL TIBI REVISION, TOTAL KNEE ARTHROPLASTY, W/WO ALLOGRAFT; REVISION, TOTAL KNEE ARTHROPLASTY; FEMORAL AND ENT REMOVAL, KNEE PROSTHESIS, METHYLMETHACRYLATE W/WO PROPHYLACTIC TREATMENT (NAIL/PIN/PLATE/WIRE), FEMU DECOMPRESSION FASCIOTOMY, THIGH AND/OR KNEE, 1 COM 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 27497 27498 27499 27500 27501 27502 27503 27506 27507 27508 27509 27510 27511 27513 27514 27516 27517 27519 27520 27524 27530 27532 27535 27536 27538 27540 27550 27552 27556 27557 27558 27560 27562 27566 27570 27580 27590 27591 27592 27594 27596 27598 27599 27600 27601 27602 27603 27604 27605 27606 27607 27610 27612 27613 27614 27615 27616 27618 27619 27620 27625 27626 27630 27632 27634 27635 27637 27638 27640 27641 27645 27646 27647 27648 27650 27652 27654 27656 27658 27659 27664 27665 27675 27676 27680 27681 27685 27686 27687 27690 27691 DECOMPRESSION FASCIOTOMY, THIGH AND/OR KNEE, 1 COM DECOMPRESSION FASCIOTOMY, THIGH AND/OR KNEE, MULTI DECOMPRESSION FASCIOTOMY, THIGH AND/OR KNEE, MULTI CLOSED TREATMENT, FEMORAL SHAFT FX, W/O MANIPULATI CLOSED TREATMENT, SUPRACONDYLAR/TRANSCONDYLAR FEMO CLOSED TREATMENT, FEMORAL SHAFT FX, W/MANIPULATION CLOSED TREATMENT, SUPRACONDYLAR/TRANSCONDYLAR FEMO OPEN TREATMENT, FEMORAL SHAFT FX, W/INSERTION, INT OPEN TREATMENT, FEMORAL SHAFT FX W/PLATE/SCREWS, W CLOSED TREATMENT, FEMORAL FX, DISTAL END, MEDIAL/L PERCUTANEOUS SKELETAL FIXATION, FEMORAL FX, DISTAL CLOSED TREATMENT, FEMORAL FX, DISTAL END, MEDIAL/L OPEN TREATMENT, FEMORAL SUPRACONDYLAR/TRANSCONDYLA OPEN TREATMENT, FEMORAL SUPRACONDYLAR/TRANSCONDYLA OPEN TREATMENT, FEMORAL FX, DISTAL END, MEDIAL/LAT CLOSED TREATMENT, DISTAL FEMORAL EPIPHYSEAL SEPARA CLOSED TREATMENT, DISTAL FEMORAL EPIPHYSEAL SEPARA OPEN TREATMENT, DISTAL FEMORAL EPIPHYSEAL SEPARATI CLOSED TREATMENT, PATELLAR FX, W/O MANIPULATION OPEN TREATMENT, PATELLAR FX, W/INT FIXATION/PATELL CLOSED TREATMENT, TIBIAL FX, PROXIMAL; W/O MANIPUL CLOSED TREATMENT, TIBIAL FX, PROXIMAL; W/WO MANIPU OPEN TREATMENT, TIBIAL FX, PROXIMAL; UNICONDYLAR, OPEN TREATMENT, TIBIAL FX, PROXIMAL; BICONDYLAR, W CLOSED TREATMENT, INTERCONDYLAR SPINE/TUBEROSITY F OPEN TREATMENT, INTERCONDYLAR SPINE/TUBEROSITY FX, CLOSED TREATMENT, KNEE DISLOCATION; W/O ANESTHESIA CLOSED TREATMENT, KNEE DISLOCATION; REQUIRING ANES OPEN TREATMENT, KNEE DISLOCATION W/WO INT/EXT FIX; OPEN TREATMENT, KNEE DISLOCATION, W/WO INT/EXT FIX OPEN TREATMENT, KNEE DISLOCATION, W/WO INT/EXT FIX CLOSED TREATMENT, PATELLAR DISLOCATION; W/O ANESTH CLOSED TREATMENT, PATELLAR DISLOCATION; REQUIRING OPEN TREATMENT, PATELLAR DISLOCATION, W/WO PARTIAL MANIPULATION, KNEE JOINT UNDER GENERAL ANESTHESIA ARTHRODESIS, KNEE, ANY TECHNIQUE AMPUTATION, THIGH, THROUGH FEMUR, ANY LEVEL; AMPUTATION, THIGH, THROUGH FEMUR, ANY LEVEL; IMMED AMPUTATION, THIGH, THROUGH FEMUR, ANY LEVEL; OPEN, AMPUTATION, THIGH, THROUGH FEMUR, ANY LEVEL; SECON AMPUTATION, THIGH, THROUGH FEMUR, ANY LEVEL; RE-AM DISARTICULATION AT KNEE UNLISTED PROC, FEMUR/KNEE DECOMPRESSION FASCIOTOMY, LEG; ANTERIOR AND/OR LAT DECOMPRESSION FASCIOTOMY, LEG; POSTERIOR COMPARTME DECOMPRESSION FASCIOTOMY, LEG; ANTERIOR AND/OR LAT INCISION AND DRAINAGE, LEG/ANKLE; DEEP ABSCESS/HEM INCISION AND DRAINAGE, LEG/ANKLE; INFECTED BURSA TENOTOMY, PERCUTANEOUS, ACHILLES TENDON (SEP PROC) TENOTOMY, PERCUTANEOUS, ACHILLES TENDON (SEP PROC) INCISION, LEG/ANKLE ARTHROTOMY, ANKLE, W/EXPLORATION, DRAINAGE/REMOVAL ARTHROTOMY, POSTERIOR CAPSULAR RELEASE, ANKLE, W/W BX, SOFT TISSUE, LEG/ANKLE AREA; SUPERFICIAL BX, SOFT TISSUE, LEG/ANKLE AREA; DEEP (SUBFASCIAL/ RAD RESECTION TUMOR SOFT TISSUE LEG/ANKLE <5CM RAD RESECTION TUMOR SOFT TISSUE LEG/ANKLE 5 CM/> EXCISION, TUMOR, LEG/ANKLE AREA; SUBQ TISSUE EXCISION, TUMOR, LEG/ANKLE AREA; DEEP (SUBFASCIAL/ ARTHROTOMY, ANKLE, W/JOINT EXPLORATION W/WO BX/REM ARTHROTOMY, W/SYNOVECTOMY, ANKLE; ARTHROTOMY, W/SYNOVECTOMY, ANKLE; W/TENOSYNOVECTOM EXCISION, LESION, TENDON SHEATH/CAPSULE, LEG AND/O EXCISION TUMOR SOFT TISSUE LEG/ANKLE SUBQ 3+CM EXC TUMOR SOFT TISSUE LEG/ANKLE SUBFASC 5+CM EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, TIBIA/ EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, TIBIA/ EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, TIBIA/ PARTIAL EXCISION, BONE; TIBIA PARTIAL EXCISION, BONE; FIBULA RADICAL RESECTION OF TUMOR TIBIA RADICAL RESECTION, TUMOR, BONE; FIBULA RADICAL RESECTION OF TUMOR TALUS OR CALCANEUS INJECTION PROC, ANKLE ARTHROGRAPHY REPAIR, PRIMARY, OPEN/PERCUTANEOUS, RUPTURED ACHIL REPAIR, PRIMARY, OPEN/PERCUTANEOUS, RUPTURED ACHIL REPAIR, SECONDARY, ACHILLES TENDON, W/WO GRAFT REPAIR, FASCIAL DEFECT, LEG REPAIR, FLEXOR TENDON, LEG; PRIMARY, W/O GRAFT, EA REPAIR, FLEXOR TENDON, LEG; SECONDARY, W/WO GRAFT, REPAIR, EXTENSOR TENDON, LEG; PRIMARY, W/O GRAFT, REPAIR, EXTENSOR TENDON, LEG; SECONDARY, W/WO GRAF REPAIR, DISLOCATING PERONEAL TENDONS; W/O FIBULAR REPAIR, DISLOCATING PERONEAL TENDONS; W/FIBULAR OS TENOLYSIS, FLEXOR/EXTENSOR TENDON, LEG AND/OR ANKL TENOLYSIS, FLEXOR/EXTENSOR TENDON, LEG AND/OR ANKL LENGTHENING/SHORTENING, TENDON, LEG/ANKLE; SINGLE LENGTHENING/SHORTENING, TENDON, LEG/ANKLE; MULTIPL GASTROCNEMIUS RECESSION TRANSFER/TRANSPLANT, SINGLE TENDON; SUPERFICIAL TRANSFER/TRANSPLANT, SINGLE TENDON; DEEP 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 27692 27695 27696 27698 27700 27702 27703 27704 27705 27707 27709 27712 27715 27720 27722 27724 27725 27726 27727 27730 27732 27734 27740 27742 27745 27750 27752 27756 27758 27759 27760 27762 27766 27767 27768 27769 27780 27781 27784 27786 27788 27792 27808 27810 27814 27816 27818 27822 27823 27824 27825 27826 27827 27828 27829 27830 27831 27832 27840 27842 27846 27848 27860 27870 27871 27880 27881 27882 27884 27886 27888 27889 27892 27893 27894 27899 28001 28002 28003 28005 28008 28010 28011 28020 28022 28024 28035 28039 28041 28043 28045 TRANSFER/TRANSPLANT, SINGLE TENDON; ADDL TENDON REPAIR, PRIMARY, DISRUPTED LIGAMENT, ANKLE; COLLAT REPAIR, PRIMARY, DISRUPTED LIGAMENT, ANKLE; BOTH C REPAIR, SECONDARY DISRUPTED LIGAMENT, ANKLE, COLLA ARTHROPLASTY, ANKLE; ARTHROPLASTY, ANKLE; W/IMPLANT (TOTAL ANKLE) ARTHROPLASTY, ANKLE; REVISION, TOTAL ANKLE REMOVAL, ANKLE IMPLANT OSTEOTOMY; TIBIA OSTEOTOMY; FIBULA OSTEOTOMY; TIBIA AND FIBULA OSTEOTOMY; MULTIPLE, W/REALIGNMENT ON INTRAMEDULLA OSTEOPLASTY, TIBIA AND FIBULA, LENGTHENING/SHORTEN REPAIR, NONUNION/MALUNION, TIBIA; W/O GRAFT REPAIR, NONUNION/MALUNION, TIBIA; W/SLIDING GRAFT REPAIR, NONUNION/MALUNION, TIBIA; W/ILIAC/OTHER AU REPAIR, NONUNION/MALUNION, TIBIA; SYNOSTOSIS, W/FI REPAIR OF FIBULA NONUNION AND/OR MALUNION W/INT FI REPAIR, CONGENITAL PSEUDARTHROSIS, TIBIA ARREST, EPIPHYSEAL (EPIPHYSIODESIS), OPEN; DISTAL ARREST, EPIPHYSEAL (EPIPHYSIODESIS), OPEN; DISTAL ARREST, EPIPHYSEAL (EPIPHYSIODESIS), OPEN; DISTAL ARREST, EPIPHYSEAL, COMBINED, PROXIMAL/DISTAL TIBI ARREST, EPIPHYSEAL, COMBINED, PROXIMAL/DISTAL TIBI PROPHYLACTIC TREATMENT, TIBIA, W/WO METHYLMETHACRY CLOSED TREATMENT, TIBIAL SHAFT FX; W/O MANIPULATIO CLOSED TREATMENT, TIBIAL SHAFT FX; W/MANIPULATION, PERCUTANEOUS SKELETAL FIXATION, TIBIAL SHAFT FX OPEN TREATMENT, TIBIAL SHAFT FX, W/PLATE/SCREWS, W TREATMENT, TIBIAL SHAFT FX, INTRAMEDULLARY IMPLANT CLOSED TREATMENT, MEDIAL MALLEOLUS FX; W/O MANIPUL CLOSED TREATMENT, MEDIAL MALLEOLUS FX; W/MANIPULAT OPEN TREATMENT, MEDIAL MALLEOLUS FX, W/WO INT/EXT CLOSED TREATMENT OF POSTERIOR MALLEOLUS FX, W/O MA CLOSED TREATMENT OF POSTERIOR MALLEOLUS FX W/MANIP OPEN TRMNT OF POSTERIOR MALLEOLUS FX, INC INTERN CLOSED TREATMENT, PROXIMAL FIBULA/SHAFT FX; W/O MA CLOSED TREATMENT, PROXIMAL FIBULA/SHAFT FX; W/MANI OPEN TREATMENT, PROXIMAL FIBULA/SHAFT FX, W/WO INT CLOSED TREATMENT, DISTAL FIBULAR FX (LATERAL MALLE CLOSED TREATMENT, DISTAL FIBULAR FX (LATERAL MALLE OPEN TREATMENT, DISTAL FIBULAR FX, W/WO INT/EXT FI CLOSED TREATMENT, BIMALLEOLAR ANKLE FX, (W/POTTS); CLOSED TREATMENT, BIMALLEOLAR ANKLE FX, (W/POTTS); OPEN TREATMENT, BIMALLEOLAR ANKLE FX, W/WO INT/EXT CLOSED TREATMENT, TRIMALLEOLAR ANKLE FX; W/O MANIP CLOSED TREATMENT, TRIMALLEOLAR ANKLE FX; W/MANIPUL OPEN TREATMENT, TRIMALLEOLAR ANKLE FX, MEDIAL/LATE OPEN TREATMENT, TRIMALLEOLAR ANKLE FX, MEDIAL/LATE CLOSED TREATMENT, FX, WT BEARING ARTICULAR PORTION CLOSED TREATMENT, FX, WT BEARING ARTICULAR PORTION OPEN TREATMENT, FX, WT BEARING ARTICULAR SURFACE/P OPEN TREATMENT, FX, WT BEARING ARTICULAR SURFACE/P OPEN TREATMENT, FX, WT BEARING ARTICULAR SURFACE/P OPEN TREATMENT, DISTAL TIBIOFIBULAR JOINT DISRUPTI CLOSED TREATMENT, PROXIMAL TIBIOFIBULAR JOINT DISL CLOSED TREATMENT, PROXIMAL TIBIOFIBULAR JOINT DISL OPEN TREATMENT, PROXIMAL TIBIOFIBULAR JOINT DISLOC CLOSED TREATMENT, ANKLE DISLOCATION; W/O ANESTHESI CLOSED TREATMENT, ANKLE DISLOCATION; W/ANESTHESIA, OPEN TREATMENT, ANKLE DISLOCATION W/WO PERC SKELET OPEN TREATMENT, ANKLE DISLOCATION W/WO PERC SKELET MANIPULATION, ANKLE UNDER GENERAL ANESTHESIA ARTHRODESIS, ANKLE, OPEN ARTHRODESIS, TIBIOFIBULAR JOINT, PROXIMAL/DISTAL AMPUTATION, LEG, THROUGH TIBIA AND FIBULA; AMPUTATION, LEG, THROUGH TIBIA AND FIBULA; W/IMMED AMPUTATION, LEG, THROUGH TIBIA AND FIBULA; OPEN, C AMPUTATION, LEG, THROUGH TIBIA AND FIBULA; SECONDA AMPUTATION, LEG, THROUGH TIBIA AND FIBULA; RE-AMPU AMPUTATION, ANKLE-MALLEOLI, TIBIA/FIBULA, W/PLASTI ANKLE DISARTICULATION DECOMPRESSION FASCIOTOMY, LEG; ANTERIOR/LATERAL CO DECOMPRESSION FASCIOTOMY, LEG; POSTERIOR COMPARTME DECOMPRESSION FASCIOTOMY, LEG; ANTERIOR/LATERAL/PO UNLISTED PROC, LEG/ANKLE INCISION AND DRAINAGE, BURSA, FOOT INCISION AND DRAINAGE BELOW FASCIA, W/WO TENDON SH INCISION AND DRAINAGE BELOW FASCIA, W/WO TENDON SH INCISION, BONE CORTEX, FOOT FASCIOTOMY, FOOT AND/OR TOE TENOTOMY, PERCUTANEOUS, TOE; SINGLE TENDON TENOTOMY, PERCUTANEOUS, TOE; MULTIPLE TENDONS ARTHROTOMY, W/EXPLORATION/DRAINAGE/REMOVAL LOOSE/F ARTHROTOMY, W/EXPLORATION/DRAINAGE/REMOVAL LOOSE/F ARTHROTOMY, W/EXPLORATION/DRAINAGE/REMOVAL LOOSE/F RELEASE, TARSAL TUNNEL (POSTERIOR TIBIAL NERVE DEC EXCISION TUMOR SOFT TISSUE FOOT/TOE SUBQ 1.5+CM EXC TUMOR SOFT TISSUE FOOT/TOE SUBFASC 1.5+CM EXCISION, TUMOR, FOOT; SUBQ TISSUE EXCISION, TUMOR, FOOT; DEEP, SUBFASCIAL, IM 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 28046 28047 28050 28052 28054 28055 28060 28062 28070 28072 28080 28086 28088 28090 28092 28100 28102 28103 28104 28106 28107 28108 28110 28111 28112 28113 28114 28116 28118 28119 28120 28122 28124 28126 28130 28140 28150 28153 28160 28171 28173 28175 28190 28192 28193 28200 28202 28208 28210 28220 28222 28225 28226 28230 28232 28234 28238 28240 28250 28260 28261 28262 28264 28270 28272 28280 28285 28286 28288 28289 28290 28292 28293 28294 28296 28297 28298 28299 28300 28302 28304 28305 28306 28307 28308 28309 28310 28312 28313 28315 28320 RAD RESECTION TUMOR SOFT TISSUE FOOT/TOE <3CM RAD RESECTION TUMOR SOFT TISSUE FOOT/TOE 3 CM/> ARTHROTOMY W/BX; INTERTARSAL/TARSOMETATARSAL JOINT ARTHROTOMY W/BX; METATARSOPHALANGEAL JOINT ARTHROTOMY W/BX; INTERPHALANGEAL JOINT Neurectomy, foot FASCIECTOMY, PLANTAR FASCIA; PARTIAL (SEP PROC) FASCIECTOMY, PLANTAR FASCIA; RADICAL (SEP PROC) SYNOVECTOMY; INTERTARSAL/TARSOMETATARSAL JOINT, EA SYNOVECTOMY; METATARSOPHALANGEAL JOINT, EACH EXCISION, INTERDIGITAL (MORTON) NEUROMA, SINGLE, E SYNOVECTOMY, TENDON SHEATH, FOOT; FLEXOR SYNOVECTOMY, TENDON SHEATH, FOOT; EXTENSOR EXCISION, LESION, TENDON SHEATH/CAPSULE; FOOT EXCISION, LESION, TENDON SHEATH/CAPSULE; TOES, EAC EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, TALUS/ EXCISION/ CURETTAGE, BONE CYST/BENIGN TUMOR, TALUS EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, TALUS/ EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, TARSAL EXCISN/CURET, BONE CYST/BENIGN TUMOR, TARSAL/METAT EXCISN/CURETTAGE, BONE CYST/BENIGN TUMOR, TARSAL/M EXCISION/CURETTAGE, BONE CYST/BENIGN TUMOR, PHALAN OSTECTOMY, PARTIAL EXCISION, 5TH METATARSAL HEAD ( OSTECTOMY, COMPLETE EXCISION; 1ST METATARSAL HEAD OSTECTOMY, COMPLETE EXCISION; OTHER METATARSAL HEA OSTECTOMY, COMPLETE EXCISION; 5TH METATARSAL HEAD OSTECTOMY, COMPLETE EXCISN; ALL METATARSAL HEADS, OSTECTOMY, EXCISION, TARSAL COALITION OSTECTOMY, CALCANEUS; OSTECTOMY, CALCANEUS; SPUR, W/WO PLANTAR FASCIAL R PARTIAL EXCISION, BONE; TALUS/CALCANEUS PARTIAL EXCISION, BONE; TARSAL/METATARSAL BONE, NO PARTIAL EXCISION, BONE; PHALANX, TOE RESECTION, PARTIAL/COMPLETE, PHALANGEAL BASE, EACH TALECTOMY (ASTRAGALECTOMY) METATARSECTOMY PHALANGECTOMY, TOE, EACH TOE RESECTION, CONDYLE(S), DISTAL END, PHALANX, EACH T HEMIPHALANGECTOMY/INTERPHALANGEAL JOINT EXCISION, RAD RESCJ TUMOR TARSAL EXCEPT TALUS/CALCANEUS RADICAL RESECTION TUMOR METATARSAL RADICAL RESECTION TUMOR PHALANX OR TOE REMOVAL, FB, FOOT; SUBQ REMOVAL, FB, FOOT; DEEP REMOVAL, FB, FOOT; COMPLICATED REPAIR, TENDON, FLEXOR, FOOT; PRIMARY/SECONDARY, W REPAIR, TENDON, FLEXOR, FOOT; SECONDARY W/FREE GRA REPAIR, TENDON, EXTENSOR, FOOT; PRIMARY/SECONDARY, REPAIR, TENDON, EXTENSOR, FOOT; SECONDARY W/FREE G TENOLYSIS, FLEXOR, FOOT; SINGLE TENDON TENOLYSIS, FLEXOR, FOOT; MULTIPLE TENDONS TENOLYSIS, EXTENSOR, FOOT; SINGLE TENDON TENOLYSIS, EXTENSOR, FOOT; MULTIPLE TENDONS TENOTOMY, OPEN, TENDON FLEXOR; FOOT, SINGLE/MULTIP TENOTOMY, OPEN, TENDON FLEXOR; TOE, SINGLE TENDON TENOTOMY, OPEN, EXTENSOR, FOOT/TOE, EACH TENDON RECONSTRUCTION, POSTERIOR TIBIAL TENDON, EXCISION, TENOTOMY, LENGTHENING/RELEASE, ABDUCTOR HALLUCIS M DIVISION, PLANTAR FASCIA AND MUSCLE (SEP PROC) CAPSULOTOMY, MIDFOOT; MEDIAL RELEASE ONLY (SEP PRO CAPSULOTOMY, MIDFOOT; W/TENDON LENGTHENING CAPSULOTOMY, MIDFOOT; EXTENSIVE, INC POST TALOTIBI CAPSULOTOMY, MIDTARSAL CAPSULOTOMY; METATARSOPHALANGEAL JOINT, W/WO TENOR CAPSULOTOMY; INTERPHALANGEAL JOINT, EACH JOINT (SE SYNDACTYLIZATION, TOES CORRECTION, HAMMERTOE CORRECTION, COCK-UP FIFTH TOE, W/PLASTIC SKIN CLOS OSTECTOMY, PARTIAL, EXOSTECTOMY/CONDYLECTOMY, META HALLUX RIGIDUS CORRECTION, W/CHEILECTOMY, DEBRIDEM HALLUX VALGUS CORRECTION, W/WO SESAMOIDECTOMY; SIM HALLUX VALGUS CORRECTION, W/WO SESAMOIDECTOMY; KEL HALLUX VALGUS CORRECTION, W/WO SESAMOIDECTOMY; RES HALLUX VALGUS CORRECTION, W/WO SESAMOIDECTOMY; W/T HALLUX VALGUS CORRECTION, W/WO SESAMOIDECTOMY; W/M HALLUX VALGUS CORRECTION, W/WO SESAMOIDECTOMY; LAP HALLUX VALGUS CORRECTION, W/WO SESAMOIDECTOMY; PHA HALLUX VALGUS CORRECTION; DOUBLE OSTEOTOMY OSTEOTOMY; CALCANEUS, W/WO INT FIXATION OSTEOTOMY; TALUS OSTEOTOMY, TARSAL BONES, OTHER THAN CALCANEUS/TALU OSTEOTOMY, TARSAL BONES, OTHER THAN CALCANEUS/TALU OSTEOTOMY, METATARSAL, W/WO LENGTHENING/SHORTENING OSTEOTOMY, METATARSAL, W/WO LENGTHENING/SHORTENING OSTEOTOMY, METATARSAL, W/WO LENGTHENING/SHORTENING OSTEOTOMY, METATARSAL, W/WO LENGTHENING/SHORTENING OSTEOTOMY, SHORTENING, ANGULAR/ROTATIONAL CORRECTI OSTEOTOMY, SHORTENING, ANGULAR/ROTATIONAL CORRECTI RECONSTRUCTION, ANGULAR DEFORMITY, TOE, SOFT TISSU SESAMOIDECTOMY, 1ST TOE (SEP PROC) REPAIR, NONUNION/MALUNION; TARSAL BONES 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 28322 28340 28341 28344 28345 28360 28400 28405 28406 28415 28420 28430 28435 28436 28445 28446 28450 28455 28456 28465 28470 28475 28476 28485 28490 28495 28496 28505 28510 28515 28525 28530 28531 28540 28545 28546 28555 28570 28575 28576 28585 28600 28605 28606 28615 28630 28635 28636 28645 28660 28665 28666 28675 28705 28715 28725 28730 28735 28737 28740 28750 28755 28760 28800 28805 28810 28820 28825 28890 28899 29000 29010 29015 29035 29040 29044 29046 29049 29055 29058 29065 29075 29085 29086 29105 29125 29126 29130 29131 29200 29240 REPAIR, NONUNION/MALUNION; METATARSAL, W/WO BONE G RECONSTRUCTION, TOE, MACRODACTYLY; SOFT TISSUE RES RECONSTRUCTION, TOE, MACRODACTYLY; REQUIRING BONE RECONSTRUCTION, TOE(S); POLYDACTYLY RECONSTRUCTION, TOE(S); SYNDACTYLY, W/WO SKIN GRAF RECONSTRUCTION, CLEFT FOOT CLOSED TREATMENT, CALCANEAL FX; W/O MANIPULATION CLOSED TREATMENT, CALCANEAL FX; W/MANIPULATION PERCUTANEOUS SKELETAL FIXATION, CALCANEAL FX, W/MA OPEN TREATMENT, CALCANEAL FX, W/WO INT/EXT FIXATIO OPEN TREATMENT, CALCANEAL FX, W/WO INT/EXT FIXATIO CLOSED TREATMENT, TALUS FX; W/O MANIPULATION CLOSED TREATMENT, TALUS FX; W/MANIPULATION PERCUTANEOUS SKELETAL FIXATION, TALUS FX, W/MANIPU OPEN TREATMENT, TALUS FX, W/WO INT/EXT FIXATION OPEN OSTEOCHONDRAL AUTOGRAFT, TALUS(INCL OBTAINING TREATMENT, TARSAL BONE FX (EXCEPT TALUS AND CALCAN TREATMENT, TARSAL BONE FX (EXCEPT TALUS AND CALCAN PERCUTANEOUS SKELETAL FIXATION, TARSAL FX, W/MANIP OPEN TREATMENT, TARSAL FX, W/WO INT/EXT FIXATION, CLOSED TREATMENT, METATARSAL FX; W/O MANIPULATION, CLOSED TREATMENT, METATARSAL FX; W/MANIPULATION, E PERCUTANEOUS SKELETAL FIXATION, METATARSAL FX, W/M OPEN TREATMENT, METATARSAL FX, W/WO INT/EXT FIXATI CLOSED TREATMENT, FX GREAT TOE, PHALANX/PHALANGES; CLOSED TREATMENT, FX GREAT TOE, PHALANX/PHALANGES; PERCUTANEOUS SKELETAL FIXATION, FX GREAT TOE, PHAL OPEN TREATMENT, FX GREAT TOE, PHALANX/PHALANGES W/ CLOSED TREATMENT, FX, PHALANX/PHALANGES, NOT GREAT CLOSED TREATMENT, FX, PHALANX/PHALANGES, NOT GREAT OPEN TREATMENT, FX, PHALANX/PHALANGES, NOT GREAT T CLOSED TREATMENT, SESAMOID FX OPEN TREATMENT, SESAMOID FX, W/WO INT FIXATION CLOSED TREATMENT, TARSAL BONE DISLOCATION, OTHER T CLOSED TREATMENT, TARSAL BONE DISLOCATION, OTHER T PERCUTANEOUS SKELETAL FIXATION, TARSAL DISLOCATION OPEN TREATMENT, TARSAL BONE DISLOCATION, W/WO INT/ CLOSED TREATMENT, TALOTARSAL JOINT DISLOCATION; W/ CLOSED TREATMENT, TALOTARSAL JOINT DISLOCATION; RE PERCUTANEOUS SKELETAL FIXATION, TALOTARSAL JOINT D OPEN TREATMENT, TALOTARSAL JOINT DISLOCATION, W/WO CLOSED TREATMENT, TARSOMETATARSAL JOINT DISLOCATIO CLOSED TREATMENT, TARSOMETATARSAL JOINT DISLOCATIO PERCUTANEOUS SKELETAL FIXATION, TARSOMETATARSAL JO OPEN TREATMENT, TARSOMETATARSAL JOINT DISLOCATION CLOSED TREATMENT, METATARSOPHALANGEAL JOINT DISLOC CLOSED TREATMENT, METATARSOPHALANGEAL JOINT DISLOC PERCUTANEOUS SKELETAL FIXATION, METATARSOPHALANGEA OPEN TREATMENT, METATARSOPHALANGEAL JOINT DISLOCAT CLOSED TREATMENT, INTERPHALANGEAL JOINT DISLOCATIO CLOSED TREATMENT, INTERPHALANGEAL JOINT DISLOCATIO PERCUTANEOUS SKELETAL FIXATION, INTERPHALANGEAL JO OPEN TREATMENT, INTERPHALANGEAL JOINT DISLOCATION ARTHRODESIS; PANTALAR ARTHRODESIS; TRIPLE ARTHRODESIS; SUBTALAR ARTHRODESIS, MIDTARSAL/TARSOMETATARSAL, MULTIPLE/T ARTHRODESIS, MIDTARSAL/TARSOMETATARSAL, MULTIPLE/T ARTHRODESIS, W/TENDON LENGTHENING/ADVANCEMENT, MID ARTHRODESIS, MIDTARSAL/TARSOMETATARSAL, SINGLE JOI ARTHRODESIS, GREAT TOE; METATARSOPHALANGEAL JOINT ARTHRODESIS, GREAT TOE; INTERPHALANGEAL JOINT ARTHRODESIS, GREAT TOE, INTERPHALANGEAL, W/EXTENSO AMPUTATION, FOOT; MIDTARSAL AMPUTATION, FOOT; TRANSMETATARSAL AMPUTATION, METATARSAL, W/TOE, SINGLE AMPUTATION, TOE; METATARSOPHALANGEAL JOINT AMPUTATION, TOE; INTERPHALANGEAL JOINT ESWT HI NRG PHYS/QHP W/US GDN INVG PLNTAR FASCIA UNLISTED PROC, FOOT/TOES APPLICATION, HALO TYPE BODY CAST (SEE 20661-20663, APPLICATION, RISSER JACKET, LOCALIZER, BODY; ONLY APPLICATION, RISSER JACKET, LOCALIZER, BODY; W/HEA APPLICATION, BODY CAST, SHOULDER TO HIPS; APPLICATION, BODY CAST, SHOULDER TO HIPS; W/HEAD, APPLICATION, BODY CAST, SHOULDER TO HIPS; W/ONE TH APPLICATION, BODY CAST, SHOULDER TO HIPS; W/BOTH T APPLICATION, CAST; FIGURE-OF-EIGHT APPLICATION, CAST; SHOULDER SPICA APPLICATION, CAST; PLASTER VELPEAU APPLICATION, CAST; SHOULDER TO HAND (LONG ARM) APPLICATION, CAST; ELBOW TO FINGER (SHORT ARM) APPLICATION, CAST; HAND AND LOWER FOREARM (GAUNTLE APPLICATION, CAST; FINGER (CONTRACTURE) APPLICATION, LONG ARM SPLINT (SHOULDER TO HAND) APPLICATION, SHORT ARM SPLINT (FOREARM TO HAND); S APPLICATION, SHORT ARM SPLINT (FOREARM TO HAND); D APPLICATION, FINGER SPLINT; STATIC APPLICATION, FINGER SPLINT; DYNAMIC STRAPPING; THORAX STRAPPING; SHOULDER (VELPEAU) 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 29260 29280 29305 29325 29345 29355 29358 29365 29405 29425 29435 29440 29445 29450 29505 29515 29520 29530 29540 29550 29580 29581 29582 29583 29584 29700 29705 29710 29720 29730 29740 29750 29799 29800 29804 29805 29806 29807 29819 29820 29821 29822 29823 29824 29825 29826 29827 29828 29830 29834 29835 29836 29837 29838 29840 29843 29844 29845 29846 29847 29848 29850 29851 29855 29856 29860 29861 29862 29863 29866 29867 29868 29870 29871 29873 29874 29875 29876 29877 29879 29880 29881 29882 29883 29884 29885 29886 29887 29888 29889 29891 STRAPPING; ELBOW/WRIST STRAPPING; HAND/FINGER APPLICATION, HIP SPICA CAST; ONE LEG APPLICATION, HIP SPICA CAST; ONE AND ONE-HALF SPIC APPLICATION, LONG LEG CAST (THIGH TO TOES); APPLICATION, LONG LEG CAST (THIGH TO TOES); WALKER APPLICATION, LONG LEG CAST BRACE APPLICATION, CYLINDER CAST (THIGH TO ANKLE) APPLICATION, SHORT LEG CAST (BELOW KNEE TO TOES); APPLICATION, SHORT LEG CAST (BELOW KNEE TO TOES); APPLICATION, PATELLAR TENDON BEARING CAST ADDING WALKER TO PREVIOUSLY APPLIED CAST APPLICATION, RIGID TOTAL CONTACT LEG CAST APPLICATION, CLUBFOOT CAST W/MOLDING/MANIPULATION, APPLICATION, LONG LEG SPLINT (THIGH TO ANKLE/TOES) APPLICATION, SHORT LEG SPLINT (CALF TO FOOT) STRAPPING; HIP STRAPPING; KNEE STRAPPING; ANKLE AND/OR FOOT STRAPPING; TOES STRAPPING; UNNA BOOT APPL MLTLAYR COMPRES LEG BELOW KNEE W/ANKLE FOOT APPL MLTLAYR COMPRES THGH LEG ANKLE FT WHEN DONE APPL MLTLAYR COMPRES SYSTEM UPPER & LOWER ARM APPL MLTLAYR COMPRES SYS UPARM LWARM HAND&FINGER REMOVAL/BIVALVING; GAUNTLET, BOOT/BODY CAST REMOVAL/BIVALVING; FULL ARM/FULL LEG CAST REMOVAL/BIVALVING; SHOULDER/HIP SPICA, MINERVA/RIS REPAIR, SPICA, BODY CAST/JACKET WINDOWING, CAST WEDGING, CAST (EXCEPT CLUBFOOT CASTS) WEDGING, CLUBFOOT CAST UNLISTED PROC, CASTING/STRAPPING ARTHROSCOPY, TEMPOROMANDIBULAR JOINT, DX W/WO SYNO ARTHROSCOPY, TEMPOROMANDIBULAR JOINT, SURGICAL ARTHROSCOPY, SHOULDER, DX, W/WO SYNOVIAL BX (SEP P ARTHROSCOPY, SHOULDER, SURGICAL; CAPSULORRHAPHY ARTHROSCOPY, SHOULDER, SURGICAL; REPAIR, SLAP LESI ARTHROSCOPY, SHOULDER, SURGICAL; W/REMOVAL, LOOSE/ ARTHROSCOPY, SHOULDER, SURGICAL; SYNOVECTOMY, PART ARTHROSCOPY, SHOULDER, SURGICAL; SYNOVECTOMY, COMP ARTHROSCOPY, SHOULDER, SURGICAL; DEBRIDEMENT, LIMI ARTHROSCOPY, SHOULDER, SURGICAL; DEBRIDEMENT, EXTE ARTHROSCOPY, SHOULDER, SURGICAL; DISTAL CLAVICULEC ARTHROSCOPY, SHOULDER, SURGICAL; W/LYSIS AND RESEC SHOULDER SCOPE BONE SHAVING ARTHROSCOPY, SHOULDER, SURGICAL; W/ROTATOR CUFF RE ARTHROSCOPY SHOULDER SURGICAL BICEPS TENODESIS ARTHROSCOPY, ELBOW, DX, W/WO SYNOVIAL BX (SEP PROC ARTHROSCOPY, ELBOW, SURGICAL; W/REMOVAL, LOOSE/FOR ARTHROSCOPY, ELBOW, SURGICAL; SYNOVECTOMY, PARTIAL ARTHROSCOPY, ELBOW, SURGICAL; SYNOVECTOMY, COMPLET ARTHROSCOPY, ELBOW, SURGICAL; DEBRIDEMENT, LIMITED ARTHROSCOPY, ELBOW, SURGICAL; DEBRIDEMENT, EXTENSI ARTHROSCOPY, WRIST, DX, W/WO SYNOVIAL BX (SEP PROC ARTHROSCOPY, WRIST, SURGICAL; INFECTION, LAVAGE AN ARTHROSCOPY, WRIST, SURGICAL; SYNOVECTOMY, PARTIAL ARTHROSCOPY, WRIST, SURGICAL; SYNOVECTOMY, COMPLET ARTHROSCOPY, WRIST, SURGICAL; EXCISION/REPAIR, TRI ARTHROSCOPY, WRIST, SURGICAL; INT FIXATION, FX/INS ENDOSCOPY, WRIST, SURGICAL, W/RELEASE, TRANSVERSE ARTHROSCOPICALLY AIDED TREATMENT, FX, KNEE W/WO MA ARTHROSCOPICALLY AIDED TREATMENT, FX, KNEE W/WO MA ARTHROSCOPICALLY AIDED TREATMENT, TIBIAL FX, PROXI ARTHROSCOPICALLY AIDED TREATMENT, TIBIAL FX, PROXI ARTHROSCOPY, HIP, DX W/WO SYNOVIAL BX (SEP PROC) ARTHROSCOPY, HIP, SURGICAL; W/REMOVAL, LOOSE/FOREI ARTHROSCOPY, HIP, SURGICAL; W/CHONDROPLASTY/ARTHRO ARTHROSCOPY, HIP, SURGICAL; W/SYNOVECTOMY ARTHOSCOPY, KNEE, SURGICAL, OSTEOCHONDRAL AUTOGRAP ARTHOSCOPY, KNEE, SURGICAL, OSTEPCJRPMDRA; ALLOGRA ARTHOSCOPY, KNEE, SURGICAL, MENISCAL TRANSPLANTATI ARTHROSCOPY, KNEE, DX, W/WO SYNOVIAL BX (SEP PROC) ARTHROSCOPY, KNEE, SURGICAL; INFECTION, LAVAGE AND ARTHROSCOPY, KNEE, SURGICAL; W/LATERAL RELEASE ARTHROSCOPY, KNEE, SURGICAL; REMOVAL, LOOSE/FB ARTHROSCOPY, KNEE, SURGICAL; SYNOVECTOMY, LIMITED ARTHROSCOPY, KNEE, SURGICAL; SYNOVECTOMY, MAJOR, O ARTHROSCOPY, KNEE, SURGICAL; DEBRIDEMENT/SHAVING, ARTHROSCOPY, KNEE, SURGICAL; ABRASION ARTHROPLASTY ARTHRS KNEE W/MENISCECTOMY MED&LAT W/SHAVING ARTHRS KNE SURG W/MENISCECTOMY MED/LAT W/SHVG ARTHROSCOPY, KNEE, SURGICAL; W/MENISCUS REPAIR, ME ARTHROSCOPY, KNEE, SURGICAL; W/MENISCUS REPAIR, ME ARTHROSCOPY, KNEE, SURGICAL; W/LYSIS, ADHESIONS, W ARTHROSCOPY, KNEE, SURGICAL; DRILL, OSTEOCHONDRITI ARTHROSCOPY, KNEE, SURGICAL; DRILLING, INTACT OSTE ARTHROSCOPY, KNEE, SURGICAL; DRILLING, INTACT OSTE ARTHROSCOPICALLY AIDED ANTERIOR CRUCIATE LIGAMENT ARTHROSCOPICALLY AIDED POSTERIOR CRUCIATE LIGAMENT ARTHROSCOPY, ANKLE, SURGICAL; EXCISION OSTEOCHONDR 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 29892 29893 29894 29895 29897 29898 29899 29900 29901 29902 29904 29905 29906 29907 29914 29915 29916 29999 30000 30020 3006F 3008F 30100 30110 30115 30117 30118 3011F 30120 30124 30125 30130 30140 3014F 30150 3015F 30160 3016F 3017F 3018F 3019F 30200 3020F 30210 3021F 30220 3022F 3023F 3025F 3027F 3028F 30300 30310 30320 3035F 3037F 3038F 30400 3040F 30410 30420 3042F 30430 30435 3044F 30450 3045F 30460 30462 30465 3046F 3048F 3049F 3050F 30520 30540 30545 3055F 30560 3056F 30580 30600 3060F 3061F 30620 3062F 30630 3066F 3072F 3073F 3074F ARTHROSCOPICALLY AIDED REPAIR, OSTEOCHONDRITIS/TAL ENDOSCOPIC PLANTAR FASCIOTOMY ARTHROSCOPY, ANKLE (TIBIOTALAR AND FIBULOTALAR JOI ARTHROSCOPY, ANKLE (TIBIOTALAR AND FIBULOTALAR JOI ARTHROSCOPY, ANKLE (TIBIOTALAR AND FIBULOTALAR JOI ARTHROSCOPY, ANKLE (TIBIOTALAR AND FIBULOTALAR JOI ARTHROSCOPY, ANKLE, SURGICAL; W/ANKLE ARTHRODESIS ARTHROSCOPY, METACARPOPHALANGEAL JOINT, DIAGNOSTIC ARTHROSCOPY, METACARPOPHALANGEAL JOINT, SURGICAL; ARTHROSCOPY, METACARPOPHALANGEAL JOINT, SURGICAL; ARTHROSCOPY SUBTALAR JOINT, SURG, W/REMOVAL LOOSE ARTHROSCOPY SUBTALAR JOINT WITH SYNOVECTOMY ARTHROSCOPY SUBTALAR JOINT WITH DEBRIDEMENT ARTHROSCOPY SUBTALAR JOINT SUBTALAR ARTHRODESIS ARTHROSCOPY HIP W/FEMOROPLASTY ARTHROSCOPY HIP W/ACETABULOPLASTY ARTHROSCOPY HIP W/LABRAL REPAIR UNLISTED PROC, ARTHROSCOPY DRAINAGE ABSCESS/HEMATOMA, NASAL, INT APPROACH DRAINAGE ABSCESS/HEMATOMA, NASAL SEPTUM Cxr doc rev BODY MASS INDEX (BMI) DOCUMENTED BX, INTRANASAL EXCISION, NASAL POLYP(S), SIMPLE EXCISION, NASAL POLYP(S), EXTENSIVE EXCISION/DESTRUCTION, INTRANASAL LESION; INT APPRO EXCISION/DESTRUCTION, INTRANASAL LESION; EXT APPRO Lipid panel doc rev EXCISION/SURGICAL PLANING, SKIN, NOSE, RHINOPHYMA EXCISION DERMOID CYST, NOSE; SIMPLE, SKIN, SUBQ EXCISION DERMOID CYST, NOSE; COMPLEX, UNDER BONE/C EXCISION TURBINATE, PARTIAL/COMPLETE, ANY METHOD SUBMUCOUS RESECTION TURBINATE, PARTIAL/COMPLETE, A Screen mammo doc rev RHINECTOMY; PARTIAL CERV CANCER SCREENING RESULTS DOCD AND RVWD RHINECTOMY; TOTAL PT SCRND UNHLTHY OH USE BY SYSTMTC SCRNG METHD Colorectal ca screen doc rev PRE-PRXD RSK ET AL DOCD LVEF ASSESSMENT PLANNED POST DISCHARGE INJECTION INTO TURBINATE(S), THERAPEUTIC LEFT VENTRICULAR FUNCTION ASSESSMENT DOCUMENTED DISPLACEMENT THERAPY (PROETZ TYPE) Lvef mod/sever deprs syst INSERTION, NASAL SEPTAL PROSTHESIS (BUTTON) Lvef =40% systolic Spirom doc rev Spirom fev/fvc<70% w copd Spirom fev/fvc=70%/ w/o copd O2 saturation doc rev REMOVAL FB, INTRANASAL; OFFICE TYPE PROC REMOVAL FB, INTRANASAL; REQUIRING GENERAL ANESTHES REMOVAL FB, INTRANASAL; LATERAL RHINOTOMY O2 saturation =88% /pa0 =55 O2 saturation> 88% /pao>55 PULMONARY FXN TEST DONE WITHIN 12 MON B4 SURGERY RHINOPLASTY, PRIMARY; LATERAL / ALAR CARTILAGES AN Fev<40% predicted value RHINOPLASTY, PRIMARY; COMPLETE, EXT PARTS W/BONY P RHINOPLASTY, PRIMARY; W/MAJOR SEPTAL REPAIR Fev= 40% predicted value RHINOPLASTY, SECONDARY; MINOR REVISION (SMALL AMOU RHINOPLASTY, SECONDARY; INTERMEDIATE REVISION (BON HG a1c level < 7.0% RHINOPLASTY, SECONDARY; MAJOR REVISION (NASAL TIP HG a1c level 7.0-9.0% RHINOPLASTY, NASAL DEFORMITY SECONDARY TO CONG CLE RHINOPLASTY, NASAL DEFORM SEC TO CONG CLEFT LIP/PA REPAIR, NASAL VESTIBULAR STENOSIS (SPREADER GRAFTI Hemoglobin a1c level > 9.0% MOST RECENT LDL-C < 100 MG/DL MOST RECENT LDL-C 100-129 MG/DL MOST RECENT LDL-C >= 130 MG/DL SEPTOPLASTY/SUBMUCOUS RESECTION W/WO CARTILAGE SCO REPAIR CHOANAL ATRESIA; INTRANASAL REPAIR CHOANAL ATRESIA; TRANSPALATINE LVEF LESS THAN OR EQUAL TO 35% LYSIS INTRANASAL SYNECHIA LVEF GREATER THAN 35% REPAIR FISTULA; OROMAXILLARY (COMBINE W/31030 IF A REPAIR FISTULA; ORONASAL Pos microalbuminuria rev Neg microalbuminuria rev SEPTAL/OTHER INTRANASAL DERMATOPLASTY (DOES NOT IN Pos macroalbuminuria rev REPAIR NASAL SEPTAL PERFORATIONS Nephropathy doc tx Low risk for retinopathy DOCUMENTED LENGTH CORNEAL POWER & LENS POWER MOST RECENT SYSTOLIC BLOOD PRESSURE <130 MM HG 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2010 1/1/2004 1/1/2009 1/1/2007 1/1/2009 1/1/2012 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2010 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2007 1/1/2010 1/1/2007 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N Y N Y Y N Y N Y Y N N N N N Y N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 3075F 3077F 3078F 3079F 30801 30802 3080F 3082F 3083F 3084F 3085F 3088F 3089F 30901 30903 30905 30906 3090F 30915 3091F 30920 3092F 30930 3093F 3095F 3096F 30999 31000 31002 3100F 31020 31030 31032 31040 31050 31051 31070 31075 31080 31081 31084 31085 31086 31087 31090 3110F 3111F 3112F 3115F 3117F 3118F 3119F 31200 31201 31205 3120F 31225 31230 31231 31233 31235 31237 31238 31239 31240 31254 31255 31256 31267 3126F 31276 31287 31288 31290 31291 31292 31293 31294 31295 31296 31297 31299 31300 31320 31360 31365 31367 31368 31370 31375 31380 MOST RECENT SYSTOLIC BLOOD PRESS 130-139MM HG MOST RECENT SYSTOLIC BLOOD PRESSURE >= 140 MM HG MOST RECENT DIASTOLIC BLOOD PRESSURE < 80 MM HG MOST RECENT DIASTOLIC BLOOD PRESSURE 80-89 MM HG ABLTJ SOF TISS INF TURBS UNI/BI SUPFC ABLTJ SOF TISS INF TURBS UNI/BI SUPFC INTRAMURAL MOST RECENT DIASTOLIC BLOOD PRESSURE >= 90 MM HG Kt/V <1.2 Kt/V >= 1.2 and <1.7 Kt/V >= 1.7 SUICIDE RISK ASSESSED MDD, mild MDD, moderate CONTROL NASAL HEMORRHAGE, ANTERIOR, SIMPLE (LIMITE CONTROL NASAL HEMORRHAGE, ANTERIOR, COMPLEX (EXTEN CONTROL NASAL HEMORRHAGE, POSTERIOR, W/POST NASAL CONTROL NASAL HEMORRHAGE, POSTERIOR, W/POST NASAL MDD, severe; w/o psych LIGATION ARTERIES; ETHMOIDAL MDD, severe; w/psych LIGATION ARTERIES; INT MAXILLARY ARTERY, TRANSANTR MDD, in remission FX NASAL TURBINATE(S), THERAPEUTIC Doc new diag 1st/addl. MDD CENTRAL DXA RESULTS DOCUMENTED CENTRAL DXA ORDERED UNLISTED PROC, NOSE LAVAGE, CANNULATION; MAXILLARY SINUS (ANTRUM PUNCT LAVAGE, CANNULATION; SPHENOID SINUS Carotid imaging study report (includes direct or indirect reference to measurements of distal in SINUSOTOMY, MAXILLARY (ANTROTOMY); INTRANASAL SINUSOTOMY, MAXILLARY (ANTROTOMY); RADICAL (CALDWE SINUSOTOMY, MAXILLARY (ANTROTOMY); RADICAL (CALDWE PTERYGOMAXILLARY FOSSA SURGERY, ANY APPROACH SINUSOTOMY, SPHENOID, W/WO BX; SINUSOTOMY, SPHENOID, W/WO BX; W/MUCOSAL STRIPPING SINUSOTOMY FRONTAL; EXT, SIMPLE (TREPHINE OPERATIO SINUSOTOMY FRONTAL; TRANSORBITAL, UNILAT (FOR MUCO SINUSOTOMY FRONTAL; OBLITERATIVE W/O OSTEOPLASTIC SINUSOTOMY FRONTAL; OBLITERATIVE, W/O OSTEOPLASTIC SINUSOTOMY FRONTAL; OBLITERATIVE, W/OSTEOPLASTIC F SINUSOTOMY FRONTAL; OBLITERATIVE, W/OSTEOPLASTIC F SINUSOTOMY FRONTAL; NONOBLITERATIVE, W/OSTEOPLASTI SINUSOTOMY FRONTAL; NONOBLITERATIVE, W/OSTEOPLASTI SINUSOTOMY, UNILAT, OVER 3 PARANASAL SINUSES DOC CT MRI RPRT OF BLDNG LESION ACUTE INFARCTION CT OR MRI BRAIN DONE W/IN 24 HRS HOSP ARRIVAL CT/MRI BRAIN DONE > 24 HRS AFTER HOSP ARRIVAL QUANT RESULTS EVAL CURR LEVEL ACTIVITY CLIN SYMP HF DISEASE SPECIFIC ASSESSMENT TOOL COMPLETED NEW YORK HEART ASSOCIATION (NYHA) CLASS DOCD NO EVAL LEVEL OF ACTIVITY OR CLINICAL SYMPTOMS ETHMOIDECTOMY; INTRANASAL, ANTERIOR ETHMOIDECTOMY; INTRANASAL, TOTAL ETHMOIDECTOMY; EXTRANASAL, TOTAL 12-LEAD ECG PERFORMED (EM) MAXILLECTOMY; W/O ORBITAL EXENTERATION MAXILLECTOMY; W/ORBITAL EXENTERATION (EN BLOC) NASAL ENDOSCOPY, DX, UNILAT/BILAT (SEP PROC) NASAL/SINUS ENDOSCOPY, DX W/MAXILLARY SINUSOSCOPY NASAL/SINUS ENDOSCOPY, DX W/SPHENOID SINUSOSCOPY NASAL/SINUS ENDOSCOPY, SURGICAL; W/BX, POLYPECTOMY NASAL/SINUS ENDOSCOPY, SURGICAL; W/CONTROL, NASAL NASAL/SINUS ENDOSCOPY, SURGICAL; W/DACRYOCYSTORHIN NASAL/SINUS ENDOSCOPY, SURGICAL; W/CONCHA BULLOSA NASAL/SINUS ENDOSCOPY, SURGICAL; W/ETHMOIDECTOMY, NASAL/SINUS ENDOSCOPY, SURGICAL; W/ETHMOIDECTOMY, NASAL/SINUS ENDOSCOPY, SURGICAL, W/MAXILLARY ANTRO NASAL/SINUS ENDOSCOPY, SURGICAL, W/MAXILLARY ANTRO ESOPH BX RPRT W/DYSPLAS INFO AND APPROP GRADING NASAL/SINUS ENDOSCOPY, SURGICAL W/FRONTAL SINUS EX NASAL/SINUS ENDOSCOPY, SURGICAL, W/SPHENOIDOTOMY; NASAL/SINUS ENDOSCOPY, SURGICAL, W/SPHENOIDOTOMY; NASAL/SINUS ENDOSCOPY, SURGICAL, W/REPAIR, CEREBRO NASAL/SINUS ENDOSCOPY, SURGICAL, W/REPAIR, CEREBRO NASAL/SINUS ENDOSCOPY, SURGICAL; W/MEDIAL/INFERIOR NASAL/SINUS ENDOSCOPY, SURGICAL; W/MEDIAL AND INFE NASAL/SINUS ENDOSCOPY, SURGICAL; W/OPTIC NERVE DEC NSL/SINUS NDSC SURG W/DILAT MAXILLARY SINUS NSL/SINUS NDSC SURG W/DILAT FRONTAL SINUS NSL/SINUS NDSC SURG W/DILAT SPHENOID SINUS UNLISTED PROC, ACCESSORY SINUSES LARYNGOTOMY (THYROTOMY, LARYNGOFISSURE); W/REMOVAL LARYNGOTOMY (THYROTOMY, LARYNGOFISSURE); DX LARYNGECTOMY; TOTAL, W/O RADICAL NECK DISSECTION LARYNGECTOMY; TOTAL, W/RADICAL NECK DISSECTION LARYNGECTOMY; SUBTOTAL SUPRAGLOTTIC, W/O RADICAL N LARYNGECTOMY; SUBTOTAL SUPRAGLOTTIC, W/RADICAL NEC PARTIAL LARYNGECTOMY (HEMILARYNGECTOMY); HORIZONTA PARTIAL LARYNGECTOMY (HEMILARYNGECTOMY); LATEROVER PARTIAL LARYNGECTOMY (HEMILARYNGECTOMY); ANTEROVER 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2010 1/1/2010 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 31382 31390 31395 31400 31420 31500 31502 31505 31510 31511 31512 31513 31515 31520 31525 31526 31527 31528 31529 31530 31531 31535 31536 31540 31541 31545 31546 31560 31561 31570 31571 31575 31576 31577 31578 31579 31580 31582 31584 31587 31588 31590 31595 31599 31600 31601 31603 31605 31610 31611 31612 31613 31614 31615 31620 31622 31623 31624 31625 31626 31627 31628 31629 31630 31631 31632 31633 31634 31635 31636 31637 31638 31640 31641 31643 31645 31646 31647 31648 31649 31651 31652 31653 31654 31660 31661 31717 31720 31725 31730 31750 PARTIAL LARYNGECTOMY (HEMILARYNGECTOMY); ANTERO-LA PHARYNGOLARYNGECTOMY, W/RADICAL NECK DISSECTION; W PHARYNGOLARYNGECTOMY, W/RADICAL NECK DISSECTION; W ARYTENOIDECTOMY/ARYTENOIDOPEXY, EXT APPROACH EPIGLOTTIDECTOMY INTUBATION, ENDOTRACHEAL, EMERGENCY PROC TRACHEOTOMY TUBE CHANGE PRIOR TO ESTABLISHMENT, FI LARYNGOSCOPY, INDIRECT; DIAGNOSTIC (SEP PROC) LARYNGOSCOPY, INDIRECT; W/BX LARYNGOSCOPY, INDIRECT; W/REMOVAL, FB LARYNGOSCOPY, INDIRECT; W/REMOVAL, LESION LARYNGOSCOPY, INDIRECT; W/VOCAL CORD INJECTION LARYNGOSCOPY DIRECT, W/WO TRACHEOSCOPY; ASPIRATION LARYNGOSCOPY DIRECT, W/WO TRACHEOSCOPY; DX, NEWBOR LARYNGOSCOPY DIRECT, W/WO TRACHEOSCOPY; DX, EXCEPT LARYNGOSCOPY DIRECT, W/WO TRACHEOSCOPY; DX, W/OPER LARYNGOSCOPY DIRECT, W/WO TRACHEOSCOPY; W/INSERTIO LARYNGOSCOPY DIRECT, W/WO TRACHEOSCOPY; W/DILATATI LARYNGOSCOPY DIRECT, W/WO TRACHEOSCOPY; W/DILATATI LARYNGOSCOPY, DIRECT, OPERATIVE, W/FB REMOVAL; LARYNGOSCOPY, DIRECT, OPERATIVE, W/FB REMOVAL; W/O LARYNGOSCOPY, DIRECT, OPERATIVE, W/BX; LARYNGOSCOPY, DIRECT, OPERATIVE, W/BX; W/OPERATING LARYNGOSCOPY, DIRECT, OPERATIVE, W/EXCISION, TUMOR LARYNGOSCOPY, DIRECT, OPERATIVE, W/EXCISION, TUMOR OPERATIVE LARYNGOSCOPY OPERATIVE LARYNGOSCOPY LARYNGOSCOPY, DIRECT, OPERATIVE, W/ARYTENOIDECTOMY LARYNGOSCOPY, DIRECT, OPERATIVE, W/ARYTENOIDECTOMY LARYNGOSCOPY, DIRECT, W/INJECTION INTO VOCAL CORD( LARYNGOSCOPY, DIRECT, W/INJECTION INTO VOCAL CORD, LARYNGOSCOPY, FLEXIBLE FIBEROPTIC; DX LARYNGOSCOPY, FLEXIBLE FIBEROPTIC; W/BX LARYNGOSCOPY, FLEXIBLE FIBEROPTIC; W/REMOVAL, FB LARYNGOSCOPY, FLEXIBLE FIBEROPTIC; W/REMOVAL, LESI LARYNGOSCOPY, FLEXIBLE/RIGID FIBEROPTIC, W/STROBOS LARYNGOPLASTY; LARYNGEAL WEB, TWO STAGE, W/KEEL IN LARYNGOPLASTY; LARYNGEAL STENOSIS, W/GRAFT/CORE MO LARYNGOPLASTY; W/OPEN REDUCTION, FX LARYNGOPLASTY, CRICOID SPLIT LARYNGOPLASTY, NOS LARYNGEAL REINNERVATION, NEUROMUSCULAR PEDICLE SECTION RECURRENT LARYNGEAL NERVE, THERAPEUTIC (SE UNLISTED PROC, LARYNX TRACHEOSTOMY, PLANNED (SEP PROC); TRACHEOSTOMY, PLANNED (SEP PROC); UNDER AGE 2 TRACHEOSTOMY, EMERGENCY PROC; TRANSTRACHEAL TRACHEOSTOMY, EMERGENCY PROC; CRICOTHYROID MEMBRAN TRACHEOSTOMY, FENESTRATION PROC W/SKIN FLAPS CONSTRUCTION, TRACHEOESOPHAGEAL FISTULA, W/SUBSEQU TRACHEAL PUNCTURE, PERCUTANEOUS W/TRANSTRACHEAL AS TRACHEOSTOMA REVISION; SIMPLE, W/O FLAP ROTATION TRACHEOSTOMA REVISION; COMPLEX, W/FLAP ROTATION TRACHEOBRONCHOSCOPY THROUGH ESTABLISHED TRACHEOSTO ENDOBRACHIAL ULTRASOUND DURING BRONCHOSCOPIC DIAGN BRNCHSC INCL FLUOR GID DX W/CELL WASHG SPX BRONCHOSCOPY, RIGID/FLEX, W/WO FLUORO GUID; W/BRUS BRONCHOSCOPY, RIGID/FLEX, W/WO FLUORO GUID; W/BRON BRONCHOSCOPY, RIGID/FLEX, W/WO FLUORO GUID; W/BRON BRNCHSC W/PLMT FIDUCIAL MARKERS 1/MLT BRNCHSC W/CPTR-ASST IMAGE-GUIDED NAVIGATION BRONCHOSCOPY, RIGID/FLEX, W/WO FLUORO GUID; W/TRAN BRONCHOSCOPY, RIGID/FLEX, W/WO FLUORO GUID; W/TRAN BRONCHOSCOPY, RIGID/FLEX, W/WO FLUORO GUID; W/TRAC BRONCHOSCOPY, RIGID/FLEX, W/WO FLUORO GUID; W/TRAC BRNCHSC W/TRANSBRNCL LUNG BX EA LOBE BRNCHSC W/TRANSBRNCL NDL ASPIR BX EA LOBE BRONCHOSCOPY BALLOON OCCLUSION BRONCHOSCOPY, RIGID/FLEX, W/WO FLUORO GUID; W/REMO BRONCHOSCOPY, WITH PLACEMENT OF BRONCHIAL STENTS BRONCHOSCOPY, EACH ADDITIONAL MAJOR BRONCHUS STENT BRONCHOSCOPY, WITH REVISION OF TRACHEAL OR BRONCHI BRONCHOSCOPY, RIGID/FLEX, W/WO FLUORO GUID; W/EXCI BRNCHSC W/DSTRJ TUM RELIEF STENOSIS OTH/THN EXC BRNCHSC W/PLMT CATH INTRCV RADIOELMNT APPL BRNCHSC W/THER ASPIR TRACHEOBRNCL TREE 1ST BRNCHSC W/THER ASPIR TRACHEOBRNCL TREE SBSQ BRNCHSC OCCLUSION&INSERT BRONCH VALVE INIT LOBE BRNCHSC REMOVAL BRONCHIAL VALVE INITIAL BRNCHSC OCCLUSION&INSERT BRONCH VALVE ADDL LOBE BRNCHSC REMOVAL BRONCHIAL VALVE EA ADDL BRONCHOSCOPY, RIGID OR FLEXIBLE, INCLUDING FLUOROSCOPIC GUIDANCE, WHEN PERFORM BRONCHOSCOPY, RIGID OR FLEXIBLE, INCLUDING FLUOROSCOPIC GUIDANCE, WHEN PERFORM BRONCHOSCOPY, RIGID OR FLEXIBLE, INCLUDING FLUOROSCOPIC GUIDANCE, WHEN PERFORM BRONCHOSCOPIC THERMOPLASTY ONE LOBE BRONCHOSCOPIC THERMOPLASTY 2/> LOBES CATHETERIZATION W/BRONCHIAL BRUSH BX CATHETER ASPIRATION (SEP PROC); NASOTRACHEAL CATHETER ASPIRATION (SEP PROC); TRACHEOBRONCHIAL W TRANSTRACHEAL INTRODUCTION, NEEDLE WIRE DILATOR/ST TRACHEOPLASTY; CERVICAL 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 7/1/2005 7/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2016 1/1/2016 1/1/2016 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 31755 31760 31766 31770 31775 31780 31781 31785 31786 31800 31805 31820 31825 31830 31899 32035 32036 32096 32097 32098 32100 32110 32120 32124 32140 32141 32150 32151 32160 32200 32215 32220 32225 32310 32320 32400 32405 32440 32442 32445 32480 32482 32484 32486 32488 32491 32501 32503 32504 32505 32506 32507 32540 32550 32551 32552 32553 32554 32555 32556 32557 32560 32561 32562 32601 32604 32606 32607 32608 32609 3260F 32650 32651 32652 32653 32654 32655 32656 32658 32659 3265F 32661 32662 32663 32664 32665 32666 32667 32668 32669 3266F TRACHEOPLASTY; TRACHEOPHARYNGEAL FISTULIZATION, EA TRACHEOPLASTY; INTRATHORACIC CARINAL RECONSTRUCTION BRONCHOPLASTY; GRAFT REPAIR BRONCHOPLASTY; EXCISION STENOSIS AND ANASTOMOSIS EXCISION TRACHEAL STENOSIS AND ANASTOMOSIS; CERVIC EXCISION TRACHEAL STENOSIS AND ANASTOMOSIS; CERVIC EXCISION, TRACHEAL TUMOR/CARCINOMA; CERVICAL EXCISION, TRACHEAL TUMOR/CARCINOMA; THORACIC SUTURE, TRACHEAL WOUND/INJURY; CERVICAL SUTURE, TRACHEAL WOUND/INJURY; INTRATHORACIC SURGICAL CLOSURE TRACHEOSTOMY/FISTULA; W/O PLASTIC SURGICAL CLOSURE TRACHEOSTOMY/FISTULA; W/PLASTIC R REVISION, TRACHEOSTOMY SCAR UNLISTED PROC, TRACHEA, BRONCHI THORACOSTOMY; W/RIB RESECTION, EMPYEMA THORACOSTOMY; W/OPEN FLAP DRAINAGE, EMPYEMA THORACTOMY W/DX BX LUNG INFILTRATE UNILATERAL THORACTOMY W/DX BX LUNG NODULE/MASS UNILATERAL THORACOTOMY W/BIOPSY OF PLEURA THORACOTOMY, MAJOR; W/EXPLORATION AND BX THORCOM CTRL TRAUMTC HEMRRG&/RPR LNG TEAR THORACOTOMY, MAJOR; POSTOPERATIVE COMPLICATIONS THORACOTOMY, MAJOR; W/OPEN INTRAPLEURAL PNEUMONOLY THORACOTOMY, MAJOR; W/CYST(S) REMOVAL, W/WO A PLEU THORACOTOMY W/RESECTION BULLAE THORCOM W/RMVL INTRAPLEURAL FB/FIBRIN DEP THORCOM W/RMVL IPUL FB THORACOTOMY, MAJOR; W/CARDIAC MASSAGE PNEUMONOSTOMY W/OPEN DRAINAGE ABSCESS/CYST PLEURAL SCARIFICATION, REPEAT PNEUMOTHORAX DECORTICATION, PULMONARY (SEP PROC); TOTAL DECORTICATION, PULMONARY (SEP PROC); PARTIAL PLEURECTOMY, PARIETAL (SEP PROC) DECORTICATION AND PARIETAL PLEURECTOMY BX, PLEURA; PERCUTANEOUS NEEDLE BX, LUNG/MEDIASTINUM, PERCUTANEOUS NEEDLE REMOVAL OF LUNG PNEUMONECTOMY REMOVAL LUNG PNEUMONECTOMY RESXN SGMNT TRACHEA REMOVAL LUNG PNEUMONECTOMY EXTRAPLEURAL RMVL LUNG OTHER THAN PNEUMONECTOMY 1 LOBE LOBECT RMVL LUNG OTHER THAN PNEUMONECT 2 LOBES BILOBEC RMVL LUNG OTHER THAN PNEUMONECT 1 SEGMENTECTOMY RMVL LUNG XCP TOT PNEUMONECTOMY SLEEVE LOBECTOMY RMVL LUNG OTHER/THAN PNUMEC COMPLETION PNUMEC RMVL LUNG OTH/THN PNUMEC RESXN-PLCTJ EMPHY LUNG RESECTION/REPAIR, PORTION, BRONCHUS, DURING LOBECT RESECTION OF APICAL LUNG TUMOR; WHEN PERFORMED; WI RESECTION OF APICAL LUNG TUMOR; WHEN PERFORMED; WI THORACOTOMY W/THERAPEUTIC WEDGE RESEXN INITIAL THORACOTOMY W/THERAP WEDGE RESEXN ADDL IPSILATRL THORACOTOMY W/DX WEDGE RESEXN &ANTOM LUNG RESEXN EXTRAPLEURAL ENUCLEATION, EMPYEMA (EMPYEMECTOMY) INSERTION INDWELLING TUNNELED PLEURAL CATH W/CUFF TUBE THORACOSTOMY INCLUDES WATER SEAL (SEP PROC) RMVL NDWELLG TUN PLEURAL CATH W/CUFF PLMT NTRSTL DEV RADJ THX GID PRQ INTRATHRC 1/MLT THORACENTESIS NEEDLE/CATH PLEURA W/O IMAGING THORACENTESIS NEEDLE/CATH PLEURA W/IMAGING PERQ DRAINAGE PLEURA INSERT CATH W/O IMAGING PERQ DRAINAGE PLEURA INSERT CATH W/IMAGING INSTLJ VIA CHEST TUBE/CATH AGENT FOR PLEURODESIS INSTLJ VIA CH TUBE/CATH AGENT FBRNLYSIS 1ST DAY INSTLJ CH TUBE/CATH AGENT FBRNLYSIS SBSQ DAY THORSC DX LUNGS/PERICAR/MED/PLEURAL SPACE W/O BX THORACOSCOPY, DX (SEP PROC); PERICARDIAL SAC, W/BX THORACOSCOPY, DX (SEP PROC); MEDIASTINAL SPACE, W/ THORACOSCOPY W/DX BX OF LUNG INFILTRATE UNILATRL THORACOSCOPY W/DX BX OF LUNG NODULES UNILATRL THORACOSCOPY WITH BIOPSYIES OF PLEURA PT CATEGORY PN CATEGORY AND HISTORIC GRADE DOCUMEN THORACOSCOPY, SURGICAL; W/PLEURODESIS (MECHANICAL/ THORACOSCOPY, SURGICAL; W/PARTIAL PULMONARY DECORT THORACOSCOPY, SURGICAL; W/TOTAL PULMONARY DECORTIC THORACOSCOPY, SURGICAL; W/REMOVAL, INTRAPLEURAL FB THORACOSCOPY, SURGICAL; W/CONTROL, TRAUMATIC HEMOR THORACOSCOPY W/RESECTION BULLAE W/WO PLEURAL PX THORACOSCOPY, SURGICAL; W/PARIETAL PLEURECTOMY THORACOSCOPY, SURGICAL; W/REMOVAL, CLOT/FB, PERICA THORACOSCOPY, SURGICAL; W/CREATION, PERICARDIAL WI RNA TESTING FOR HEP C VIREMIA ORDERED/DOCD THORACOSCOPY, SURGICAL; W/EXCISION, PERICARDIAL CY THORACOSCOPY, SURGICAL; W/EXCISION, MEDIASTINAL CY THORACOSCOPY, SURGICAL; W/LOBECTOMY, TOTAL/SEGMENT THORACOSCOPY, SURGICAL; W/THORACIC SYMPATHECTOMY THORACOSCOPY, SURGICAL; W/ESOPHAGOMYOTOMY THORACOSCOPY W/THERA WEDGE RESEXN INITIAL UNILAT THORACOSCOPY W/THERA WEDGE RESEXN ADDL IPSILATRL THORACOSCOPY W/DX WEDGE RESEXN ANATO LUNG RESEXN THORACOSCOPY W/SEGMENTECTOMY HEP C GENO TSTNG DOCD B/4 ANTIVRL TXMNT HEP C 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2008 1/1/2008 1/1/2010 1/1/2010 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2008 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 32670 32671 32672 32673 32674 3267F 3268F 3269F 32701 3270F 3271F 3272F 3273F 3274F 3278F 3279F 32800 3280F 32810 32815 3281F 32820 3284F 32850 32851 32852 32853 32854 32855 32856 3285F 3288F 32900 32905 32906 3290F 3291F 3292F 3293F 32940 3294F 32960 32997 32998 32999 3300F 33010 33011 33015 3301F 33020 33025 33030 33031 33050 33120 33130 3313F 33140 33141 3314F 3315F 3316F 3317F 3318F 3319F 33202 33203 33206 33207 33208 3320F 33210 33211 33212 33213 33214 33215 33216 33217 33218 3321F 33220 33221 33222 33223 33224 33225 33226 33227 33228 THORACOSCOPY W/BILOBECTOMY THORACOSCOPY W/PNEUMONECTOMY THORACOSCOPY W/RESEXN-PLICAJ EMPHYSEMA LUNG UNIL THORACOSCOPY RESEXN THYMUS UNI/BILATERAL THORCOSCPY W/MEDIASTINL &REGIONL LYMPHADENECTOMY PATH RPRT INCLUDES PT + PN CAT GLEASON + MARGIN PSA 1 TM T STG & GLEASON SCORE DOCD BE/R TXMNT BONE SCAN DONE B/4 TXMNT/AFTR DIAG OF PRST CNCR THORAX STEREOTACTIC RADIATION TARGET W/TX COURSE BONE SCAN NOT DONE B/4 TXMNT/AFTR DIAG PRST CNCR LOW RISK OF RECURRENCE PROSTATE CANCER INTERMED RISK OF RECURRENCE PROSTATE CANCER HIGH RISK OF RECURRENCE PROSTATE CANCER PROST CANCER RSK RECUR NOT KNWN/NOT LOW-HIGH SERUM LEVELS: CA P INTACT PTH & LIPID PROF HB LEVEL GREATER >= TO 13 G/DL REPAIR LUNG HERNIA THROUGH CHEST WALL HB LEVEL 11 G/DL TO 12.9 G/DL CLOSURE, CHEST WALL FOLLOWING OPEN FLAP DRAINAGE, OPEN CLOSURE, MAJOR BRONCHIAL FISTULA HB LEVEL< 11 G/DL MAJOR RECONSTRUCTION, CHEST WALL (POSTTRAUMATIC) IOP REDUCED >= 15% PRE-INTERVENTION LEVEL DONOR PNEUMONECTOMY(IES) W/PREPARATION AND MAINTEN LUNG TRANSPLANT, SINGLE; W/O CARDIOPULMONARY BYPAS LUNG TRANSPLANT, SINGLE; W/CARDIOPULMONARY BYPASS LUNG TRANSPLANT, DOUBLE (BILAT SEQUENTIAL/EN BLOC) LUNG TRANSPLANT, DOUBLE (BILAT SEQUENTIAL/EN BLOC) PREP OF CADAVER DONOR LUNG ALLOGRAFT PRIOR TO TRAN PREP OF CADAVER DONOR LUNG ALLOGRAFT PRIOR TO TRAN IOP REDUCED < 15% PRE-INTERVENTION LEVEL FALLS RISK ASSESSMENT DOCUMENTED RESECTION, RIBS, EXTRAPLEURAL, ALL STAGES THORACOPLASTY, SCHEDE TYPE/EXTRAPLEURAL (ALL STAGE THORACOPLASTY, SCHEDE TYPE/EXTRAPLEURAL (ALL STAGE PATIENT IS D (RH) NEGATIVE AND UNSENSITIZED PATIENT IS D (RH) POSITIVE OR SENSITIZED HIV TSTNG ASK/DOCD/RVWD AT 1ST/2ND PRENATAL VST ABO AND RH BLOOD TYPING DOCUMENTED AS PERFORMED PNEUMONOLYSIS, EXTRAPERIOSTEAL, W/FILLING/PACKING GBS SCRNING DOCD DONE DURING WK 35-37 GESTATION PNEUMOTHORAX, THERAPEUTIC, INTRAPLEURAL INJECTION, TOTAL LUNG LAVAGE (UNILAT) Perq rf ablate tx, pul tumor UNLISTED PROC, LUNGS AND PLEURA AJCC STAGE DOCD & RVWD B/4 STARTING THERAPY PERICARDIOCENTESIS; INITIAL PERICARDIOCENTESIS; SUBSEQUENT TUBE PERICARDIOSTOMY CNCR STG DOCD AS METAST & RVWD B/4 THXPY PERICARDIOTOMY, REMOVAL, CLOT/FB (PRIMARY PROC) CREATION, PERICARDIAL WINDOW/PARTIAL RESECTION, DR PERICARDIECTOMY, SUBTOTAL/COMPLETE; W/O CARDIOPULM PERICARDIECTOMY, SUBTOTAL/COMPLETE; W/CARDIOPULMON RESECTION PERICARDIAL CYST/TUMOR EXCISION, INTRACARDIAC TUMOR, RESECTION W/CARDIOPU RESECTION, EXT CARDIAC TUMOR AJCC CANCER STAGE IVB DOCUMENTED TRANSMYOCARDIAL LASER REVASCULARIZATION, BY THORAC TRANSMYOCARDIAL LASER REVASCULARIZATION, BY THORAC AJCC CANCER STAGE IVC DOCUMENTED ER POSITIVE OR PR POSITIVE BREAST CANCER ER OR PR BREAST CANCER PATH RPT MALIG CANCER DOCD PATH RPT MALIG CANCER DOCD X-RAY/CT/ULTRSND ET AL ORDD Insert epicard eltrd, open Insert epicard eltrd, endo INS NEW/RPLCMT PRM PACEMAKR W/TRANS ELTRD ATRIAL INS NEW/RPLC PRM PACEMAKER W/TRANSV ELTRD VENTR INS NEW/RPLCMT PRM PM W/TRANSV ELTRD ATRIAL&VENT NO XRAY/CT/ ET AL ORDD INSERTION/REPLACEMENT, TEMPORARY TRANSVENOUS SINGL INSERTION/REPLACEMENT, TEMPORARY TRANSVENOUS DUAL INS PM PLS GEN W/EXIST SINGLE LEAD INS PACEMAKER PULSE GEN ONLY W/EXIST DUAL LEADS UPGRADE, IMPLANTED PACEMAKER, CONVERSION, SINGLE T REPOSITIONING, PREVIOUSLY IMPLANTED TRANSVENOUS EL INSERTION, TRANSVENOUS ELECTRODE; SINGLE CHAMBER P INSERTION/REPOSITIONING, TRANSVENOUS ELECTRODE; DU RPR 1 ELTRD PRM PM/PACING CVDFB AJCC CANCER STAGE 0 OR 1A MELANOMA DOCD RPR 2 ELTRDS PRM PM/PACING CVDFB INS PACEMAKER PULSE GEN ONLY W/EXIST MULT LEADS RELOCATION OF SKIN POCKET FOR PACEMAKER RELOCATE SKIN POCKET CARDIOVERTER-DEFIBRILLATOR INSJ ELTRD CAR VEN SYS ATTCH PM/CVDFB PLS GEN INSJ ELTRD CAR VEN SYS TM INSJ CVDFB/PM PLS GEN RPSG PREV IMPLTED CAR VEN SYS L VENTR ELTRD REMVL PERM PM PLSE GEN W/REPL PLSE GEN SNGL LEAD REMVL PERM PM PLS GEN W/REPL PLSE GEN 2 LEAD SYS 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 7/1/2011 1/1/2008 1/1/2008 1/1/2013 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2010 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N Y N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 33229 3322F 33230 33231 33233 33234 33235 33236 33237 33238 3323F 33240 33241 33243 33244 33249 3324F 33250 33251 33254 33255 33256 33257 33258 33259 3325F 33261 33262 33263 33264 33265 33266 33270 33271 33272 33273 33282 33284 3328F 33300 33305 3330F 33310 33315 3331F 33320 33321 33322 33330 33335 33361 33362 33363 33364 33365 33366 33367 33368 33369 33400 33401 33403 33404 33405 33406 3340F 33410 33411 33412 33413 33414 33415 33416 33417 33418 33419 3341F 33420 33422 33425 33426 33427 3342F 33430 3343F 3344F 3345F 33460 33463 33464 33465 REMVL PERM PM PLS GEN W/REPL PLSE GEN MULT LEAD MELANOMA GREATER THAN AJCC STAGE 0 OR IA INS PACNG CVDFB PLS GEN ONLY W/EXIST DUAL LEADS INS PACNG CVDFB PLS GEN ONLY W/EXIST MULTI LEADS REMOVAL, PERMANENT PACEMAKER PULSE GENERATOR REMOVAL, TRANSVENOUS PACEMAKER ELECTRODE(S); SINGL REMOVAL, TRANSVENOUS PACEMAKER ELECTRODE(S); DUAL REMOVAL, PERMANENT EPICARDIAL PACEMAKER/ELECTRODES REMOVAL, PERMANENT EPICARDIAL PACEMAKER AND ELECTR REMOVAL, PERMANENT TRANSVENOUS ELECTRODE(S), THORA CLIN TUMOR NODE METASTASES STAGING DOCD B4 SURG INSJ 1/2 CHMBR PACING CARDIOVERTERDEFIB PLS GEN REMVL PAC CVDFB PLS GEN ONLY REMOVAL, SINGLE/DUAL CHAMBER PACING CARDIOVERTER-D REMOVAL, SINGLE/DUAL CHAMBER PACING CARDIOVERTER-D INS/REP PAC PERM CVDFB TRNSVEN LEADS 1/2 CHAMBER MRI CT SCAN ORDERED RVWD OR REQUESTED OPERATIVE ABLATION, SUPRAVENTRICULAR ARRHYTHMOGENI OPERATIVE ABLATION, SUPRAVENTRICULAR ARRHYTHMOGENI Ablate atria, lmtd Ablate atria w/o bypass, ext Ablate atria w/bypass, exten ATRIA ABLATE & RCNSTJ W OTHER PROCEDURE LIMITED ATRIA ABLTJ & RCNSTJ W OTHER PX EXTENSIVE W/O BYP ATRIA ABLATE & RCNSTJ W OTHER PX EXTENSIVE W BYP PREOP ASSES 4 CATARACT SURG OPERATIVE ABLATION, VENTRICULAR ARRHYTHMOGENIC FOC REM PAC CVDFB PLSE GEN &REPL PLSE GEN SNGL LEAD REM PAC CVDFB PLSE GEN &REPL PLSE GEN DUAL LEAD REM PAC CVDFB PLS GEN &REPL PLSE GEN MULTI LEAD Ablate atria w/bypass, endo Ablate atria w/o bypass endo INS/RPLCMNT PERM SUBQ IMPLTBL DFB W/SUBQ ELTRD INSJ OF SUBQ IMPLANTABLE DEFIBRILLATOR ELECTRODE RMVL OF SUBQ IMPLANTABLE DEFIBRILLATOR ELECTRODE REPOS PREVIOUSLY IMPLANTED SUBQ IMPLANTABLE DFB IMPLANTATION OF PATIENT-ACTIVATED CARDIAC EVENT RE REMOVAL OF AN IMPLANTABLE, PATIENT-ACTIVATED CARDI PERFORMANCE STATUS DOCD RVWD IN 2 WKS B4 SURG REPAIR, CARDIAC WOUND; W/O BYPASS REPAIR, CARDIAC WOUND; W/CARDIOPULMONARY BYPASS IMAGING STUDY ORDERED (BKP) CARDIOTOMY, EXPLORATORY W/REMOVAL FB/THROMBUS; W/O CARDIOTOMY, EXPLORATORY W/REMOVAL, FB; W/CARDIOPUL BK IMAGING TST NOT ORDERED SUTURE REPAIR, AORTA/GREAT VESSELS; W/O SHUNT/CARD SUTURE REPAIR, AORTA/GREAT VESSELS; W/SHUNT BYPASS SUTURE REPAIR, AORTA/GREAT VESSELS; W/CARDIOPULMON INSERTION, GRAFT, AORTA/GREAT VESSELS; W/O SHUNT/C INSERTION, GRAFT, AORTA/GREAT VESSELS; W/CARDIOPUL REPLACE AORTIC VALVE PERQ FEMORAL ARTRY APPROACH REPLACE AORTIC VALVE OPENFEMORAL ARTERY APPROACH REPLACE AORTIC VALVE OPEN AXILLRY ARTRY APPROACH REPLACE AORTIC VALVE OPEN ILIAC ARTERY APPROACH REPLACE AORTIC VALVE OPEN TRANSAORTIC APPROACH TRANSCATHETER TRANSAPICAL REPLACEMT AORTIC VALVE REPLACE AORTIC VALVE W/BYP PRQ ART/VENOUS APPRCH REPLACE AORTIC VALVE W/BYP OPEN ART/VENOUS APRCH REPLACE AORTA VALVE W/BYP CNTRL ART/VENOUS APRCH VALVULOPLASTY, AORTIC VALVE; OPEN, W/CARDIOPULMONA VALVULOPLASTY, AORTIC VALVE; OPEN, W/INFLOW OCCLUS VALVULOPLASTY, AORTIC VALVE; USING TRANSVENTRICULA CONSTRUCTION, APICAL-AORTIC CONDUIT REPLACEMENT AORTIC VALVE W/CARDIOPULM BYPASS; PROS REPLACEMENT AORTIC VALVE W/CARDIOPULM BYPASS; W/AL BRST IMAG-RPT/DATACAT 0 DOCD REPLACEMENT AORTIC VALVE W/CARDIOPULM BYPASS; W/ST RPLCMT AORTIC VALVE ANNULUS ENLGMENT NONC SINUS REPLACEMENT, AORTIC VALVE; W/TRANSVENTRICULAR AORT REPLACEMENT, AORTIC VALVE; TRANSLOCATION, AUTOLOGO REPAIR, LEFT VENTRICULAR OUTFLOW TRACT OBSTRUCTION RESECTION/INCISION, SUBVALVULAR TISSUE, DISCRETE S VENTRICULOMYOTOMY/MYECTOMY, IDIOPATHIC HYPERTROPHI AORTOPLASTY (GUSSET), SUPRAVALVULAR STENOSIS TCAT MITRAL VALVE REPAIR INITIAL PROSTHESIS TCAT MITRAL VALVE REPAIR ADDL PROSTHESIS BRST IMAG-RPT/DATACAT 1 DOCD VALVOTOMY, MITRAL VALVE; CLOSED HEART VALVOTOMY, MITRAL VALVE; OPEN HEART, W/CARDIOPULMO VALVULOPLASTY, MITRAL VALVE, W/CARDIOPULMONARY BYP VALVULOPLASTY, MITRAL VALVE, W/CARDIOPULMONARY BYP VALVULOPLASTY, MITRAL VALVE, W/CARDIOPULMONARY BYP BRST IMAG-RPT/DATACAT 2 DOCD REPLACEMENT, MITRAL VALVE, W/CARDIOPULMONARY BYPAS BRST IMAG-RPT/DATACAT 3 DOCD BRST IMAG-RPT/DATACAT 4 DOCD BRST IMAG-RPT/DATACAT 5 DOCD VALVECTOMY, TRICUSPID VALVE, W/CARDIOPULMONARY BYP VALVULOPLASTY, TRICUSPID VALVE; W/O RING INSERTION VALVULOPLASTY, TRICUSPID VALVE; W/RING INSERTION REPLACEMENT, TRICUSPID VALVE, W/CARDIOPULMONARY BY 1/1/2012 10/1/2008 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2007 1/1/2007 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2014 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N Y Y N N N N N N N Y Y Y Y Y N N N N N N N N N N N Y Y Y N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 33468 33470 33471 33474 33475 33476 33477 33478 33496 33500 33501 33502 33503 33504 33505 33506 33507 33508 33510 33511 33512 33513 33514 33516 33517 33518 33519 3351F 33521 33522 33523 3352F 33530 33533 33534 33535 33536 3353F 33542 33545 33548 3354F 33572 33600 33602 33606 33608 33610 33611 33612 33615 33617 33619 33620 33621 33622 33641 33645 33647 33660 33665 33670 33675 33676 33677 33681 33684 33688 33690 33692 33694 33697 33702 3370F 33710 33720 33722 33724 33726 3372F 33730 33732 33735 33736 33737 3374F 33750 33755 33762 33764 33766 TRICUSPID VALVE REPOSITIONING AND PLICATION, EBSTE VALVOTOMY, PULMONARY VALVE, CLOSED HEART; TRANSVEN VALVOTOMY, PULMONARY VALVE, CLOSED HEART; VIA PULM VALVOTOMY, PULMONARY VALVE, OPEN HEART; W/CARDIOPU REPLACEMENT, PULMONARY VALVE RIGHT VENTRICULAR RESECTION, INFUNDIBULAR STENOSIS TRANSCATHETER PULMONARY VALVE IMPLANTATION, PERCUTANEOUS APPROACH, INCLUDIN OUTFLOW TRACT AUGMENTATION (GUSSET), W/WO COMMISSU REPAIR, PROSTHETIC VALVE DYSFUNCTION W/CARDIOPULMO REPAIR, CORONARY AV/ARTERIOCARDIAC CHAMBER FISTULA REPAIR, CORONARY AV/ARTERIOCARDIAC CHAMBER FISTULA REPAIR, ANOMALOUS CORONARY ARTERY; LIGATION REPAIR, ANOMALOUS CORONARY ARTERY; GRAFT, W/O CARD REPAIR, ANOMALOUS CORONARY ARTERY; GRAFT, W/CARDIO REPAIR, ANOMALOUS CORONARY ARTERY; W/CONSTRUCTION, REPAIR, ANOMALOUS CORONARY ARTERY; TRANSLOCATION, REPAIR OF ANOMALOUS CORONARY ARTERY BY UNROOFING O ENDOSCOPY W/VIDEO-ASSISTED VEIN HARVEST, CABG PROC CORONARY ARTERY BYPASS, VEIN ONLY; SINGLE CORONARY CORONARY ARTERY BYPASS, VEIN ONLY; 2 CORONARY VENO CORONARY ARTERY BYPASS, VEIN ONLY; 3 CORONARY VENO CORONARY ARTERY BYPASS, VEIN ONLY; 4 CORONARY VENO CORONARY ARTERY BYPASS, VEIN ONLY; 5 CORONARY VENO CORONARY ARTERY BYPASS, VEIN ONLY; OVER 6 CORONARY CORONARY ARTERY BYPASS, VENOUS/ARTERIAL GRAFTS; 1 CORONARY ARTERY BYPASS, VENOUS/ARTERIAL GRAFTS; 2 CORONARY ARTERY BYPASS, VENOUS/ARTERIAL GRAFTS; 3 NEG DEP SYMP CAT USING STAND DEP ASSESS TOOL CORONARY ARTERY BYPASS, VENOUS/ARTERIAL GRAFTS; 4 CORONARY ARTERY BYPASS, VENOUS/ARTERIAL GRAFTS; 5 CORONARY ARTERY BYPASS, VENOUS/ARTERIAL GRAFTS; OV NO SIGNIF DEP SYMP CAT BY STAND DEP ASSESS TOOL REOPERATION, CORONARY ARTERY BYPASS/VALVE PROC, MO CORONARY ARTERY BYPASS, USING ARTERIAL GRAFT(S); S CORONARY ARTERY BYPASS, USING ARTERIAL GRAFT(S); 2 CORONARY ARTERY BYPASS, USING ARTERIAL GRAFT(S); 3 CORONARY ARTERY BYPASS, USING ARTERIAL GRAFT(S); O MILD TO MOD DEP SYMP BY STAND DEP ASSESS TOOL MYOCARDIAL RESECTION (VENTRICULAR ANEURYSMECTOMY) REPAIR, POSTINFARCTION VENTRICULAR SEPTAL DEFECT, SURG VENTRICULAR RESTORATION PROCEDURE; INCLUDES P CLIN SIGN DEP SYMP BY STAND DEP ASSESS TOOL CORONARY ENDARTERECTOMY, OPEN, LAD/CIRCUMFLX/RCA W CLOSURE, ATRIOVENTRICULAR VALVE (MITRAL/TRICUSPID) CLOSURE, SEMILUNAR VALVE (AORTIC/PULMONARY), SUTUR ANASTOMOSIS, PULMONARY ARTERY TO AORTA REPAIR, COMPLEX CARDIAC ANOMALY, NON-PULM ATRESIA, REPAIR, COMPLEX CARDIAC ANOMALIES, SURG ENLARGEMEN REPAIR, DOUBLE OUTLET RIGHT VENTRICLE W/INTRAVENTR REPAIR, DOUBLE OUTLET RIGHT VENTRICLE W/INTRAVENT REPAIR, COMPLEX CARDIAC ANOMALIES, CLOSURE, ATRIAS REPAIR, COMPLEX CARDIAC ANOMALIES, MODIFIED FONTAN REPAIR, SINGLE VENTRICLE W/AORTIC OUTFLOW OBSTRUCT APPLICATION RIGHT & LEFT PULMONARY ARTERY BANDS TTHRC CATHETER INSERT FOR STENT PLACEMENT RECONSTRUCTION COMPLEX CARDIAC ANOMALY REPAIR, ATRIAL SEPTAL DEFECT, SECUNDUM, W/CARDIOPU DIRECT/PATCH CLOSURE, SINUS VENOSUS, W/WO ANOMALOU REPAIR, ATRIAL SEPTAL DEFECT AND VENTRICULAR SEPTA REPAIR, INCOMPLETE/PARTIAL ATRIOVENTRICULAR CANAL, REPAIR, INTERMEDIATE/TRANSITIONAL ATRIOVENTRICULAR REPAIR, COMPLETE ATRIOVENTRICULAR CANAL, W/WO PROS Close mult vsd Close mult vsd w/resection Cl mult vsd w/rem pul band CLOSURE, VENTRICULAR SEPTAL DEFECT, W/WO PATCH CLOSURE, VENTRICULAR SEPTAL DEFECT, W/WO PATCH; W/ CLOSURE, VENTRICULAR SEPTAL DEFECT, W/WO PATCH; W/ BANDING, PULMONARY ARTERY COMPLETE REPAIR TETRALOGY, FALLOT W/O PULMONARY AT COMPLETE REPAIR TETRALOGY, FALLOT W/O PULMONARY AT REPAIR TETRALOGY, FALLOT W/PULM ATRESIA W/CONSTRUC REPAIR SINUS, VALSALVA FISTULA, W/CARDIOPULMONARY AJCC BREAST CANCER STAGE 0 DOCD REPAIR SINUS, VALSALVA FISTULA, W/CARDIOPULMONARY REPAIR SINUS, VALSALVA ANEURYSM, W/CARDIOPULMONARY CLOSURE, AORTICO-LEFT VENTRICULAR TUNNEL Repair venous anomaly Repair pul venous stenosis AJCC BRST CANCER STAGE 1 T1MIC T1A OR T1B DOCD COMPLETE REPAIR, ANOMALOUS VENOUS RETURN (SUPRACAR REPAIR, COR TRIATRIATUM/SUPRAVALVULAR MITRAL RING, ATRIAL SEPTECTOMY/SEPTOSTOMY; CLOSED HEART ATRIAL SEPTECTOMY/SEPTOSTOMY; OPEN HEART, W/CARDIO ATRIAL SEPTECTOMY/SEPTOSTOMY; OPEN HEART, W/INFLOW AJCC BRST CANCER STAGE 1 TIC (&GT;1-2 CM) DOCD SHUNT; SUBCLAVIAN TO PULMONARY ARTERY SHUNT; ASCENDING AORTA TO PULMONARY ARTERY SHUNT; DESCENDING AORTA TO PULMONARY ARTERY SHUNT; CENTRAL, W/PROSTHETIC GRAFT SHUNT; SUPERIOR VENA CAVA TO PULMONARY ARTERY, FLO 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 7/1/2008 1/1/2004 1/1/2004 1/1/2004 7/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 7/1/2008 1/1/2004 1/1/2004 1/1/2006 7/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 33767 33768 3376F 33770 33771 33774 33775 33776 33777 33778 33779 33780 33781 33782 33783 33786 33788 3378F 33800 33802 33803 3380F 33813 33814 33820 33822 33824 3382F 33840 33845 3384F 33851 33852 33853 33860 33863 33864 3386F 33870 33875 33877 33880 33881 33883 33884 33886 33889 3388F 33891 3390F 33910 33915 33916 33917 33920 33922 33924 33925 33926 33930 33933 33935 33940 33944 33945 33946 33947 33948 33949 3394F 33951 33952 33953 33954 33955 33956 33957 33958 33959 3395F 33962 33963 33964 33965 33966 33967 33968 33969 33970 33971 33973 SHUNT; SUPERIOR VENA CAVA TO PULMONARY ARTERY, FLO ANASTOMOSIS, CAVOPULMONARY, SECOND SUPERIOR VENA C AJCC BREAST CANCER STAGE II DOCD REPAIR, TRANSPOSITION GREAT ARTERIES; W/O SURGICAL REPAIR, TRANSPOSITION GREAT ARTERIES; W/SURGICAL E REPAIR, TRANSPOSITION GREAT ARTERIES, ATRIAL BAFFL REPAIR, TRANSPOSITION GREAT ARTERIES, ATRIAL BAFFL REPAIR, TRANSPOSITION GREAT ARTERIES, ATRIAL BAFFL REPAIR, TRANSPOSITION GREAT ARTERIES, ATRIAL BAFFL REPAIR, TRANSPOSITION GREAT ARTERIES, AORTOPULMONA REPAIR, TRANSPOSITION GREAT ARTERIES, AORTOPULMONA REPAIR, TRANSPOSITION GREAT ARTERIES, AORTOPULMONA REPAIR, TRANSPOSITION GREAT ARTERIES, AORTOPULMONA A-ROOT TLCJ VSD PULM STNS RPR W/O C OST RIMPLTJ A-ROOT TLCJ VSD PULM STNS RPR W/ RIMPLTJ C OSTIA TOTAL REPAIR, TRUNCUS ARTERIOSUS REIMPLANTATION, ANOMALOUS PULMONARY ARTERY AJCC BREAST CANCER STAGE III DOCD AORTIC SUSPENSION, TRACHEAL DECOMPRESSION (SEP PRO DIVISION, ABERRANT VESSEL (VASCULAR RING); DIVISION, ABERRANT VESSEL (VASCULAR RING); W/REANA AJCC BREAST CANCER STAGE IV DOCD OBLITERATION, AORTOPULMONARY SEPTAL DEFECT; W/O CA OBLITERATION, AORTOPULMONARY SEPTAL DEFECT; W/CARD REPAIR, PATENT DUCTUS ARTERIOSUS; LIGATION REPAIR, PATENT DUCTUS ARTERIOSUS; DIVISION, UNDER REPAIR, PATENT DUCTUS ARTERIOSUS; DIVISION, OVER A AJCC COLON CANCER STAGE 0 DOCD EXCISION, COARCTATION, AORTA W/WO PATENT DUCTUS AR EXCISION, COARCTATION, AORTA W/WO PATENT DUCTUS AR AJCC COLON CANCER STAGE I DOCD EXCISION, COARCTATION, AORTA; REPAIR W/LEFT SUBCLA REPAIR, HYPOPLASTIC AORTIC ARCH W/AUTOGENOUS/PROST REPAIR, HYPOPLASTIC AORTIC ARCH W/AUTOGENOUS/PROST ASCENDING AORTA GRF W/CARD BYP & VALVE SSP AS-AORT GRF W/CARD BYP & AORTIC ROOT RPLCMT ASCENDING AORTA GRF VALVE SPARE ROOT REMODEL AJCC COLON CANCER STAGE II DOCD TRANSVERSE ARCH GRAFT, W/CARDIOPULMONARY BYPASS DESCENDING THORACIC AORTA GRAFT, W/WO BYPASS REPAIR, THORACOABDOMINAL AORTIC ANEURYSM W/GRAFT, ENDOVASCULAR REPAIR OF DESCENDING THORACIC AORTA; ENDOVASCULAR REPAIR OF DESCENDING THORACIC AORTA; PLACEMENT OF PROXIMAL EXT PROSTHESIS FOR ENDOVASCU PLACEMENT OF PROXIMAL EXT PROSTHESIS FOR ENDOVASCU PLACEMENT OF DISTAL EXT PROSTHESIS OPEN SUBCLAVIAN TO CAROTID ARTERY TRANSPOSITION; U AJCC COLON CANCER STAGE III DOCD BYPASS GRAFT, TRANSCERVICAL CAROTID-CAROTID; BY NE AJCC COLON CANCER STAGE IV DOCD PULMONARY ARTERY EMBOLECTOMY; W/CARDIOPULMONARY BY PULMONARY ARTERY EMBOLECTOMY; W/O CARDIOPULMONARY PULMONARY ENDARTERECTOMY, W/WO EMBOLECTOMY, W/CARD REPAIR, PULMONARY ARTERY STENOSIS, RECONSTRUCTION REPAIR, PULMONARY ATRESIA, W/CONSTRUCT/REPLACE CON TRANSECTION, PULMONARY ARTERY W/CARDIOPULMONARY BY LIGATION/TAKEDOWN, SYSTEMIC-TO-PULMONARY ARTERY SH REPAIR OF PULMONARY ARTERY ARBORIZATION; WITHOUT C REPAIR OF PULMONARY ARTERY ARBORIZATION; WITH CARD DONOR CARDIECTOMY-PNEUMONECTOMY, W/PREPARATION AND PREP OF CADAVER DONOR HEART/LUNG ALLOGRAFT HEART-LUNG TRANSPLANT W/RECIPIENT CARDIECTOMY-PNEU DONOR CARDIECTOMY, W/PREPARATION AND MAINTENANCE, BACKBENCH STANDARD PREPARATION OF CADAVER DONOR HE HEART TRANSPLANT, W/WO RECIPIENT CARDIECTOMY ECMO/ECLS INITIATION VENO-VENOUS ECMO/ECLS INITIATION VENO-ARTERIAL ECMO/ECLS DAILY MANAGEMENT EACH DAY VENO-VENOUS ECMO/ECLS DAILY MANAGEMENT EA DAY VENO-ARTERIAL QUANT HER2 IHC EVAL OF BRST CANCER - ASCO/CAP ECMO/ECLS INSJ OF PRPH CANNULA BIRTH-5 YRS PERQ ECMO/ECLS INSJ OF PRPH CANNULA 6 YRS&OLDER PERQ ECMO/ECLS INSJ OF PRPH CANNULA BIRTH-5 YRS OPEN ECMO/ECLS INSJ OF PRPH CANNULA 6 YRS&OLDER OPEN ECMO/ECLS INSJ OF CENTRAL CANNULA BIRTH-5 YRS ECMO/ECLS INSJ OF CENTRAL CANNULA 6 YRS & OLDER ECMO/ECLS REPOS PERIPH CANNULA PERQ BIRTH-5 YRS ECMO/ECLS REPOS PERPH CANNULA PRQ 6 YRS & OLDER ECMO/ECLS REPOS PERPH CANNULA OPEN BIRTH-5 YRS QUANT NON-HER2 IHC EVAL OF BRST CANCER PERFORMED ECMO/ECLS REPOS PERPH CANNULA OPEN 6 YRS & OLDER ECMO/ECLS REPOS CENTRAL PERPH CANNULA BIRTH-5YRS ECMO/ECLS ECLS REPOS CENTRAL CNULA 6YRS & OLDER ECMO/ECLS RMVL OF PERPH CANNULA PERQ BIRTH-5 YRS ECMO/ECLS RMVL OF PRPH CANNULA PRQ 6 YRS & OLDER INSERTION, INTRA-AORTIC BALLOON ASSIST DEVICE, PER REMOVAL, INTRA-AORTIC BALLOON ASSIST DEVICE, PERCU ECMO/ECLS RMVL OF PERPH CANNULA OPEN BIRTH-5 YRS INSERTION, INTRA-AORTIC BALLOON ASSIST DEVICE THRO REMOVAL, INTRA-AORTIC BALLOON ASSIST DEVICE W/REPA INSERTION, INTRA-AORTIC BALLOON ASSIST DEVICE THRO 1/1/2004 1/1/2006 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 10/1/2008 1/1/2006 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2015 7/1/2011 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 7/1/2011 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y N N N N Y N N N N N N N N N Y N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 33974 33975 33976 33977 33978 33979 33980 33981 33982 33983 33984 33985 33986 33987 33988 33989 33990 33991 33992 33993 33999 34001 34051 34101 34111 34151 34201 34203 34401 34421 34451 34471 34490 34501 34502 3450F 34510 3451F 34520 3452F 34530 3455F 3470F 3471F 3472F 3475F 3476F 34800 34802 34803 34804 34805 34806 34808 34812 34813 34820 34825 34826 34830 34831 34832 34833 34834 34839 34841 34842 34843 34844 34845 34846 34847 34848 34900 3490F 3491F 3492F 3493F 3494F 3495F 3496F 3497F 3498F 35001 35002 35005 3500F 35011 35013 35021 35022 REMOVAL, INTRA-AORTIC BALLOON ASSIST DEVICE, ASCEN INSERTION, VENTRICULAR ASSIST DEVICE; EXTRACORPORE INSERTION, VENTRICULAR ASSIST DEVICE; EXTRACORPORE REMOVAL, VENTRICULAR ASSIST DEVICE; EXTRACORPOREAL REMOVAL, VENTRICULAR ASSIST DEVICE; EXTRACORPOREAL INSERTION, VENTRICULAR ASSIST DEVICE, IMPLANTABLE REMOVAL, VENTRICULAR ASSIST DEVICE, IMPLANTABLE IN RPLCMT XTRCORP VAD 1/BIVENTR PUMP 1/EA PUMP RPLCMT VAD PMP IMPLTBL ICORP 1 VNTR W/O CARD BYP RPLCMT VAD PMP IMPLTBL ICORP 1 VNTR W/CARD BYP ECMO/ECLS RMVL PRPH CANNULA OPEN 6 YRS & OLDER ECMO/ECLS REMOVAL OF CENTRAL CANNULA BIRTH-5 YRS ECMO/ECLS RMVL OF CENTRAL CANNULA 6 YRS & OLDER ARTERY EXPOS/GRAFT ARTERY PERFUSION ECMO/ECLS INSERT LEFT HEART VENT BY THORACIC INC ECMO/ECLS RMVL LEFT HEART VENT BY THORACIC INCIS ECMO/ECLS INSJ PERQ VAD W/IMAGING ARTERY ACCESS ONLY INSJ PERQ VAD TRNSPTAL W/IMAGE ART&VENOUS ACCESS REMOVAL PERCUTANEOUS VAD DIFFERENT SESSION REPOSITION VAD W/IMAGING DIFFERENT SESSION UNLISTED PROC, CARDIAC SURGERY EMBOLECTOMY/THROMBECTOMY; CAROTID/SUBCLAVIAN/INNOM EMBOLECTOMY/THROMBECTOMY; INNOMINATE/SUBCLAVIAN AR EMBOLECTOMY/THROMBECTOMY; AXILLARY/BRACHIAL/INNOMI EMBOLECTOMY/THROMBECTOMY; RADIAL/ULNAR ARTERY, ARM EMBOLECTOMY/THROMBECTOMY; RENAL/CELIAC/MESENTERY/A EMBOLECTOMY/THROMBECTOMY; FEMOROPOPLITEAL/AORTOILI EMBOLECTOMY/THROMBECTOMY; POPLITEAL-TIBIO-PERONEAL THROMBECTOMY, DIRECT/W/CATHETER; VENA CAVA, ILIAC THROMBECTOMY, DIRECT/W/CATHETER; VENA CAVA, ILIAC, THROMBECTOMY, DIRECT/W/CATHETER; VENA CAVA/ILIAC/F THROMBECTOMY, DIRECT/W/CATHETER; SUBCLAVIAN VEIN, THROMBECTOMY, DIRECT/W/CATHETER; AXILLARY AND SUBC VALVULOPLASTY, FEMORAL VEIN RECONSTRUCTION, VENA CAVA, ANY METHOD DYSPNEA SCRND NO-MILD DYSP VENOUS VALVE TRANSPOSITION, ANY VEIN DONOR DYSPNEA SCRND MOD-HIGH DYSP CROSS-OVER VEIN GRAFT TO VENOUS SYSTEM DYSPNEA NOT SCREENED SAPHENOPOPLITEAL VEIN ANASTOMOSIS TB SCRNG DONE-INTERPD 6MON RA DISEASE ACTIVITY LOW RA DISEASE ACTIVITY MOD RA DISEASE ACTIVITY HIGH DISEASE PROGN RA POOR DOCD DISEASE PROGN RA GOOD DOCD REPAIR, ENDOVASC, INFRARENAL ABDOM AORTIC ANEURYSM REPAIR, ENDOVASC, INFRARENAL ABDOM AORTIC ANEURYSM ENDOVASCULAR REPAIR OF INFRARENAL ABD AORTIC ANEUR REPAIR, ENDOVASC, INFRARENAL ABDOM AORTIC ANEURYSM REPAIR, ENDOVASC, INFRARENAL ABDOM AORTIC ANEURYSM TRANSCATH PLCMT PHYSIOLGIC SENSOR ANEURYSMAL SAC PLACEMENT, ENDOVASC, ILIAC ARTERY OCCLUSION DEVICE OPEN EXPOSURE, FEM ARTERY, ENDOVASC PROSTH DELIVER PLACEMENT, FEM-FEM PROSTH GRAFT DURING ENDOVASCULA OPEN EXPOSURE, ILIAC ART, ENDOVASC PROSTH/ILIAC OC PROX/DISTAL EXTN PROSTH, INFRARENAL ABDOM AORTIC/I PROX/DISTAL EXTN PROSTH, INFRARENAL ABDOM AORTIC/I OPEN REPAIR, INFRARENAL AORTIC ANUERS/DISSEC, AND OPEN REPAIR, INFRARENAL AORTIC ANEUR/DISSEC AND RE OPEN REPAIR, INFRARENAL AORTIC ANEUR/DISSEC AND RE OPEN ILIAC ARTERY EXPOSE W/CONDUIT, INFRARENAL AOR OPEN BRACHIAL ARTERY EXPOSE, DEPLOY INFRARENAL AOR PLNNING PT SPEC FENEST VISCERAL AORTIC GRAFT ENDOVASC VISCER AORTA REPAIR FENEST 1 ENDOGRAFT ENDOVASC VISCER AORTA REPAIR FENEST 2 ENDOGRAFT ENDOVASC VISCER AORTA REPAIR FENEST 3 ENDOGRAFT ENDOVASC VISCER AORTA REPR FENEST 4+ ENDOGRAFT VISCER AND INFRARENAL ABDOM AORTA 1 PROSTHESIS VISCER AND INFRARENAL ABDOM AORTA 2 PROSTHESIS VISCER AND INFRARENAL ABDOM AORTA 3 PROSTHESIS VISCER AND INFRARENAL ABDOM AORTA 4+ PROSTHESIS EVASC RPR ILIAC ART ILIO-ILIAC PROSTHESIS HISTORY - AIDS-DEFINING COND HIV UNSURE BABY OF HIV+MOMS HISTORY OF NADIR CD4+ CELL COUNT <350 CELLS/MM3 NO HIST NADIR CD4+ CELL CNT <350 OR AIDS-INDICA CD4+ CELL COUNT <200 CELLS/MM CD4+ CELL COUNT 200 - 499 CELLS/MM (HIV) CD4+ CELL COUNT /EQUAL 500 CELLS/MM CD4+ CELL PERCENTAGE <15% CD4+ CELL PERCENTAGE >=15% REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT; R REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN CD4+CELL CNT/CD4+CELL % DOCD AS DONE REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT INS REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2009 1/1/2004 1/1/2009 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N Y Y Y Y N N N N N N N N N N N N N N N Y N Y N Y N N Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 3502F 3503F 35045 35081 35082 35091 35092 35102 35103 3510F 35111 35112 3511F 35121 35122 3512F 35131 35132 3513F 35141 35142 3514F 35151 35152 3515F 3517F 35180 35182 35184 35188 35189 35190 35201 35206 35207 3520F 35211 35216 35221 35226 35231 35236 35241 35246 35251 35256 35261 35266 35271 35276 35281 35286 35301 35302 35303 35304 35305 35306 35311 35321 35331 35341 35351 35355 35361 35363 35371 35372 35390 35400 35450 35452 35458 35460 35471 35472 35475 35476 35500 35501 35506 35508 35509 3550F 35510 35511 35512 35515 35516 35518 3551F HIV RNA VIRAL LOAD <LIMITS OF QUANTIF HIV RNA VRL LDNOT<LMTS QUNTF REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN DOC THAT TB SCREENING DONE-RESULTS INTERPRETED REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN CHLAMYDIA/GONORRHEA TSTS DOCD AS DONE REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN SYPHILIS SCREENING DOCUMENTED AS DONE REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN HEPATITIS B SCREENING DOCUMENTED AS PERFORMED REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN HEP C SCREENING DOCD AS PERFORMED REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN REPAIR DIRECT/FALSE ANEURYSM/EXCISION AND GRAFT IN PATIENT HAS DOCUMENTED IMMUNITY TO HEP C HBV STATUS ASSESSED W/ RESULTS IN 1 YR REPAIR, CONGENITAL ARTERIOVENOUS FISTULA; HEAD AND REPAIR, CONGENITAL ARTERIOVENOUS FISTULA; THORAX A REPAIR, CONGENITAL ARTERIOVENOUS FISTULA; EXTREMIT REPAIR, ACQUIRED/TRAUMATIC ARTERIOVENOUS FISTULA; REPAIR, ACQUIRED/TRAUMATIC ARTERIOVENOUS FISTULA; REPAIR, ACQUIRED/TRAUMATIC ARTERIOVENOUS FISTULA; REPAIR BLOOD VESSEL, DIRECT; NECK REPAIR BLOOD VESSEL, DIRECT; UPPER EXTREMITY REPAIR BLOOD VESSEL, DIRECT; HAND, FINGER CLOSTRIDIUM DIFFICILE TESTING PERFORMED REPAIR BLOOD VESSEL, DIRECT; INTRATHORACIC, W/BYPA REPAIR BLOOD VESSEL, DIRECT; INTRATHORACIC, W/O BY REPAIR BLOOD VESSEL, DIRECT; INTRA-ABDOMINAL REPAIR BLOOD VESSEL, DIRECT; LOWER EXTREMITY REPAIR BLOOD VESSEL W/VEIN GRAFT; NECK REPAIR BLOOD VESSEL W/VEIN GRAFT; UPPER EXTREMITY REPAIR BLOOD VESSEL W/VEIN GRAFT; INTRATHORACIC, W REPAIR BLOOD VESSEL W/VEIN GRAFT; INTRATHORACIC, W REPAIR BLOOD VESSEL W/VEIN GRAFT; INTRA-ABDOMINAL REPAIR BLOOD VESSEL W/VEIN GRAFT; LOWER EXTREMITY REPAIR BLOOD VESSEL W/GRAFT OTHER THAN VEIN; NECK REPAIR BLOOD VESSEL W/GRAFT OTHER THAN VEIN; UPPER REPAIR BLOOD VESSEL W/GRAFT OTHER THAN VEIN; INTRA REPAIR BLOOD VESSEL W/GRAFT OTHER THAN VEIN; INTRA REPAIR BLOOD VESSEL W/GRAFT OTHER THAN VEIN; INTRA REPAIR BLOOD VESSEL W/GRAFT OTHER THAN VEIN; LOWER THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; CAROTID, Rechanneling of artery Rechanneling of artery Rechanneling of artery Rechanneling of artery Rechanneling of artery THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; SUBCLAVIA THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; AXILLARYTHROMBOENDARTERECTOMY, W/WO PATCH GRAFT; ABDOMINAL THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; MESENTERI THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; ILIAC THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; ILIOFEMOR THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; COMBINED THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; COMBINED THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; COMMON FE THROMBOENDARTERECTOMY, W/WO PATCH GRAFT; DEEP (PRO REOPERATION, CAROTID, THROMBOENDARTERECTOMY, MORE ANGIOSCOPY, NON-CORONARY, DURING THERAPEUTIC INTER TRANSLUMINAL BALLOON ANGIOPLASTY, OPEN; RENAL/OTHE TRANSLUMINAL BALLOON ANGIOPLASTY, OPEN; AORTIC TRANSLUMINAL BALLOON ANGIOPLASTY, OPEN; BRACHIOCEP TRANSLUMINAL BALLOON ANGIOPLASTY, OPEN; VENOUS TRANSLUMINAL BALLOON ANGIOPLASTY, PERCUTANEOUS; RE TRANSLUMINAL BALLOON ANGIOPLASTY, PERCUTANEOUS, AO TRANSLUMINAL BALLOON ANGIOPLASTY, PERCUTANEOUS, BR TRANSLUMINAL BALLOON ANGIOPLASTY, PERCUTANEOUS, VE HARVEST VEIN, UPPER EXTREMITY, ONE SEGMENT, LOWER BYPASS GRAFT, W/VEIN; CAROTID BYPASS GRAFT, W/VEIN; CAROTID-SUBCLAVIAN BYPASS GRAFT, W/VEIN; CAROTID-VERTEBRAL BYPASS GRAFT, W/VEIN; CAROTID-CAROTID LOW RISK FOR THROMBOEMBOLISM BYPASS GRAFT, W/VEIN; CAROTID-BRACHIAL BYPASS GRAFT, W/VEIN; SUBCLAVIAN-SUBCLAVIAN BYPASS GRAFT, W/VEIN; SUBCLAVIAN-BRACHIAL BYPASS GRAFT, W/VEIN; SUBCLAVIAN-VERTEBRAL BYPASS GRAFT, W/VEIN; SUBCLAVIAN-AXILLARY BYPASS GRAFT, W/VEIN; AXILLARY-AXILLARY INTERMEDIATE RISK FOR THROMBOEMBOLISM 10/1/2008 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 10/1/2008 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 35521 35522 35523 35525 35526 3552F 35531 35533 35535 35536 35537 35538 35539 35540 35556 35558 3555F 35560 35563 35565 35566 35570 35571 35572 35583 35585 35587 35600 35601 35606 35612 35616 35621 35623 35626 35631 35632 35633 35634 35636 35637 35638 35642 35645 35646 35647 35650 35654 35656 35661 35663 35665 35666 35671 35681 35682 35683 35685 35686 35691 35693 35694 35695 35697 35700 35701 3570F 35721 3572F 3573F 35741 35761 35800 35820 35840 35860 35870 35875 35876 35879 35881 35883 35884 35901 35903 35905 35907 36000 36002 36005 36010 BYPASS GRAFT, W/VEIN; AXILLARY-FEMORAL BYPASS GRAFT, W/VEIN; AXILLARY-BRACHIAL BYPASS GRAFT WITH VEIN BRACHIAL-ULNAR/-RADIAL BYPASS GRAFT, W/VEIN; BRACHIAL-BRACHIAL BYPASS W/VEIN AORTOSUBCLAV/CAROTID/INNOMINATE HIGH RISK FOR THROMBOEMBOLISM BYPASS GRAFT, W/VEIN; AORTOCELIAC/AORTOMESENTERIC BYPASS GRAFT, W/VEIN; AXILLARY-FEMORAL-FEMORAL BYPASS GRAFT WITH VEIN HEPATORENAL BYPASS GRAFT, W/VEIN; SPLENORENAL Artery bypass graft Artery bypass graft Artery bypass graft Artery bypass graft BYPASS GRAFT, W/VEIN; FEMORAL-POPLITEAL BYPASS GRAFT, W/VEIN; FEMORAL-FEMORAL PT HAD INR MEASUREMENT PERFORMED BYPASS GRAFT, W/VEIN; AORTORENAL BYPASS GRAFT, W/VEIN; ILIOILIAC BYPASS GRAFT, W/VEIN; ILIOFEMORAL BYPASS GRAFT, W/VEIN; FEMORAL-ANT TIBIAL/POST TIBI BYP TIBL-TIBL/PRONEAL-TIBL/TIBL/PRONEAL TRK-TIBL BYPASS GRAFT, W/VEIN; POPLITEAL-TIBIAL, -PERONEAL HARVEST, FEMOROPOPLITEAL VEIN, ONE SEGMENT, VASCUL IN-SITU VEIN BYPASS; FEMORAL-POPLITEAL IN-SITU VEIN BYPASS; FEMORAL-ANTERIOR TIBIAL, POST IN-SITU VEIN BYPASS; POPLITEAL-TIBIAL, PERONEAL HARVEST, UPPER EXTREMITY ART, 1 SEGMENT, CORONARY BYPASS GRAFT, W/OTHER THAN VEIN; CAROTID BYPASS GRAFT, W/OTHER THAN VEIN; CAROTID-SUBCLAVIA BYPASS GRAFT, W/OTHER THAN VEIN; SUBCLAVIAN-SUBCLA BYPASS GRAFT, W/OTHER THAN VEIN; SUBCLAVIAN-AXILLA BYPASS GRAFT, W/OTHER THAN VEIN; AXILLARY-FEMORAL BYPASS GRAFT, W/OTHER THAN VEIN; AXILLARY-POPLITEA BYPASS NOT VEIN AORTOSUBCLA/CAROTID/INNOMINATE BYPASS GRAFT, W/OTHER THAN VEIN; AORTOCELIAC, AORT BYPASS GRAFT W/OTHER THAN VEIN ILIO-CELIAC BYPASS GRAFT W/OTHER THAN VEIN ILIO-MESENTERIC BYPASS GRAFT W/OTHER THAN VEIN ILIORENAL BYPASS GRAFT, W/OTHER THAN VEIN; SPLENORENAL (SPLE Artery bypass graft Artery bypass graft BYPASS GRAFT, W/OTHER THAN VEIN; CAROTID-VERTEBRAL BYPASS GRAFT, W/OTHER THAN VEIN; SUBCLAVIAN-VERTEB BYPASS GRAFT, W/OTHER THAN VEIN; AORTOBIFEMORAL BYPASS GRAFT, W/OTHER THAN VEIN; AORTOFEMORAL BYPASS GRAFT, W/OTHER THAN VEIN; AXILLARY-AXILLARY BYPASS GRAFT, W/OTHER THAN VEIN; AXILLARY-FEMORALBYPASS GRAFT, W/OTHER THAN VEIN; FEMORAL-POPLITEAL BYPASS GRAFT, W/OTHER THAN VEIN; FEMORAL-FEMORAL BYPASS GRAFT, W/OTHER THAN VEIN; ILIOILIAC BYPASS GRAFT, W/OTHER THAN VEIN; ILIOFEMORAL BYPASS GRAFT, W/OTHER THAN VEIN; FEMORAL-ANT TIBIA BYPASS GRAFT, W/OTHER THAN VEIN; POPLITEAL-TIBIAL/ BYPASS GRAFT; COMPOSITE/PROSTHETIC/VEIN BYPASS GRAFT; AUTOGENOUS COMPOSITE, 2 SEGMENTS, 2 BYPASS GRAFT; AUTOGENOUS COMPOSITE, 3 OR MORE SEGM PLACEMENT, VEIN PATCH/CUFF, DISTAL ANASTOMOSIS BYP CREATION, DISTAL ARTERIOVENOUS FISTULA, LOWER EXTR TRANSPOSITION AND/OR REIMPLANTATION; VERTEBRAL TO TRANSPOSITION AND/OR REIMPLANTATION; VERTEBRAL TO TRANSPOSITION AND/OR REIMPLANTATION; SUBCLAVIAN TO TRANSPOSITION AND/OR REIMPLANTATION; CAROTID TO SU REIMPLANTATION, VISCERAL ARTERY-INFRARENAL AORTIC REOPERATION, FEMORAL-POPLITEAL/FEMORAL/OTHER DISTA EXPLORATION, NOT FOLLOWED, SURGICAL REPAIR, W/WO A RPRT BONE SCINT X-REF W/ X-RAY 4 SAME REGION EXPLORATION, NOT FOLLOWED, SURGICAL REPAIR, W/WO A PT CONSIDERED POSS RISK FX 4 WT-BEARING SITE PT NOT CONSID POSS RISK FX 4 WT-BEARING SITE EXPLORATION, NOT FOLLOWED, SURGICAL REPAIR, W/WO A EXPLORATION, NOT FOLLOWED, SURGICAL REPAIR, W/WO A EXPLORATION, POSTOPERATIVE HEMORRHAGE, THROMBOSIS/ EXPLORATION, POSTOPERATIVE HEMORRHAGE, THROMBOSIS/ EXPLORATION, POSTOPERATIVE HEMORRHAGE, THROMBOSIS/ EXPLORATION, POSTOPERATIVE HEMORRHAGE, THROMBOSIS/ REPAIR, GRAFT-ENTERIC FISTULA THROMBECTOMY, ARTERIAL/VENOUS GRAFT (OTHER THAN HE THROMBECTOMY, ARTERIAL/VENOUS GRAFT (OTHER THAN HE REVISION, LOWER EXTREMITY ARTERY BYPASS W/O THROMB REVISION, LOWER EXTREMITY ARTERY BYPASS W/O THROMB Revise graft w/nonauto graft Revise graft w/vein EXCISION, INFECTED GRAFT; NECK EXCISION, INFECTED GRAFT; EXTREMITY EXCISION, INFECTED GRAFT; THORAX EXCISION, INFECTED GRAFT; ABDOMEN INTRODUCTION, NEEDLE/INTRACATHETER, VEIN INJECTION, (THROMBIN) PERCUTANEOUS TREATMENT EXTRE INJECTION PROC, EXTREMITY, VENOGRAPHY, W/INTRO NEE INTRODUCTION, CATHETER, SUPERIOR/INFERIOR VENA CAV 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 1/1/2004 10/1/2008 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 36011 36012 36013 36014 36015 36100 36120 36140 36147 36148 36160 36200 36215 36216 36217 36218 36221 36222 36223 36224 36225 36226 36227 36228 36245 36246 36247 36248 36251 36252 36253 36254 36260 36261 36262 36299 36400 36405 36406 36410 36415 36416 36420 36425 36430 36440 36450 36455 36460 36468 36470 36471 36475 36476 36478 36479 36481 36500 3650F 36510 36511 36512 36513 36514 36515 36516 36522 36555 36556 36557 36558 36560 36561 36563 36565 36566 36568 36569 36570 36571 36575 36576 36578 36580 36581 36582 36583 36584 36585 36589 36590 SELECTIVE CATHETER PLACEMENT, VENOUS SYSTEM; 1ST O SELECTIVE CATHETER PLACEMENT, VENOUS SYSTEM; OR M INTRODUCTION, CATHETER, RIGHT HEART/MAIN PULMONARY SELECTIVE CATHETER PLACEMENT, LEFT/RIGHT PULMONARY SELECTIVE CATHETER PLACEMENT, SEGMENTAL/SUBSEGMENT INTRODUCTION, NEEDLE/INTRACATHETER; CAROTID/VERTEB INTRODUCTION, NEEDLE/INTRACATHETER; RETROGRADE BRA INTRODUCTION, NEEDLE/INTRACATHETER; EXTREMITY ARTE INTRO NDL/CATH AV SHUNT IST ACCESS W/ RAD EVAL INTRO NDL/CATH AV SHUNT ADDL ACCESS THER IVNTJ INTRODUCTION, NEEDLE/INTRACATHETER; AORTIC, TRANSL INTRODUCTION OF CATHETER, AORTA SELECTIVE CATHETERIZATION, ARTERIAL; 1ST ORDER THO SELECTIVE CATHETERIZATION, ARTERIAL; 2ND ORDER THO SELECTIVE CATHETERIZATION, ARTERIAL; 3RD OR MORE O SELECTIVE CATHETERIZATION, ARTERIAL; ADDL 2ND OR M NONSLCTV CATH THOR AORTA ANGIO INTR/XTRCRANL ART SLCTV CATH CAROTID/INNOM ART ANGIO XTRCRANL ART SLCTV CATH CAROTID/INNOM ART ANGIO INTRCRANL ART SLCTV CATH INTRNL CAROTID ART ANGIO INTRCRNL ART SLCTV CATH SUBCLAVIAN ART ANGIO VERTEBRAL ARTERY SLCTV CATH VERTEBRAL ART ANGIO VERTEBRAL ARTERY SLCTV CATH XTRNL CAROTID ANGIO XTRNL CAROTD CIRC SLCTV CATH INTRCRNL BRNCH ANGIO INTRL CAROT/VERT SELECTIVE CATHETERIZATION, ARTERIAL; 1ST ORDER ABD SELECTIVE CATHETERIZATION, ARTERIAL; 2ND ORDER ABD SELECTIVE CATHETERIZATION, ARTERIAL; 3RD OR MORE A SELECTIVE CATHETERIZATION, ARTERIAL; ADDL 2ND OR M SLCTV CATH 1STORD W/WO ART PUNCT/FLUORO/S&I UNI SLCTV CATH 1STORD W/WO ART PUNCT/FLUOR/S&I BILAT SUPSLCTV CATH 2ND+ORD RENL&ACCES ARTERY/S&I UNI SUPSLCTV CATH 2ND+ORD RENL&ACCES ARTER/S&I BILAT INSERTION, IMPLANTABLE INTRA-ARTERIAL INFUSION PUM REVISION, IMPLANTED INTRA-ARTERIAL INFUSION PUMP REMOVAL, IMPLANTED INTRA-ARTERIAL INFUSION PUMP UNLISTED PROC, VASCULAR INJECTION VNPNXR <3 YEARS PHY/QHP SKILL FEMRAL/JUGLAR VEIN VNPNXR <3 YEARS PHYS/QHP SKILL SCALP VEIN VNPNXR <3 YEARS PHYS/QHP SKILL OTHER VEIN VNPNXR 3 YEARS/> PHYS/QHP SKILL COLLECTION, VENOUS BLOOD, VENIPUNCTURE COLLECTION, CAPILLARY BLOOD SPECIMEN VENIPUNCTURE, CUTDOWN; UNDER AGE 1 VENIPUNCTURE, CUTDOWN; OVER AGE 1 TRANSFUSION, BLOOD/BLOOD COMPONENTS PUSH TRANSFUSION, BLOOD, AGE 2OR UNDER EXCHANGE TRANSFUSION, BLOOD; NEWBORN EXCHANGE TRANSFUSION, BLOOD; OTHER THAN NEWBORN TRANSFUSION, INTRAUTERINE, FETAL SINGLE/MULTIPLE INJECTIONS, SCLEROSING SOLUTIONS, INJECTION, SCLEROSING SOLUTION; SINGLE VEIN INJECTION, SCLEROSING SOLUTION; MULTIPLE VEINS, SA ENDOVENOUS ABLATION THERAPY OF INCOMPETENT VEIN, E ENDOVENOUS ABLATION THERAPY OF INCOMPETENT VEIN ENDOVENOUS ABLATION THERAPY OF INCOMPETENT VEIN, E ENDOVENOUS ABLATION THERAPY OF INCOMPETENT VEIN, L PERCUTANEOUS PORTAL VEIN CATHETERIZATION, ANY METH VENOUS CATHETERIZATION, SELECTIVE ORGAN BLOOD SAMP ELECTROENCEPHALOGRAM ORDERED RVWD OR REQ CATHETERIZATION, UMBILICAL VEIN, DX/THERAPY, NEWBO THERAPEUTIC APHERESIS; WHITE BLOOD CELLS THERAPEUTIC APHERESIS; RED BLOOD CELLS THERAPEUTIC APHERESIS; PLATELETS THERAPEUTIC APHERESIS; PLASMA PHERESIS THERAPEUTIC APHERESIS; W/EXTRACORPOREAL IMMUNOADSO THERAPEUTIC APHERESIS; W/EXTRACORPRL SELECTVE ADSO PHOTOPHERESIS, EXTRACORPOREAL INSERTION, NON-TUNNELED CENTRALLY INSERTED VENOUS INSERTION, NON-TUNNELED CENTRALLY INSERTED VENOUS INSERTION, TUNNELED CENTRALLY INSERTED VENOUS CATH INSERTION, TUNNELED CENTRALLY INSERTED VENOUS CATH INSERTION, TUNNELED CENTRALLY INSERTED VENOUS ACCE INSERTION, TUNNELED CENTRALLY INSERTED VENOUS ACCE INSERTION, TUNNELED CENTRALLY INSERTED VENOUS ACCE INSERTION, TUNNELED VENOUS ACCESS DEVICE, REQ 2 CA INSERTION, TUNNELED VENOUS ACCESS DEVICE, REQ 2 CA INSERTION, PERIPHERAL CENTRAL VENOUS CATHETER, W/O INSERTION, PERIPHERAL CENTRAL VENOUS CATHETER, W/O INSERTION, PERIPHERAL CENTRAL VENOUS ACCESS DEVICE INSERTION, PERIPHERAL CENTRAL VENOUS ACCESS DEVICE REPAIR, TUNNELED/NON-TUNNELED CVA CATHETER, W/O SU REPAIR, CVA DEVICE, W/SUBQ PORT/PUMP REPLACEMENT, CATHETER, CVA DEVICE, W/SUBQ PORT/PUM REPLACEMENT, COMPLETE, NON-TUNNELED CENTRAL VENOUS REPLACEMENT, COMPLETE, TUNNELED CENTRAL VENOUS CAT REPLACEMENT, COMPLETE, TUNNELED CVA DEVICE, W/SUBQ REPLACEMENT, COMPLETE, TUNNELED CVA DEVICE, W/SUBQ REPLACEMENT, COMPLETE, PERIPHERAL CENTRAL VENOUS C REPLACEMENT, COMPLETE, PERIPHERAL CVA DEVICE, W/SU REMOVAL OF TUNNELED CENTRAL VENOUS CATHETER, W/O S REMOVAL OF TUNNELED CVA DEVICE, WITH SUBQ PORT OR 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 36591 36592 36593 36595 36596 36597 36598 36600 36620 36625 36640 36660 36680 36800 36810 36815 36818 36819 36820 36821 36823 36825 36830 36831 36832 36833 36835 36838 36860 36861 36870 3700F 37140 37145 37160 37180 37181 37182 37183 37184 37185 37186 37187 37188 37191 37192 37193 37195 37197 37200 37202 3720F 37211 37212 37213 37214 37215 37216 37217 37218 37220 37221 37222 37223 37224 37225 37226 37227 37228 37229 37230 37231 37232 37233 37234 37235 37236 37237 37238 37239 37241 37242 37243 37244 37250 37251 37252 37253 3725F 37500 37501 COLLECT BLOOD FROM IMPLANT VENOUS ACCESS DEVICE COLLECT BLOOD FROM CATHETER VENOUS NOS DECLOT BY THROMBOLYTIC AGENT IMPLANT DEVICE/CATH MECHANICAL REMOVAL, PERICATHETER OBSTRUCTIVE MATER MECHANICAL REMOVAL, INTRALUMINAL OBSTRUCTIVE MATER REPOSITIONING, PREVIOUSLY PLACED CV CATHETER, W/FL CONTRACT INJ; INCLUDING FLUOROSCOPY; IMAGE DOCUMEN ARTERIAL PUNCTURE, WITHDRAWAL, BLOOD, DX ARETRIAL CATHETERIZATION OR CANNULATION FOR SAMPLI ARTERIAL CATHETERIZATION/CANNULATION, MONITORING/T ARTERIAL CATHETERIZATION, PROLONGED INFUSION THERA CATHETERIZATION, UMBILICAL ARTERY, NEWBORN, DX/THE PLACEMENT, NEEDLE, INTRAOSSEOUS INFUSION INSERTION, CANNULA, HEMODIALYSIS (SEP PROC); VEIN INSERTION, CANNULA, HEMODIALYSIS (SEP PROC); AV, E INSERTION, CANNULA, HEMODIALYSIS (SEP PROC); AV, E ARTERIOVENOUS ANASTOMOSIS, OPEN;BY UPPER ARM CEPHA ARTERIOVENOUS ANASTOMOSIS, OPEN; BY UPPER ARM BASI ARTERIOVENOUS ANASTOMOSIS, OPEN; FOREARM VEIN TRAN ARTERIOVENOUS ANASTOMOSIS, OPEN; DIRECT, ANY SITE INSERT, CANNULA, ISOLATED EXTRACORPOREAL CIRCULATI CREATION, AV FISTULA, NON-DIRECT (SEP PROC); AUTOG CREATION, AV FISTULA, NON-DIRECT (SEP PROC); NON-A THROMBECTOMY, OPEN, AV FISTULA, W/O REVISION, AUTO REVISION, OPEN, AV FISTULA; W/O THROMBECTOMY, AUTO REVISION, OPEN, AV FISTULA; W/THROMBECTOMY, AUTOGE INSERTION, THOMAS SHUNT (SEP PROC) DISTAL REVASCULARIZATION AND INTERVAL LIGATION, UP EXTERNAL CANNULA DECLOTTING (SEP PROC); W/O BALLOO EXTERNAL CANNULA DECLOTTING (SEP PROC); W/BALLOON THROMBEC, PERCUT, AV FIST, AUTOG/NONAUTOG GRAFT (M PSYCHIATRIC DISORDERS/DISTURBANCES ASSESSED VENOUS ANASTOMOSIS, OPEN; PORTOCAVAL VENOUS ANASTOMOSIS, OPEN; RENOPORTAL VENOUS ANASTOMOSIS, OPEN; CAVALMESENTERIC VENOUS ANASTOMOSIS, OPEN; SPLENORENAL, PROXIMAL VENOUS ANASTOMOSIS, OPEN; SPLENORENAL, DISTAL INSERTION, TRANSVENOUS INTRAHEPATIC PORTOSYSTEMIC REVISION, TRANSVENOUS INTRAHEPATIC PORTOSYSTEMIC S PRIMARY PERCUTANEOUS TRANSLUMINAL MECH THROBECTOMY PRIMARY PERCUTANEOUS TRANSLUMINAL MECH THROBECTOMY SECONDARY PERCUTANEOUS TRANSLUMINAL THROMBECTOMY' PERCUTANEOUS TRANSLUMINAL MECH THROMBECTMY; VEINS, PERCUTANEOUS TRANSLUMINAL MECH THROMBECTOMY; VEINS INS INTRVAS VC FILTR W/WO VAS ACS VSL SELXN RS&I REPSNG INTRVAS VC FILTR W/WO ACS VSL SELXN RS&I RTRVL INTRVAS VC FILTR W/WO ACS VSL SELXN RS&I THROMBOLYSIS, CEREBRAL, IV INFUSION PRQ TRANSCATHETER RTRVL INTRVAS FB WITH IMAGING TRANSCATHETER BX TRANSCATHETER THERAPY, INFUSION NON-THROMBOLYSIS COGNITIVE IMPAIRMENT/DYSFUNCTION ASSESSED THROMBOLYSIS ARTERIAL INFUSION W/IMAGING INIT TX THROMBOLYSIS VENOUS INFUSION W/IMAGING INIT TX THROMBOLYSIS ART/VENOUS INFSN W/IMAGE SUBSQ TX CESSATION THROMBOLYTIC THRPY W/CATHETER REMOVAL TRANSCATHETER PLACEMENT OF INTRAVASCULAR STENTS WI TRANSCATHETER PLACEMENT OF INTRAVASCULAR STENTS WI TCATH STENT PLACEMT RETROGRAD CAROTID/INNOMINATE TCATH STENT PLACEMT ANTEGRADE CAROTID/INNOMINATE REVASCULARIZATION ILIAC ARTERY ANGIOP 1ST VSL REVSC OPN/PRQ ILIAC ART W/STNT PLMT & ANGIOP UNI REVASCULARIZATION ILIAC ART ANGIOP EA IPSI VSL REVSC OPN/PRQ ILIAC ART W/STNT & ANGIOP IPSI VSL REVSC OPN/PRG FEM/POP W/ANGIOPLASTY UNI REVSC OPN/PRQ FEM/POP W/ATHRC/ANGIOP SM VSL REVSC OPN/PRQ FEM/POP W/STNT/ANGIOP SM VSL REVSC OPN/PRQ FEM/POP W/STNT/ATHRC/ANGIOP SM VSL REVSC OPN/PRQ TIB/PERO W/ANGIOPLASTY UNI REVSC OPN/PRQ TIB/PERO W/ATHRC/ANGIOP SM VSL REVSC OPN/PRQ TIB/PERO W/STNT/ANGIOP SM VSL REVSC OPN/PRQ TIB/PERO W/STNT/ATHR/ANGIOP SM VSL REVSC OPN/PRQ TIB/PERO W/ANGIOPLASTY UNI EA VSL REVSC OPN/PRQ TIB/PERO W/ATHRC/ANGIOP UNI EA VSL REVSC OPN/PRQ TIB/PERO W/STNT/ANGIOP UNI EA VSL REVSC OPN/PRQ TIB/PERO W/STNT/ATHR/ANGIOP EA VSL OPEN/PERQ PLACEMENT INTRAVASCULAR STENT INITIAL OPEN/PERQ PLACEMENT INTRAVASCULAR STENT EA ADDL OPEN/PERQ PLACEMENT INTRAVASCULAR STENT SAME 1ST OPEN/PERQ PLACEMT INTRAVASC STENT SAME EA ADDL VASCULAR EMBOLIZATION OR OCCLUSION VENOUS RS&I VASCULAR EMBOLIZATION OR OCCLUSION ARTERIAL RS&I VASCULAR EMBOLIZE/OCCLUDE ORGAN TUMOR INFARCT VASCULAR EMBOLIZATION OR OCCLUSION HEMORRHAGE INTRAVASCULAR ULTRASOUND (NON-CORONARY) DURING DX/ INTRAVASCULAR ULTRASOUND (NON-CORONARY) DURING DX/ INTRAVASCULAR ULTRASOUND (NONCORONARY VESSEL) DURING DIAGNOSTIC EVALUATION A INTRAVASCULAR ULTRASOUND (NONCORONARY VESSEL) DURING DIAGNOSTIC EVALUATION A SCREENING FOR DEPRESSION PERFORMED VASCULAR ENDOSCOPY, SURGICAL, W/LIGATION, PERFORAT UNLISTED VASCULAR ENDOSCOPY PROC 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2013 1/1/2004 1/1/2004 10/1/2010 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2014 1/1/2015 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2012 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 3750F 3751F 3752F 3753F 3754F 3755F 37565 3756F 3757F 3758F 3759F 37600 37605 37606 37607 37609 3760F 37615 37616 37617 37618 37619 3761F 3762F 3763F 37650 37660 37700 37718 37722 37735 37760 37761 37765 37766 37780 37785 37788 37790 37799 38100 38101 38102 38115 38120 38129 38200 38204 38205 38206 38207 38208 38209 38210 38211 38212 38213 38214 38215 38220 38221 38230 38232 38240 38241 38242 38243 38300 38305 38308 38380 38381 38382 38500 38505 38510 38520 38525 38530 38542 38550 38555 38562 38564 38570 38571 38572 38589 38700 38720 38724 PT NOT RCVNG DOSE CORTICOSTEROIDS >/= 10MG/DAY ELECTRODIAG STUDIES DSP DOCD RVWD W/IN 6 MONTHS ELECTRODIAG STUDIES DSP NOT DOCD RVWD W/IN 6 MON PT HAS CLINICAL SYMP+SIGNS NEUROPATHY W/ CAUSE SCREENING TSTS DIABETES MELLITUS RVWD RQSTD ORD COGNITIVE+BEHAVIORAL IMPAIRMENT SCRNG PERFORMED LIGATION, INT JUGULAR VEIN PT HAS PSEUDOBULBAR AFFECT/SIALORRHEA/ALS SYMP NO PSEUDOBULBAR AFFECT/SIALORRHEA/ALS SYMP PULM FUNC TESTING/PEAK COUGH EXPIRATORY FLOW PT SCRND DYSPHAGIA+WT LOSS+IMPAIRED NUTRITION LIGATION; EXT CAROTID ARTERY LIGATION; INT/COMMON CAROTID ARTERY LIGATION; INT/COMMON CAROTID ARTERY, W/GRADUAL OCC LIGATION/BANDING, ANGIOACCESS ARTERIOVENOUS FISTUL LIGATION/BX, TEMPORAL ARTERY DYSPHAG+WT LOSS+IMPAIRED NUTRITION LIGATION, MAJOR ARTERY; NECK LIGATION, MAJOR ARTERY; CHEST LIGATION, MAJOR ARTERY; ABDOMEN LIGATION, MAJOR ARTERY; EXTREMITY INS INTRVAS VC FILTR W/WO VAS ACS VSL SELXN RS&I NO DYSPHAG+WT LOSS+IMPAIRED NUTRITION PATIENT IS DYSARTHRIC PATIENT IS NOT DYSARTHRIC LIGATION, FEMORAL VEIN LIGATION, COMMON ILIAC VEIN LIGATION AND DIVISION, LONG SAPHENOUS VEIN, SAPHEN LIGATION, DIVISION, AND STRIPPING, SHORT SAPHENOUS LIGATION, DIVISION, AND STRIPPING, LONG SAPHENOUS LIGATN/DIVISN/STRPG, LNG/SHRT SAPHENOUS VEINS W/RA LIG PRFRATR VEIN SUBFSCAL RAD INCL SKN GRF 1 LEG LIG PRFRATR VEIN SUBFSCAL OPEN INCL US GID 1 LEG STAB PHLEBECTOMY, VARICOSE VEINS, 1 EXTREMITY; LES STAB PHLEBECTOMY, VARICOSE VEINS, 1 EXTREMITY; OVE LIGATION/ DIVISION, SHORT SAPHENOUS VEIN, SAPHENOP LIGATION/DIVISION/EXCISION, VARICOSE VEIN CLUSTER( PENILE REVASCULARIZATION, ARTERY, W/WO VEIN GRAFT PENILE VENOUS OCCLUSIVE PROC UNLISTED PROC, VASCULAR SURGERY SPLENECTOMY; TOTAL (SEP PROC) SPLENECTOMY; PARTIAL (SEP PROC) SPLENECTOMY; TOTAL, EN BLOC, EXTENSIVE DISEASE, W/ REPAIR, RUPTURED SPLEEN (SPLENORRHAPHY) W/WO PARTI LAPAROSCOPY, SURGICAL, SPLENECTOMY UNLISTED LAPAROSCOPY PROC, SPLEEN INJECTION PROC, SPLENOPORTOGRAPHY MANAGEMENT, RECIPIENT HEMATOPOIETIC PROGENITOR CEL BLOOD-DERIVED HEMATOPOIETIC PROGENITOR CELL HARVES BLOOD-DERIVED HEMATOPOIETIC PROGENITOR CELL HARVES TRANSPLANT PREPARATION, HEMATOPOIETIC PROGENITOR C TRNSPL PREP HEMATOP PROGEN THAW PREV HRV PER DNR TRNSP PREP HMATOP PROG THAW PREV HRV WSH PER DNR TRANSPLANT PREP, HEMATOPOIETIC PROGENITOR CELLS; S TRANSPLANT PREPARATION, HEMATOPOIETIC PROGENITOR C TRANSPLANT PREPARATION, HEMATOPOIETIC PROGENITOR C TRANSPLANT PREPARATION, HEMATOPOIETIC PROGENITOR C TRANSPLANT PREPARATION, HEMATOPOIETIC PROGENITOR C TRANSPLANT PREP, HEMATOIEPOTIC PROGENITOR CELLS; C BONE MARROW; ASPIRATION ONLY BONE MARROW BX, NEEDLE/TROCAR BONE MARROW HARVEST TRANSPLANTATION ALLOGENEIC BONE MARROW HARVEST TRANSPLANTATION AUTOLOGOUS TRNSPLJ ALLOGENEIC HEMATOPOIETIC CELLS PER DONOR TRNSPLJ AUTOLOGOUS HEMATOPOIETIC CELLS PER DONOR ALLOGENEIC LYMPHOCYTE INFUSIONS TRNSPLJ HEMATOPOIETIC CELL BOOST DRAINAGE, LYMPH NODE ABSCESS/LYMPHADENITIS; SIMPLE DRAINAGE, LYMPH NODE ABSCESS/LYMPHADENITIS; EXTENS LYMPHANGIOTOMY/OTHER OPERATIONS ON LYMPHATIC CHANN SUTURE AND/OR LIGATION, THORACIC DUCT; CERVICAL AP SUTURE AND/OR LIGATION, THORACIC DUCT; THORACIC AP SUTURE AND/OR LIGATION, THORACIC DUCT; ABDOMINAL A BX/EXCISION, LYMPH NODE(S); SUPERFICIAL BX/EXCISION, LYMPH NODE(S); NEEDLE, SUPERFICIAL BX/EXCISION, LYMPH NODE(S); OPEN, DEEP CERVICAL NO BX/EXCISION, LYMPH NODE(S); OPEN, DEEP CERVICAL NO BX/EXCISION, LYMPH NODE(S); OPEN, DEEP AXILLARY NO BX/EXCISION, LYMPH NODE(S); INT MAMMARY NODE(S) DISSECTION, DEEP JUGULAR NODE(S) EXCISION, CYSTIC HYGROMA, AXILLARY/CERVICAL; W/O D EXCISION, CYSTIC HYGROMA, AXILLARY/CERVICAL; W/DEE LIMITED LYMPHADENECTOMY, STAGING (SEP PROC); PELVI LIMITED LYMPHADENECTOMY, STAGING (SEP PROC); RETRO LAPAROSCOPY, SURGICAL; W/RETROPERITONEAL LYMPH NOD LAPAROSCOPY, SURGICAL; W/BILAT TOTAL PELVIC LYMPHA LAPAROSCOPY, SURGICAL; W/BILAT TOTAL PELVIC LYMPHA LAPAROSCOPY, LYMPHATIC SYSTEM, UNLISTED PROC SUPRAHYOID LYMPHADENECTOMY CERVICAL LYMPHADENECTOMY (COMPLETE) CERVICAL LYMPHADENECTOMY (MODIFIED RADICAL NECK DI 1/1/2012 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N Y N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 38740 38745 38746 38747 38760 38765 38770 38780 38790 38792 38794 38900 38999 39000 39010 39200 39220 39400 39401 39402 39499 39501 39503 39540 39541 39545 39560 39561 39599 4000F 4001F 4004F 4005F 4008F 4010F 4011F 4013F 4019F 4025F 4030F 4033F 4035F 4037F 4040F 4041F 4044F 4045F 4046F 4047F 4048F 40490 4049F 40500 4050F 40510 4051F 40520 40525 40527 4052F 40530 4053F 4054F 4055F 4056F 4058F 4060F 4062F 4063F 4064F 40650 40652 40654 4065F 4066F 4067F 4069F 40700 40701 40702 40720 40761 40799 40800 40801 40804 40805 40806 40808 40810 40812 AXILLARY LYMPHADENECTOMY; SUPERFICIAL AXILLARY LYMPHADENECTOMY; COMPLETE THORCOM THRC W/MEDSTNL & REGIONAL LMPHADEC ABD LYMPHADENEC, REGIONAL, W/CELIAC, GASTRC, PORTL INGUINOFEMORAL LYMPHADENECTOMY, SUPERFICIAL, W/CLO INGUINOFEMORAL LYMPHADENECTOMY, SUPERFICL W/PELVIC PELVIC LYMPHADENECTOMY, W/EXT ILIAC/HYPOGASTRIC/OB RETROPERITONEAL TRANSABDOMINAL LYMPHADENECTOMY, EX INJECTION PROC; LYMPHANGIOGRAPHY INJECTION PROC; IDENTIFICATION, SENTINEL NODE CANNULATION, THORACIC DUCT INTRAOP SENTINEL LYMPH ID W/DYE NJX UNLISTED PROC, HEMIC/LYMPHATIC SYSTEM MEDIASTINOTOMY W/EXPLORATION, DRAINAGE AND REMOVAL MEDIASTINOTOMY W/EXPLORATION, DRAINAGE AND REMOVAL RESECTION OF MEDIASTINAL CYST RESECTION MEDIASTINAL TUMOR MEDIASTINOSCOPY INCL BIOPSIES WHEN PERFORMED MEDIASTINOSCOPY; INCLUDES BIOPSY(IES) OF MEDIASTINAL MASS (EG, LYMPHOMA), WHEN P MEDIASTINOSCOPY; WITH LYMPH NODE BIOPSY(IES) (EG, LUNG CANCER STAGING) UNLISTED PROC, MEDIASTINUM REPAIR, LACERATION, DIAPHRAGM, ANY APPROACH REPAIR, NEONATAL DIAPHRAGMATIC HERNIA, W/WO CHEST REPAIR, DIAPHRAGMATIC HERNIA (OTHER THAN NEONATAL) REPAIR, DIAPHRAGMATIC HERNIA (OTHER THAN NEONATAL) IMBRICATION, DIAPHRAGM, EVENTRATION, TRANSTHORACIC RESECTION, DIAPHRAGM; W/SIMPLE REPAIR RESECTION, DIAPHRAGM; W/COMPLEX REPAIR UNLISTED PROC, DIAPHRAGM TOBACCO USE CESSATION IVNTJ COUNSELING TOBACCO USE CESSATION IVNTJ PHARMACOLOGIC THER PT SCRND TOBACCO USE RCVD TOBACCO CESSATION TALK PHARMACOLOGIC OSTEOPOROSIS THERAPY PRESCRIBED BETA- BLOCKER THERAPY RXD/CURRENTLY BEING TAKEN ACE INHIBITOR/ARB THERAPY RXD/CURRENTLY TAKEN ORAL ANTIPLATELET TX, RX STATIN THERAPY RXD/CURRENTLY TAKEN Doc recpt counsl vit d/calc+ Inhaled bronchodilator rx Oxygen therapy rx Pulmonary rehab rec INFLUENZA IMMUNIZATION RECOMMENDED INFLUENZA IMMUNIZATION ORDERED OR ADMINISTERED PNEUMOCOCCAL VACCINE ADMIN RCVD PRIOR DOC FOR ORDER FOR CEFAZOLIN OR CEFUROXIME DOCUMENTATION THAT AN ORDER WAS GIVEN TO DISCONTIN Empiric antibiotic rx DOCUMENTATION THAT ANTIBIOTICS WERE GIVEN DOCUMENTATION OF ORDER GIVEN FOR ANTIBIOTICS DOC ADMIN PROPHYL PAREN ANTIBIO IN 1HR B4 CUT BX, LIP DOCUMENTATION THAT AN ORDER WAS GIVEN TO DISCONTIN VERMILIONECTOMY (LIP SHAVE), W/MUCOSAL ADVANCEMENT HYPERTENSION PLAN OF CARE DOCUMENTED EXCISION, LIP; TRANSVERSE WEDGE EXCISION W/PRIMARY Referred for an AV fistula EXCISION, LIP; V-EXCISION W/PRIMARY DIRECT LINEAR EXCISION, LIP; FULL THICKNESS, RECONSTRUCTION W/LO EXCISION, LIP; FULL THICKNESS, RECONSTRUCTION W/CR Hemodialysis via AV fistula RESECTION, LIP, MORE THAN ONE-FOURTH, W/O RECONSTR Hemodialysis via AV graft Hemodialysis via catheter Pt. rcvng periton dialysis Approp. oral rehyd. Recommended Ped gastro ed given, caregvr PSYCHOTHERPAY SERVICES PROVIDED (MDD, MD ADOL) PATIENT REFERRAL FOR PSYCHOTHERAPY DOCUMENTED ( MD ANTIDEP RXTHXY CONSIDERED AND NOT RXD ANTIDEPRESSANT PHARMACOTHERAPY PRESCRIBED (MDD, MD REPAIR LIP, FULL THICKNESS; VERMILION ONLY REPAIR LIP, FULL THICKNESS; UP TO HALF VERTICAL HE REPAIR LIP, FULL THICKNESS; OVER ONE-HALF VERTICAL Antipsychotic rx ECT provided Pt referral for ECT documented VENOUS THROMBOEMBOLISM (VTE) PROPHYLAXIS RCVD PLASTIC REPAIR, CLEFT LIP/NASAL DEFORMITY; PRIMARY PLASTIC REPAIR, CLEFT LIP/NASAL DEFORMITY; PRIMARY PLASTIC REPAIR, CLEFT LIP/NASAL DEFORMITY; PRIMARY PLASTIC REPAIR, CLEFT LIP/NASAL DEFORMITY; SECONDA PLASTIC REPAIR, CLEFT LIP/NASAL DEFORMITY; W/CROSS UNLISTED PROC, LIPS DRAINAGE, ABSCESS, CYST, HEMATOMA, VESTIBULE, MOUT DRAINAGE, ABSCESS, CYST, HEMATOMA, VESTIBULE, MOUT REMOVAL, EMBEDDED FB, VESTIBULE, MOUTH; SIMPLE REMOVAL, EMBEDDED FB, VESTIBULE, MOUTH; COMPLICATE INCISION, LABIAL FRENUM (FRENOTOMY) BX, VESTIBULE, MOUTH EXCISION, LESION, MUCOSA AND SUBMUCOSA, VESTIBULE, EXCISION, LESION, MUCOSA AND SUBMUCOSA, VESTIBULE, 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2007 1/1/2012 1/1/2012 1/1/2004 1/1/2012 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2008 1/1/2009 1/1/2007 1/1/2009 1/1/2009 1/1/2010 1/1/2004 1/1/2009 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 10/1/2009 1/1/2010 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 40814 40816 40818 40819 40820 40830 40831 40840 40842 40843 40844 40845 4086F 40899 41000 41005 41006 41007 41008 41009 4100F 41010 41015 41016 41017 41018 41019 41100 41105 41108 41110 41112 41113 41114 41115 41116 41120 41130 41135 41140 41145 41150 41153 41155 4124F 41250 41251 41252 4140F 4142F 4144F 4145F 4148F 4149F 41500 41510 41512 41520 41530 4153F 41599 4163F 4164F 4165F 4167F 4168F 4169F 4171F 4172F 4174F 4175F 4176F 4177F 4178F 4179F 41800 41805 41806 4180F 4181F 41820 41821 41822 41823 41825 41826 41827 41828 4182F 41830 41850 EXCISION, LESION, MUCOSA AND SUBMUCOSA, VESTIBULE, EXCISION, LESION, MUCOSA AND SUBMUCOSA, VESTIBULE, EXCISION, MUCOSA, VESTIBULE, MOUTH AS DONOR GRAFT EXCISION, FRENUM, LABIAL/BUCCAL (FRENUMECTOMY, FRE DESTRUCTION, LESION/SCAR, MOUTH, PHYSICAL METHOD CLOSURE, LACERATION, VESTIBULE, MOUTH; 2.5 CM OR L CLOSURE, LACERATION, VESTIBULE, MOUTH; OVER 2.5 CM VESTIBULOPLASTY; ANTERIOR VESTIBULOPLASTY; POSTERIOR, UNILAT VESTIBULOPLASTY; POSTERIOR, BILAT VESTIBULOPLASTY; ENTIRE ARCH VESTIBULOPLASTY; COMPLEX (W/RIDGE EXTENSION, MUSCL ASPIRIN OR CLOPIDOGREL PRESCRIBED UNLISTED PROC, VESTIBULE, MOUTH INTRAORAL INCISION AND DRAINAGE, ABSCESS/CYST/HEMA INTRAORAL INCISION AND DRAINAGE, ABSCESS/CYST/HEMA INTRAORAL INCISION AND DRAIN, ABSCESS/CYST/HEMATOM INTRAORAL INCISION AND DRAINAGE, ABSCESS/CYST/HEMA INTRAORAL INCISION AND DRAINAGE, ABSCESS/CYST/HEMA INTRAORAL INCISION AND DRAINAGE, ABSCESS/CYST/HEMA Bisphosphonate therapy, intravenous, ordered or received (HEM) INCISION, LINGUAL FRENUM (FRENOTOMY) EXTRAORAL INCISION AND DRAINAGE, ABSCESS/CYST/HEMA EXTRAORAL INCISION AND DRAINAGE, ABSCESS/CYST/HEMA EXTRAORAL INCISION AND DRAINAGE, ABSCESS/CYST/HEMA EXTRAORAL INCISION AND DRAINAGE, ABSCESS/CYST/HEMA PLACEMENT NEEDLE HEAD/NECK RADIOELEMENT APPLICAT BX, TONGUE; ANTERIOR TWO-THIRDS BX, TONGUE; POSTERIOR ONE-THIRD BX, MOUTH, FLOOR EXCISION, LESION, TONGUE W/O CLOSURE EXCISION, LESION, TONGUE W/CLOSURE; ANTERIOR TWO-T EXCISION, LESION, TONGUE W/CLOSURE; POSTERIOR ONEEXCISION, LESION, TONGUE W/CLOSURE; W/LOCAL TONGUE EXCISION, LINGUAL FRENUM (FRENECTOMY) EXCISION, LESION, MOUTH FLOOR GLOSSECTOMY; LESS THAN ONE-HALF TONGUE GLOSSECTOMY; HEMIGLOSSECTOMY GLOSSECTOMY; PARTIAL, W/UNILAT RADICAL NECK DISSEC GLOSSECTOMY; COMPLETE/TOTAL, W/WO TRACHEOSTOMY, W/ GLOSSECTOMY; COMPLETE/TOTAL, W/WO TRACHEOSTOMY, W/ GLOSSECTOMY; COMPOSITE PROC, W/MOUTH FLOOR AND MAN GLOSSECTOMY; COMPOSITE PROC, W/MOUTH FLOOR RESECTI GLOSSECTOMY; COMPOSITE PROC, W/MOUTH FLOOR/MANDIBU ANTIBIOTIC NEITHER PRESCRIBED NOR DISPENSED. (URI,PHAR), (A-BRONCH) REPAIR, LACERATION 2.5 CM OR LESS; MOUTH FLOOR, AN REPAIR, LACERATION 2.5 CMOR LESS; POSTERIOR ONE-TH REPAIR, LACERATION, TONGUE, MOUTH FLOOR, OVER 2.6 INHALED CORTICOSTEROIDS PRESCRIBED CORTICOSTEROID SPARING THERAPY PRESCRIBED ALTERNATIVE LONG-TERM CONTROL MEDICATION RXD >=2 ANTI-HYPERTENSIVE AGENTS RXD OR TAKEN HEP A VAC INJXN ADMIN OR PREV RECVD HEPATITIS B VACCCINE ADMIN OR PREVIOSLY RECVD FIXATION, TONGUE, MECHANICAL, OTHER THAN SUTURE SUTURE, TONGUE TO LIP, MICROGNATHIA TONGUE BASE SUSPENSION PERMANENT SUTURE TQ FRENOPLASTY SUBMUCOSAL ABLTJ TONGUE RF 1/> SITES PR SESSION COMB PEGINTERF/RIBAVIRIN TX PRESCRIBED UNLISTED PROC, TONGUE, MOUTH FLOOR PT COUNS 4 TXMNT OPT PROST ADJV HRMNL THXPY RXD 3D-CRT/IMRT) RECEIVED HD BED TILTED 1ST DAY VENT PT CARE ICUAND VENT W/IN 24HRS NO PT CARE ICU/VENT IN 24HRS PT. RCVNG ESA THXPY PT. NOT RCVNG ESA THXPY COUNS. POTENT. GLAUC IMPCT VIS >= TO 20/40 W/IN 90 DAYS TALK RE UV LIGHT PT/CRGVR TALK PT/CRGVR RE: AREDSPREV ANTID GLBLN RCVD W/IN 26WKS TAMOXIFEN/AI PRESCRIBED DRAINAGE, ABSCESS, CYST, HEMATOMA, DENTOALVEOLAR S REMOVAL, EMBEDDED FB, DENTOALVEOLAR STRUCTURES; SO REMOVAL, EMBEDDED FB, DENTOALVEOLAR STRUCTURES; BO ADJV THXPYRXD/RCVD STG3A-C CONFORMAL RADN THXPY RCVD GINGIVECTOMY, EXCISION GINGIVA, EACH QUADRANT OPERCULECTOMY, EXCISION PERICORONAL TISSUES EXCISION, FIBROUS TUBEROSITIES, DENTOALVEOLAR STRU EXCISION, OSSEOUS TUBEROSITIES, DENTOALVEOLAR STRU EXCISION, LESION/TUMOR (EXCEPT LISTED ABOVE), DENT EXCISION OF LESION/TUMOR DENTOALVEOLAR STRUCTURES EXCISION, LESION/TUMOR (EXCEPT LISTED ABOVE), DENT EXCISION, HYPERPLASTIC ALVEOLAR MUCOSA, EACH QUADR NO CONFORMAL RADN THXPY ALVEOLECTOMY, W/CURETTAGE, OSTEITIS/SEQUESTRECTOMY DESTRUCTION, LESION (EXCEPT EXCISION), DENTOALVEOL 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2012 10/1/2008 10/1/2008 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2009 1/1/2008 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 4185F 4186F 41870 41872 41874 4187F 4188F 41899 4189F 4190F 4191F 4192F 4193F 4194F 4195F 4196F 42000 4200F 4201F 42100 42104 42106 42107 4210F 42120 42140 42145 42160 42180 42182 42200 42205 4220F 42210 42215 4221F 42220 42225 42226 42227 42235 42260 42280 42281 42299 42300 42305 4230F 42310 42320 42330 42335 42340 42400 42405 42408 42409 4240F 42410 42415 42420 42425 42426 4242F 42440 42450 4245F 4248F 42500 42505 42507 42509 4250F 42510 42550 4255F 4256F 42600 4260F 4261F 42650 4265F 42660 42665 4266F 4267F 4268F 42699 4269F 42700 4270F CONTINUOUS PPI OR H2RA RCVD NO CONT. PPI OR H2RA RCVD PERIODONTAL MUCOSAL GRAFTING GINGIVOPLASTY, EACH QUADRANT (SPECIFY) ALVEOLOPLASTY, EACH QUADRANT (SPECIFY) ANTI RHEUM DRUGTHXPYRXD/GVN APPROP ACE/ARB TSTNG DONE UNLISTED PROCEDURE, DENTOALVEOLAR STRUCTURES APPROP DIGOXIN TSTNG DONE APPROP DIURETIC TSTNG DONE APPROP ANTICONVULS TSTNG PT RCVNGGLUCOTHERPY PT RCVNG<10MG DAILY PREDNISO PT RCVNG>10MG DAILY PREDNISO PT RCVNG ANTI-RHEUM THXPY RA PTNOT RCVNG ANTI-RHM THXPYRA DRAINAGE, ABSCESS, PALATE, UVULA EXTERNAL BEAM TO PROST ONLY EXTRNL BEAM OTHER THAN PROST BX, PALATE, UVULA EXCISION, LESION, PALATE, UVULA; W/O CLOSURE EXCISION, LESION, PALATE, UVULA; W/SIMPLE PRIMARY EXCISION, LESION, PALATE, UVULA; W/LOCAL FLAP CLOS ACE/ARB THXPY FOR >= 6 MONS RESECTION, PALATE/EXTENSIVE RESECTION, LESION UVULECTOMY, EXCISION, UVULA PALATOPHARYNGOPLASTY DESTRUCTION, LESION, PALATE/UVULA (THERMAL, CRYO/C REPAIR, LACERATION, PALATE; UP TO 2 CM REPAIR, LACERATION, PALATE; UP TO 2 CM/COMPLEX PALATOPLASTY, CLEFT PALATE, SOFT AND/OR HARD PALAT PALATOPLASTY, CLEFT PALATE, W/CLOSURE, ALVEOLAR RI DIGOXIN THXPY FOR >= 6 MONS PALATOPLASTY, CLEFT PALATE, W/CLOSURE, ALVEOLAR RI PALATOPLASTY, CLEFT PALATE; MAJOR REVISION DIURETIC THXPY FOR >= 6 MONS PALATOPLASTY, CLEFT PALATE; SECONDARY LENGTHENING PALATOPLASTY, CLEFT PALATE; ATTACHMENT PHARYNGEAL LENGTHENING, PALATE, AND PHARYNGEAL FLAP LENGTHENING, PALATE, W/ISLAND FLAP REPAIR, ANTERIOR PALATE, W/VOMER FLAP REPAIR, NASOLABIAL FISTULA MAXILLARY IMPRESSION, PALATAL PROSTHESIS INSERTION, PIN-RETAINED PALATAL PROSTHESIS UNLISTED PROC, PALATE, UVULA DRAINAGE, ABSCESS; PAROTID, SIMPLE DRAINAGE, ABSCESS; PAROTID, COMPLICATED ANTICONV THXPY FOR >= 6 MONS DRAINAGE, ABSCESS; SUBMAXILLARY/SUBLINGUAL, INTRAO DRAINAGE, ABSCESS; SUBMAXILLARY, EXT SIALOLITHOTOMY; SUBMANDIBULAR (SUBMAXILLARY), SUBL SIALOLITHOTOMY; SUBMANDIBULAR (SUBMAXILLARY), COMP SIALOLITHOTOMY; PAROTID, EXTRAORAL/COMPLICATED INT BX, SALIVARY GLAND; NEEDLE BX, SALIVARY GLAND; INCISIONAL EXCISION, SUBLINGUAL SALIVARY CYST (RANULA) MARSUPIALIZATION, SUBLINGUAL SALIVARY CYST (RANULA INSTR THER XRCS-DR FLLWUP PT EPSD BACK PN >12 WK EXCISION, PAROTID TUMOR/PAROTID GLAND; LATERAL LOB EXCISION OF PAROTID TUMOR/GLAND;LATERAL LOBE, W/DI EXCISION, PAROTID TUMOR/PAROTID GLAND; TOTAL, W/NE EXCISION, PAROTID TUMOR/PAROTID GLAND; TOTAL, EN B EXCISION, PAROTID TUMOR/PAROTID GLAND; TOTAL, W/UN SPRVSD XRCZ BK PN >12 WEEKS EXCISION, SUBMANDIBULAR (SUBMAXILLARY) GLAND EXCISION, SUBLINGUAL GLAND PT INSTR RESUME NRML LIFEST PT INSTR-NO BD REST>= 4 DAYS PLASTIC REPAIR, SALIVARY DUCT, SIALODOCHOPLASTY; P PLASTIC REPAIR, SALIVARY DUCT, SIALODOCHOPLASTY; S PAROTID DUCT DIVERSION, BILAT PAROTID DUCT DIVERSION, BILAT; W/EXCISION, BOTH SU WRMNG 4 SURG - NORMOTHERMIA PAROTID DUCT DIVERSION, BILAT; W/LIGATION, BOTH SU INJECTION PROC, SIALOGRAPHY DURATION GEN NEUR ANESTH 60 MINS/> DOC RECORD DURATION GEN NEUR ANESTH <60 MIN DOCD RECORD CLOSURE SALIVARY FISTULA WOUND SRFC CULTURETECH USED TECH OTHER THAN SURFC CULTR DILATION SALIVARY DUCT WET-DRY DRESSINGS RX-RECMD DILATION AND CATHETERIZATION, SALIVARY DUCT, W/WO LIGATION SALIVARY DUCT, INTRAORAL NO WET-DRY DRSSINGS RX-RECMD COMPRSSION THXPY PRESCRIBED PT ED RE COMP THXPY RCVD UNLISTED PROC, SALIVARY GLANDS/DUCTS APPROPOS MTHD OFFLOADING RXD INCISION AND DRAINAGE ABSCESS; PERITONSILLAR PT RCVNG POTENT ANTI R-VIRAL THXPY 6 MON OR MORE 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2011 1/1/2008 1/1/2008 1/1/2008 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2009 1/1/2009 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2009 1/1/2004 10/1/2008 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 4271F 42720 42725 4274F 4276F 4279F 42800 42804 42806 42808 42809 4280F 42810 42815 42820 42821 42825 42826 42830 42831 42835 42836 42842 42844 42845 42860 42870 42890 42892 42894 42900 4290F 4293F 42950 42953 42955 42960 42961 42962 42970 42971 42972 42999 4300F 4301F 43020 43030 43045 4305F 4306F 43100 43101 43107 43108 43112 43113 43116 43117 43118 43121 43122 43123 43124 43130 43135 43180 43191 43192 43193 43194 43195 43196 43197 43198 43200 43201 43202 43204 43205 43206 4320F 43210 43211 43212 43213 43214 43215 43216 43217 43220 43226 PT CVNG POT ANTI R--VIRAL THXPY <6 MON/NOT RCVNG INCISION AND DRAINAGE ABSCESS; RETROPHARYNGEAL/PAR INCISION AND DRAINAGE ABSCESS; RETROPHARYNGEAL/PAR FLU IMMUNO ADMIND/PREVIOUSLY RCVD POTENT ANTIRETROVIRAL THERAPY PRESCRIBED (HIV) PCP PROPHYLAXIS RXD BX; OROPHARYNX BX; NASOPHARYNX, VISIBLE LESION, SIMPLE BX; NASOPHARYNX, SURVEY, UNKNOWN PRIMARY LESION EXCISION/DESTRUCTION, LESION, PHARYNX, ANY METHOD REMOVAL, FB, PHARYNX PCP PROPHYLAX RXD 3MON LOW % EXCISION, BRANCHIAL CLEFT CYST/VESTIGE, CONFINED T EXCISION, BRANCHIAL CLEFT CYST/VESTIGE/FISTULA, EX TONSILLECTOMY AND ADENOIDECTOMY; UNDER AGE 12 TONSILLECTOMY AND ADENOIDECTOMY; OVER AGE 12 TONSILLECTOMY, PRIMARY/SECONDARY; UNDER AGE 12 TONSILLECTOMY, PRIMARY/SECONDARY; OVER AGE 12 ADENOIDECTOMY, PRIMARY; UNDER AGE 12 ADENOIDECTOMY, PRIMARY; OVER AGE 12 ADENOIDECTOMY, SECONDARY; UNDER AGE 12 ADENOIDECTOMY, SECONDARY; OVER AGE 12 RADICAL RESECTION, TONSIL, TONSILLAR PILLARS, AND/ RADICAL RESECTION, TONSIL, TONSILLAR PILLARS, AND/ RADICAL RESECTION, TONSIL, TONSILLAR PILLARS, AND/ EXCISION, TONSIL TAGS EXCISION/DESTRUCTION LINGUAL TONSIL, ANY METHOD (S LIMITED PHARYNGECTOMY RESECTION, LATERAL PHARYNGEAL WALL/PYRIFORM SINUS, RESECTION, PHARYNGEAL WALL REQUIRING CLOSURE W/MYO SUTURE PHARYNX, WOUND/INJURY PATIENT SCREENED FOR INJECTION DRUG USE PT SCRND - HGH-RSK SEXUAL BEHAVIOR PHARYNGOPLASTY (PLASTIC/RECONSTRUCTIVE OPERATION O PHARYNGOESOPHAGEAL REPAIR PHARYNGOSTOMY (FISTULIZATION, PHARYNX, EXT, FEEDIN CONTROL OROPHARYNGEAL HEMORRHAGE; SIMPLE CONTROL OROPHARYNGEAL HEMORRHAGE; COMPLICATED, W/H CONTROL OROPHARYNGEAL HEMORRHAGE; W/SECONDARY SURG CONTROL NASOPHARYNGEAL HEMORRHAGE; SIMPLE, W/POSTE CONTROL NASOPHARYNGEAL HEMORRHAGE; COMPLICATED, W/ CONTROL NASOPHARYNGEAL HEMORRHAGE; W/SECONDARY SUR UNLISTED PROC, PHARYNX/ADENOIDS/TONSILS PT RCVNG WARFARIN THXPY - NONVALV AFIB OR AFLUT PT NOT RCVNG WARFARIN THXPY - NONVALV AFIB/AFLUT ESOPHAGOTOMY, CERVICAL APPROACH, W/REMOVAL, FB CRICOPHARYNGEAL MYOTOMY ESOPHAGOTOMY, THORACIC APPROACH, W/ REMOVAL, FB PT ED RE FT CARE INSPCT RCVD PT TLK PSYCH & RX OPD ADDIC EXCISION, LESION, ESOPHAGUS, W/PRIMARY REPAIR; CER EXCISION, LESION, ESOPHAGUS, W/PRIMARY REPAIR; THO TOTAL/NEAR TOTAL ESOPHAGECTOMY, W/O THORACOTOMY; W TOTAL/NEAR TOTAL ESOPHAGECTOMY, W/O THORACOTOMY; W TOTAL/NEAR TOTAL ESOPHAGECTOMY, W/THORACOTOMY; W/P TOTAL/NEAR TOTAL ESOPHAGECTOMY, W/THORACOTOMY; W/C PARTL ESOPHAGECTOMY, CERVICAL W/FREE INTESTINAL GR PARTL ESOPHAGECTOMY, DISTAL TWO THRDS, W/THORACOTO PARTL ESOPHAGECTOMY, DISTAL TWO THRDS, W/THORACTMY PARTL ESOPHAGECTOMY, DISTAL TWO THRDS, W/THORACOTO PARTL ESOPHAGECTOMY, THORACOABDOMINAL/ABDOMINAL AP PARTL ESOPHAGECTOMY, THORACOABDOMINAL/ABDOMINAL AP TOTAL/PARTL ESOPHAGECTOMY, W/O RECONSTRUCTION, W/C DIVERTICULECTOMY, HYPOPHARYNX/ESOPHAGUS, W/WO MYOT DIVERTICULECTOMY, HYPOPHARYNX/ESOPHAGUS, W/WO MYOT ESOPHAGOSCP RIG TRANSORAL HYPOPHARYNX CRV ESOPH ESOPHAGOSCOPY RIGID TRANSORAL DIAGNOSTIC BRUSH ESOPHAGOSCOPY RIGID TRANSORAL INJ SUBMUCOSAL ESOPHAGOSCOPY RIGID TRANSORAL WITH BIOPSY ESOPHAGOSCOPY RIG TRANSORAL REMOVAL FOREIGN BODY ESOPHAGOSCOPY RIGID TRANSORAL BALLOON DILATION ESOPHAGOSCOPY RIG TRANSORAL GUIDE WIRE DILATION ESOPHAGOSCOPY FLEXIBLE TRANSNASAL DIAGNOSTIC ESOPHAGOSCOPY FLEXIBLE TRANSNASAL WITH BIOPSY ESOPHAGOSCOPY FLEXIBLE TRANSORAL DIAGNOSTIC ESOPHAGOSCOPY FLEXIBLE TRANSORAL W SUBMUCOUS INJ ESOPHAGOSCOPY FLEXIBLE TRANSORAL WITH BIOPSY ESOPHAGOSCOPY FLEX TRANSORAL INJECTION VARICES ESPHGOSCOPY FLEX W/BAND LIGATION ESOPHGL VARICES ESOPHAGOSCOPY TRANSORAL W/OPTICAL ENDOMICROSCOPY PT TALK PSYCHSOC & PHARM TXTMNT OPTS OH DEPEND ESOPHAGOGASTRODUODENOSCOPY, FLEXIBLE, TRANSORAL; WITH ESOPHAGOGASTRIC FUNDO ESOPHAGOSCOPY FLEXIBLE TRANSORAL MUCOSAL RESEXN ESOPHAGOSCOPY TRANSORAL STENT PLACEMENT ESOPHAGOSCOPY RETROGRADE DILATE BALLOON/OTHER ESOPHAGOSCOPY DILATE ESOPHAGUS BALLOON 30 MM ESOPHAGOSCOPY FLEXIBLE REMOVAL FOREIGN BODY ESPHGSC FLEX LESION REMOVAL HOT BX FORCEPS/CAUT ESOPHAGOSCOPY FLEXIB LESION REMOVAL TUMOR SNARE ESOPHAGOSCOPY FLEX BALLOON DILAT <30 MM DIAM ESOPHAGOSCOPY FLEXIBLE GUIDE WIRE DILATION 10/1/2008 1/1/2004 1/1/2004 10/1/2008 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 6/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2008 10/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 10/1/2008 1/1/2016 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 43227 43229 4322F 43231 43232 43233 43235 43236 43237 43238 43239 43240 43241 43242 43243 43244 43245 43246 43247 43248 43249 4324F 43250 43251 43252 43253 43254 43255 43257 43259 4325F 43260 43261 43262 43263 43264 43265 43266 4326F 43270 43273 43274 43275 43276 43277 43278 43279 43280 43281 43282 43283 43289 4328F 43300 43305 4330F 43310 43312 43313 43314 43320 43325 43327 43328 43330 43331 43332 43333 43334 43335 43336 43337 43338 43340 43341 43351 43352 43360 43361 43400 43401 43405 4340F 43410 43415 43420 43425 43450 43453 43460 43496 ESOPHAGOSCOPY FLEXIBLE W BLEEDING CONTROL ESOPHAGOSCOPY FLEX TRANSORAL LESION ABLATION CRGVR PROVIDED W/ ED REFERRED ADDL RESOURCES ESOPHAGOSCOPY FLEXIBLE TRANSORAL ULTRASOUND EXAM ESOPHAGOSCOPY INTRA/TRANSMURAL NEEDLE ASPIRAT/BX EGD ESOPHAGUS BALLOON DILATION 30 MM OR LARGER ESOPHAGOGASTRODUODENOSCOPY TRANSORAL DIAGNOSTIC ESOPHAGOGASTRODUODENOSCOPY SUBMUCOSAL INJECTION ESOPHAGOGASTRODUODENOSCOPY US SCOPE W/ADJ STRXRS EGD INTRMURAL US NEEDLE ASPIRATE/BIOPSY ESOPHAGS EGD TRANSORAL BIOPSY SINGLE/MULTIPLE EGD TRANSORAL TRANSMURAL DRAINAGE PSEUDOCYST EGD INTRALUMINAL TUBE/CATHETER INSERTION EGD INTRMURAL NEEDLE ASPIR/BIOP ALTERED ANATOMY EGD INJECTION SCLEROSIS ESOPHGL/GASTRIC VARICES EGD BAND LIGATION ESOPHGEAL/GASTRIC VARICES EGD DILATION GASTRIC/DUODENAL STRICTURE EGD PERCUTANEOUS PLACEMENT GASTROSTOMY TUBE EGD FLEXIBLE FOREIGN BODY REMOVAL EGD INSERT GUIDE WIRE DILATOR PASSAGE ESOPHAGUS EGD BALLOON DILATION ESOPHAGUS <30 MM DIAM PT QUERIED PARKINSONS MED-RELATED COMPLICATION EGD FLEX REMOVAL LESION(S) BY HOT BIOPSY FORCEPS EGD REMOVAL TUMOR POLYP/OTHER LESION SNARE TECH EGD FLEX TRANSORAL W/OPTICAL ENDOMICROSCOPY EGD US GUIDED TRANSMURAL INJXN/FIDUCIAL MARKER EGD TRANSORAL ENDOSCOPIC MUCOSAL RESECTION EGD TRANSORAL CONTROL BLEEDING ANY METHOD EGD DELIVER THERMAL ENERGY SPHNCTR/CARDIA GERD EGD US EXAM SURGICAL ALTER STOM DUODENUM/JEJUNUM MED SURG TXMNT OPTIONS RVWD W/ PT-CAREGIVER ERCP DX COLLECTION SPECIMEN BRUSHING/WASHING ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY; W/ ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY; W/ ERCP W/PRESSURE MEASUREMENT SPHINCTER OF ODDI ERCP REMOVE CALCULI/DEBRIS BILIARY/PANCREAS DUCT ERCP DESTRUCTION/LITHOTRIPSY CALCULI ANY METHOD EGD ENDOSCOPIC STENT PLACEMENT W/WIRE& DILATION PT (OR CAREGIVER) QUERIED RE AUTO DYSFXN SYMTOMS EGD ABLATE TUMOR POLYP/LESION W/DILATION& WIRE ENDOSCOPIC PAPILLA CANNULATION BILE/PANCREATIC ERCP STENT PLACEMENT BILIARY/PANCREATIC DUCT ERCP REMOVE FOREIGN BODY/STENT BILIARY/PANC DUCT ERCP BILIARY/PANC DUCT STENT EXCHANGE W/DIL&WIRE ERCP BALLOON DILATE BILIARY/PANC DUCT/AMPULLA EA ERCP TUMOR/POLYP/LESION ABLATION W/DILATION&WIRE LAPS ESOPHAGOMYOTOMY W/FUNDOPLASTY IF PERFORMED LAPAROSCOPY, SURGICAL, ESOPHAGOGASTRIC FUNDOPLASTY LAPS RPR PARAESPHGL HRNA INCL FUNDPLSTY W/O MESH LAPS RPR PARAESPHGL HRNA INCL FUNDPLSTY W/MESH LAPS ESOPHAGEAL LENGTHENING ADDL UNLISTED PROC, LAPAROSCOPY, ESOPHAGUS PT (OR CAREGIVER) QUERIED RE SLEEP DISTURBANCES ESOPHAGOPLASTY, CERVICAL APPROACH; W/O REPAIR, TRA ESOPHAGOPLASTY, CERVICAL APPROACH; W/REPAIR, TRACH COUNSELING RE EPIL SPEC SAFETY FOR PT/CRGVR(S) ESOPHAGOPLASTY, THORACIC APPROACH; W/O REPAIR, TRA ESOPHAGOPLASTY, THORACIC APPROACH; W/REPAIR, TRACH ESOPHAGOPLASTY CONGENITAL DEFECT, THORACIC APPROAC ESOPHAGOPLASTY CONGENITAL DEFECT, THORACIC APPROAC ESOPHAGOGASTROSTOMY, W/WO VAGOTOMY AND PYLOROPLAST ESOPG/GSTR FUNDOPLASTY W/FUNDIC PATCH ESOPG/GSTR FUNDOPLASTY W/LAPT ESOPG/GSTR FUNDOPLASTY W/THORCOM ESOPHAGOMYOTOMY; ABDOMINAL APPROACH ESOPHAGOMYOTOMY; THORACIC APPROACH RPR PARAESOPH HIATAL HERNIA W/LAPT W/O MESH LAPT RPR PARAESOPH HIATAL HERNIA W/ MESH RPR PARAESOPH HIATAL HERNIA W/THORCOM W/O MESH RPR PARAESOPH HIATAL HERNIA W/THORCOM W/MESH RPR PARAESOPH HIATAL HERNIA THORCOABDOM W/O MESH RPR PARAESOPH HIATAL HERNIA THORCOABDOM W/MESH ESOPHAGUS LENGTHENING ESOPHAGOJEJUNOSTOMY (WITHOUT TOTAL GASTRECTOMY); A ESOPHAGOJEJUNOSTOMY (WITHOUT TOTAL GASTRECTOMY); T ESOPHAGOSTOMY, FISTULIZATION, ESOPHAGUS, EXT; THOR ESOPHAGOSTOMY, FISTULIZATION, ESOPHAGUS, EXT; CERV GI RECONSTRUCTION, PRIOR ESOPHAGECTOMY; W/STOMACH, GI RECONSTRUCTION, PRIOR ESOPHAGECTOMY; W/COLON IN LIGATION, DIRECT, ESOPHAGEAL VARICES TRANSECTION, ESOPHAGUS W/REPAIR, ESOPHAGEAL VARICE LIGATION/STAPLING AT GASTROESOPHAGEAL JUNCTION, PR COUNSELING WOMEN CHILDBEARING WITH EPILEPSY SUTURE, ESOPHAGEAL WOUND/INJURY; CERVICAL APPROACH SUTURE, ESOPHAGEAL WOUND/INJURY; TRANSTHORACIC/TRA CLOSURE, ESOPHAGOSTOMY/FISTULA; CERVICAL APPROACH CLOSURE, ESOPHAGOSTOMY/FISTULA; TRANSTHORACIC/TRAN DILATION, ESOPHAGUS, UNGUIDED SOUND/BOUGIE, SINGLE DILATION, ESOPHAGUS, OVER GUIDE WIRE ESOPHAGOGASTRIC TAMPONADE, W/BALLOON FREE JEJUNUM TRANSFER W/MICROVASCULAR ANASTOMOSIS 1/1/2004 1/1/2014 1/1/2012 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2010 1/1/2004 1/1/2004 1/1/2013 1/1/2014 1/1/2014 1/1/2004 1/1/2007 1/1/2004 10/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 10/1/2010 1/1/2014 1/1/2009 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2009 1/1/2004 1/1/2010 1/1/2010 1/1/2011 1/1/2004 10/1/2010 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 43499 43500 43501 43502 4350F 43510 43520 43605 43610 43611 43620 43621 43622 43631 43632 43633 43634 43635 43640 43641 43644 43645 43647 43648 43651 43652 43653 43659 43752 43753 43754 43755 43756 43757 43760 43761 43770 43771 43772 43773 43774 43775 43800 43810 43820 43825 43830 43831 43832 43840 43842 43843 43845 43846 43847 43848 43850 43855 43860 43865 43870 43880 43881 43882 43886 43887 43888 43999 44005 4400F 44010 44015 44020 44021 44025 44050 44055 44100 44110 44111 44120 44121 44125 44126 44127 44128 44130 44132 44133 44135 44136 UNLISTED PROC, ESOPHAGUS GASTROTOMY; W/EXPLORATION/FB REMOVAL GASTROTOMY; W/SUTURE REPAIR, BLEEDING ULCER GASTROTOMY; W/SUTURE REPAIR, PRE-EXISTING ESOPHAGO COUNSELING PROVIDED SYMP MNGMNT PALLIATION GASTROTOMY; W/ESOPHAGEAL DILATION/INSERTION PERMAN PYLOROMYOTOMY, CUTTING, PYLORIC MUSCLE BX STOMACH LAPT EXCISION, LOCAL; ULCER/BENIGN TUMOR, STOMACH EXCISION, LOCAL; MALIGNANT TUMOR, STOMACH GASTRECTOMY, TOTAL; W/ESOPHAGOENTEROSTOMY GASTRECTOMY, TOTAL; W/ROUX-EN-Y RECONSTRUCTION GASTRECTOMY, TOTAL; W/FORMATION, INTESTINAL POUCH, GASTRECTOMY, PARTIAL, DISTAL; W/GASTRODUODENOSTOMY GASTRECTOMY, PARTIAL, DISTAL; W/GASTROJEJUNOSTOMY GASTRECTOMY, PARTIAL, DISTAL; W/ROUX-EN-Y RECONSTR GASTRECTOMY, PARTIAL, DISTAL; W/FORMATION, INTESTI VAGOTOMY W/PARTIAL DISTAL GASTRECTOMY VAGOTOMY W/PYLOROPLASTY, W/WO GASTROSTOMY; TRUNCAL VAGOTOMY W/PYLOROPLASTY, W/WO GASTROSTOMY; PARIETA LAPAROSCOPY, SURGICAL GASTRIC RESTRICTIVE PROCEDUR LAPAROSCOPY, SURGICAL GASTRIC RESTRICTIVE PROCEDUR Lap impl electrode, antrum Lap revise/remv eltrd antrum LAPAROSCOPY, SURGICAL; TRANSECTION, VAGUS NERVES, LAPAROSCOPY, SURGICAL; TRANSECTION, VAGUS NERVES, LAPAROSCOPY, SURGICAL; GASTROSTOMY, W/O CONSTRUCTI UNLISTED PROC, LAPAROSCOPY, STOMACH NASO-/ORO-GASTRIC TUBE PLACEMENT, REQUIRING PHYSIC GASTRIC TUBE PLMT W/ASPIR & LAVAGE GASTRIC TUBE DX PLMT W/ASPIR 1 SPECIMEN GASTRIC TUBE DX PLMT W/ASPIR MULT SPECIMENS DUODENAL TUBE DX PLMT W/IMG GID 1 SPECIMEN DUODENAL TUBE DX PLMT W/IMG GID MULT SPECIMEN CHANGE, GASTROSTOMY TUBE REPOS NASO/ORO GASTRIC FEEDING TUBE THRU DUO LAPAROSCOPY, SURG; GASTRIC RESTRICTIVE PROCEDURE; Laparoscopy, surgical, gastric restrictive procedu LAPAROSCOPY, SURG; GASTRIC RESTRICTIVE PROCEDURE; LAPAROSCOPY, SURG; GASTRIC RESTRICTIVE PROCEDURE; LAPAROSCOPY, SURG; GASTRIC RESTRICTIVE PROCEDURE; LAPS GSTRC RSTRICTIV PX LONGITUDINAL GASTRECTOMY PYLOROPLASTY GASTRODUODENOSTOMY GASTROJEJUNOSTOMY; W/O VAGOTOMY GASTROJEJUNOSTOMY; W/VAGOTOMY, ANY TYPE GASTROSTOMY, OPEN; W/O CONSTRUCTION, GASTRIC TUBE GASTROSTOMY, OPEN; NEONATAL, FOR FEEDING GASTROSTOMY, OPEN; W/CONSTRUCTION, GASTRIC TUBE GASTRORRHAPHY, SUTURE, PERFORATED DUODENAL/GASTRIC GASTRIC RESTRICTIVE PROC, W/O GASTRIC BYPASS, MORB GASTRIC RESTRICTVE PROC, W/O GASTRIC BYPASS, MORBI GASTRIC RESTICTIVE PROCEDURE WITH PART GASTRECTOMY GASTRIC RESTRICTVE PROCEDRE, W/GASTRIC BYPASS, MOR GASTRIC RESTRICTIVE PROC, W/GASTRIC BYPASS, MORBID REVISION, GASTRIC RESTRICTIVE PROC, MORBID OBESITY REVISION, GASTRODUODENAL ANASTOMOSIS (GASTRODUODEN REVISION, GASTRODUODENAL ANASTOMOSIS (GASTRODUODEN REVISION, GASTROJEJUNAL ANASTOMOSIS W/RECONSTRUCTI REVISION, GASTROJEJUNAL ANASTOMOSIS W/RECONSTRUCTI CLOSURE, GASTROSTOMY, SURGICAL CLOSURE, GASTROCOLIC FISTULA Impl/redo electrd, antrum Revise/remove electrd antrum GASTRIC RESTRICTIVE PROCEDURE; OPEN; REVISION OF S GASTRIC RESTRICTIVE PROCEDURE; OPEN; REMOVAL OF SU GASTRIC RESTRICT PROCEDURE; OPEN; REM & REPLACEMEN UNLISTED PROC, STOMACH ENTEROLYSIS (FREEING, INTESTINAL ADHESION) (SEP PR REHAB THXPY OPTIONS DISCUSSED W/ PT-CAREGIVER DUODENOTOMY, EXPLORATION, BX(S)/FB REMOVAL TUBE/NEEDLE CATHETER JEJUNOSTOMY, ENTERAL ALIMENTA ENTEROTOMY, SMALL BOWEL, NON-DUODENUM; EXPLORATION ENTEROTOMY, SMALL BOWEL, NON-DUODENUM; DECOMPRESSI COLOTOMY, EXPLORATION, BX(S)/FB REMOVAL REDUCTION, VOLVULUS, INTUSSUSCEPTION, INT HERNIA, CORRECTION, MALROTATION, LYSIS, DUODENAL BANDS AND BX, INTESTINE, CAPSULE/TUBE/PERORAL, MORE THAN 1 S EXCISION, 1 MORE THAN LESION, SMALL/LARGE BOWEL; S EXCISION, MORE THAN 1 LESION, SMALL/LARGE BOWEL; M ENTERECTOMY, RESECTION, SMALL INTESTINE; SINGLE RE ENTERECTOMY, RESECTION, SMALL INTESTINE; ADDL RESE ENTERECTOMY, RESECTION, SMALL INTESTINE; W/ ENTERO ENTERECTOMY, RESECT SMALL INTESTINE CONGENITAL ATR ENTERECTOMY, RESECT SMALL INTESTINE CONGENITAL ATR ENTERECTOMY, RESECT SMALL INTESTINE CONGENITAL ATR ENTEROENTEROSTOMY, ANASTOMOSIS, INTESTINE, W/WO CU DONOR ENTERECTOMY, OPEN, W/PREP AND MAINTENANCE, A DONOR ENTERECTOMY, OPEN WITH PREP AND MAINTENANCE, INTESTINAL ALLOTRANSPLANTATION; FROM CADAVER DONOR INTESTINAL ALLOTRANSPLANTATION; FROM LIVING DONOR 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2010 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 10/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N Y Y N N N N N Y N N N N N N N N Y Y Y Y Y Y N N N N N N N N Y Y Y Y Y Y N N N N N N N N Y Y Y N N N N N N N N N N N N N N N N N N N N Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 44137 44139 44140 44141 44143 44144 44145 44146 44147 44150 44151 44155 44156 44157 44158 44160 44180 44186 44187 44188 44202 44203 44204 44205 44206 44207 44208 44210 44211 44212 44213 44227 44238 44300 44310 44312 44314 44316 44320 44322 44340 44345 44346 44360 44361 44363 44364 44365 44366 44369 44370 44372 44373 44376 44377 44378 44379 44380 44381 44382 44384 44385 44386 44388 44389 44390 44391 44392 44394 44401 44402 44403 44404 44405 44406 44407 44408 44500 4450F 44602 44603 44604 44605 44615 44620 44625 44626 44640 44650 44660 44661 REMOVAL OF TRANSPLANTED INTESTINAL ALLOGRAFT, COMP MOBILIZATION, SPLENIC FLEXURE, W/PARTIAL COLECTOMY COLECTOMY, PARTIAL; W/ANASTOMOSIS COLECTOMY, PARTIAL; W/SKIN LEVEL CECOSTOMY/COLOSTO COLECTOMY, PARTIAL; W/END COLOSTOMY AND CLOSURE, D COLECTOMY, PARTIAL; W/RESECTION, W/COLOSTOMY/ILEOS COLECTOMY, PARTIAL; W/COLOPROCTOSTOMY (LOW PELVIC COLECTOMY, PARTIAL; W/COLOPROCTOSTOMY (LOW PELVIC COLECTOMY, PARTIAL; ABDOMINAL AND TRANSANAL APPROA COLECTOMY, TOTAL, ABDOMINAL, W/O PROCTECTOMY; W/IL COLECTOMY, TOTAL, ABDOMINAL, W/O PROCTECTOMY; W/CO COLECTOMY, TOTAL, ABDOMINAL, W/PROCTECTOMY; W/ILEO COLECTOMY, TOTAL, ABDOMINAL, W/PROCTECTOMY; W/CONT Colectomy w/ileoanal anast Colectomy w/neo-rectum pouch COLECTOMY, PARTIAL, W/REMOVAL, TERMINAL ILEUM W/IL LAPAROSCOPY; ENTEROLYSIS LAPAROSCOPY, SURGICAL; JEJUNOSTOMY LAPAROSCOPY; SURGICAL, COLOSTOMY OR SKILL LEVEL CE Laparoscopy, surgical, colostomy or skin level cec LAPAROSCOPY, SURGICAL; ENTERECTOMY, INTESTINAL RES LAPAROSCOPY, SURGICAL; EACH ADDL SMALL INTESTINE R LAPAROSCOPY, SURGICAL; COLECTOMY, PARTIAL, W/ANAST LAPAROSCOPY, SURGICAL; COLECTOMY, PARTIAL, W/REMOV LAP, SURG; COLECTOMY, PARTIAL, W/END COLOSTOMY AND LAP, SURG; COLECTOMY, PARTIAL, W/ANASTOMOSIS, W/CO LAP, SURG; COLECTOMY, PARTIAL, W/ANASTOMOSIS, W/CO LAP, SURG; COLECTOMY, TOTAL, ABDOM, W/O PROCTECTOM LAP, SURG; COLECTOMY, ABD W/PROCTECTOMY W/ILEOANAL LAP, SURG; COLECTOMY, TOT, ABDOM, W/PROCTECTOMY, W LAPAROSCOPY; SURGICAL, MOBILIZATION OF SPLENIC FLE LAPAROSCOPY; SURGICAL, CLOSURE OF ENTEROSTOMY; LAR UNLISTED LAPAROSCOPY PROC, INTESTINE (EXCEPT RECTU ENTEROSTOMY/CECOSTOMY, TUBE (SEP PROC) ILEOSTOMY/JEJUNOSTOMY, NON-TUBE (SEP PROC) REVISION, ILEOSTOMY; SIMPLE (RELEASE, SUPERFICIAL REVISION, ILEOSTOMY; COMPLICATED (RECONSTRUCTION I CONTINENT ILEOSTOMY (KOCK PROC) (SEP PROC) COLOSTOMY/SKIN LEVEL CECOSTOMY; (SEP PROC) COLOSTOMY/SKIN LEVEL CECOSTOMY; W/MULTIPLE BIOPSIE REVISION, COLOSTOMY; SIMPLE (RELEASE, SUPERFICIAL REVISION, COLOSTOMY; COMPLICATED (RECONSTRUCTION I REVISION, COLOSTOMY; W/REPAIR, PARACOLOSTOMY HERNI ENDOSCOPY UPPER SMALL INTESTINE SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT ENTEROSCOPY > 2ND PRTN W/RMVL FOREIGN BODY SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT SM INTESTINL ENDO/ENTEROSCOPY, BEYOND 2ND PORTN DU SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT SMALL INTESTINAL ENDO/ENTEROSCOPY, BEYOND 2ND PORT SM INTESTIN ENDOSCOPY, ENTEROSCOPY BEYOND 2ND PORT ILEOSCOPY THRU STOMA DX W/COLLJ SPEC WHEN PRFMD ILEOSCOPY STOMA W/BALLOON DILATION ILEOSCOPY, THROUGH STOMA; W/BX, SINGLE/MULTIPLE ILEOSCOPY STOMA W/PLMT OF ENDOSCOPIC STENT NDSC EVAL INTSTINAL POUCH DX W/COLLJ SPEC SPX NDSC EVAL INTSTINAL POUCH W/BX SINGLE/MULTIPLE COLONOSCOPY STOMA DX INCLUDING COLLJ SPEC SPX COLONOSCOPY THROUGH STOMA; W/BX, SINGLE/MULTIPLE COLONOSCOPY STOMA W/RMVL FOREIGN BODY COLONOSCOPY STOMA CONTROL BLEEDING COLONOSCOPY STOMA RMVL LES BY HOT BIOPSY FORCEPS COLONOSCOPY THROUGH STOMA; W/REMOVAL, LESION, SNAR COLONOSCOPY STOMA ABLATION LESION COLONOSCOPY STOMA W/ENDOSCOPIC STENT PLCMT COLONOSCOPY STOMA W/ENDOSCOPIC MUCOSAL RESCJ COLONOSCOPY STOMA W/SUBMUCOSAL INJECTION COLONOSCOPY STOMA W/BALLOON DILATION COLONOSCOPY STOMA W/ENDOSCOPIC ULTRASOUND EXAM COLONOSCOPY STOMA W/US GID NDL ASPIR/BX COLONOSCOPY THROUGH STOMA WITH DECOMPRESSION INTRODUCTION, LONG GI TUBE (SEP PROC) SELF-CARE EDUCATION PROVIDED TO PATIENT SUTURE, SMALL INTESTINE; SINGLE PERFORATION SUTURE, SMALL INTESTINE; MULTIPLE PERFORATIONS SUTURE, LARGE INTESTINE; W/O COLOSTOMY SUTURE, LARGE INTESTINE; W/COLOSTOMY INTESTINAL STRICTUROPLASTY W/WO DILATION CLOSURE, ENTEROSTOMY, LARGE/SMALL INTESTINE CLOSURE, ENTEROSTOMY, LARGE/SMALL INTESTINE; W/RES CLOSURE, ENTEROSTOMY, LARGE/SMALL INTESTINE; W/RES CLOSURE, INTESTINAL CUTANEOUS FISTULA CLOSURE, ENTEROENTERIC/ENTEROCOLIC FISTULA CLOSURE, ENTEROVESICAL FISTULA; W/O INTESTINAL/BLA CLOSURE, ENTEROVESICAL FISTULA; W/INTESTINE AND/OR 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 44680 44700 44701 44705 4470F 44715 44720 44721 44799 44800 4480F 4481F 44820 44850 44899 44900 44950 44955 44960 44970 44979 45000 45005 4500F 45020 45100 45108 4510F 45110 45111 45112 45113 45114 45116 45119 45120 45121 45123 45126 45130 45135 45136 45150 45160 45171 45172 45190 4525F 4526F 45300 45303 45305 45307 45308 45309 45315 45317 45320 45321 45327 45330 45331 45332 45333 45334 45335 45337 45338 45340 45341 45342 45346 45347 45349 45350 45378 45379 45380 45381 45382 45384 45385 45386 45388 45389 45390 45391 45392 45393 45395 45397 INTESTINAL PLICATION (SEP PROC) EXCLUSION, SMALL BOWEL, PELVIS, MESH/PROSTHESIS/NA IINTRAOPERATIVE COLONIC LAVAGE (ADDL PROC) PREPARE FECAL MICROBIOTA FOR INSTILLATION IMPLANT CARDIOVERT-DEFIB (ICD) COUNSELING PROV PREP DONOR , INTESTINE ALLOGRAFT, MESENTERIC ARTER PREP DONOR , INTESTINE ALLOGRAFT, VENOUS ANASTOMOS PREP DONOR , INTESTINE ALLOGRAFT, VENOUS ANASTOMOS UNLISTED PROC, INTESTINE EXCISION, MECKELS DIVERTICULUM (DIVERTICULECTOMY)/ PT RCVNG ACE/ARB BETA-BLOCKER TX 3 MONS/LONGER PT RCVNG ACE/ARB AND BETA BLOCKER > 3 MONTHS EXCISION, LESION, MESENTERY (SEP PROC) SUTURE, MESENTERY (SEP PROC) UNLISTED PROC, MECKELS DIVERTICULUM AND MESENTERY INCISION AND DRAINAGE APPENDICEAL ABSCESS OPEN APPENDECTOMY; APPENDECTOMY; INDICATED PURPOSE, W/OTHER PROC (NOT APPENDECTOMY; RUPTURED APPENDIX W/ABSCESS/GENERALI LAPAROSCOPY, SURGICAL, APPENDECTOMY UNLISTED PROC, LAPAROSCOPY, APPENDIX TRANSRECTAL DRAINAGE, PELVIC ABSCESS INCISION AND DRAINAGE, SUBMUCOSAL ABSCESS, RECTUM REFERRED TO OUTPT CARD REHABILITATION PROGRAM INCISION AND DRAINAGE, DEEP SUPRALEVATOR, PELVIREC BX, ANORECTAL WALL, ANAL APPROACH ANORECTAL MYOMECTOMY PREVIOUS CARDIAC REHAB FOR QUAL CARD EVENT DONE PROCTECTOMY; COMPLETE, COMBINED ABDOMINOPERINEAL, PROCTECTOMY; PARTIAL RESECTION, RECTUM, TRANSABDOM PROCTECTOMY, COMBINED ABDOMINOPERINEAL, PULL-THROU PROCTECTOMY, PARTIAL, W/RECTAL MUCOSECTOMY, ILEOAN PROCTECTOMY, PARTIAL, W/ANASTOMOSIS; ABDOMINAL AND PROCTECTOMY, PARTIAL, W/ANASTOMOSIS; TRANSSACRAL A PROCTECTOMY, COMBINED ABDOMINOPERINEAL PULL-THROUG PROCTECTOMY, COMPLETE, (CONG MEGACOLON) ABD/PERINE PROCTECT, COMPLETE, (CONG MEGACOLON) ABD/PERINEAL PROCTECTOMY, PARTIAL, W/O ANASTOMOSIS, PERINEAL AP PELVIC EXENTERATION, W/PROCTECTOMY/PELVIC ORGAN RE EXCISION, RECTAL PROCIDENTIA, W/ANASTOMOSIS; PERIN EXCISION, RECTAL PROCIDENTIA, W/ANASTOMOSIS; ABDOM EXCISION, ILEOANAL RESERVOIR W/ILEOSTOMY DIVISION, STRICTURE, RECTUM EXCISION, RECTAL TUMOR, PROCTOTOMY, TRANSSACRAL/TR EXC RCT TUM NOT INCL MUSCULARIS PROPRIA EXC RCT TUM INCL MUSCULARIS PROPRIA DESTRUCTION, RECTAL TUMOR, TRANSANAL APPROACH NEUROPSYCHIATRIC INTERVENTION ORDERED NEUROPSYCHIATRIC INTERVENTION RECEIVED PROCTOSIGMOIDOSCOPY, RIGID; DX, W/WO SPECIMEN(S), PROCTOSIGMOIDOSCOPY, RIGID; W/DILATION PROCTOSIGMOIDOSCOPY, RIGID; W/BX, SINGLE/MULTIPLE PROCTOSIGMOIDOSCOPY, RIGID; W/REMOVAL, FB PROCTOSIGMOIDOSCOPY, RIGID; W/REMOVAL, SINGLE LESI PROCTOSIGMOIDOSCOPY, RIGID; W/REMOVAL, SINGLE LESI PROCTOSIGMOIDOSCOPY, RIGID; W/REMOVAL, MULTIPLE LE PROCTOSIGMOIDOSCOPY, RIGID; W/CONTROL, BLEEDING PROCTOSIGMOIDOSCOPY, RIGID; W/ABLATION, LESION, NO PROCTOSIGMOIDOSCOPY, RIGID; W/DECOMPRESSION, VOLVU PROCTOSIGMOIDOSCOPY, RIGID; W/TRANSENDOSCOPIC STEN SIGMOIDOSCOPY FLX DX W/COLLJ SPEC BR/WA IF PFRMD SIGMOIDOSCOPY, FLEXIBLE; W/BX, SINGLE/MULTIPLE SIGMOIDOSCOPY FLX W/RMVL FOREIGN BODY SIGMOIDOSCOPY FLX W/RMVL TUMOR BY HOT BX FORCEPS SIGMOIDOSCOPY FLX CONTROL BLEEDING SIGMOIDOSCOPY, FLEXIBLE; W/DIRECTED SUBMUCOSAL INJ SGMDSC FLX W/DCMPRN W/PLMT DCMPRN TUBE SIGMOIDOSCOPY, FLEXIBLE; W/REMOVAL, LESION, SNARE SIGMOIDOSCOPY FLX TNDSC BALO DILAT SIGMOIDOSCOPY, FLEXIBLE; W/ENDOSCOPIC ULTRASOUND E SIGMOIDOSCOPY, FLEXIBLE; W/TRANSENDOSCOPIC ULTRASO SIGMOIDOSCOPY FLX ABLATION TUMOR POLYP/OTH LES SIGMOIDOSCOPY FLX PLACEMENT OF ENDOSCOPIC STENT SGMDSC FLX WITH ENDOSCOPIC MUCOSAL RESECTION SIGMOIDOSCOPY FLX WITH WITH BAND LIGATION(S) COLONOSCOPY FLX DX W/COLLJ SPEC WHEN PFRMD COLONOSCOPY FLX W/REMOVAL OF FOREIGN BODY(S) COLONOSCOPY W/BIOPSY SINGLE/MULTIPLE COLSC FLX WITH DIRECTED SUBMUCOSAL NJX ANY SBST COLSC FLEXIBLE W/CONTROL BLEEDING ANY METHOD COLSC FLX W/REMOVAL LESION BY HOT BX FORCEPS COLSC FLX W/RMVL OF TUMOR POLYP LESION SNARE TQ COLSC FLEXIBLE W/TRANSENDOSCOPIC BALLOON DILAT COLONOSCOPY FLX ABLATION TUMOR POLYP/OTHER LES COLONOSCOPY FLX WITH ENDOSCOPIC STENT PLACEMENT COLONOSCOPY FLX W/ENDOSCOPIC MUCOSAL RESECTION COLSC FLX W/NDSC US XM RCTM ET AL LMTD&ADJ STRUX COLSC FLX W/US GUID NDL ASPIR/BX W/US RCTM ET AL COLONOSCOPY FLEXIBLE WITH DECOMPRESSION LAPAROSCOPY; SURGICAL, PROCTECTOMY, COMPLETE; COMB LAPAROSCOPY; SURGICAL, PROCTECTOMY, COMPLETE; WITH 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2015 1/1/2006 1/1/2006 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 45398 45399 45400 45402 4540F 4541F 45499 45500 45505 4550F 4551F 45520 4552F 4553F 45540 45541 4554F 45550 4555F 45560 45562 45563 4556F 4557F 4558F 4559F 4560F 4561F 4562F 4563F 45800 45805 45820 45825 45900 45905 45910 45915 45990 45999 46020 46030 46040 46045 46050 46060 46070 46080 46083 46200 46220 46221 46230 46250 46255 46257 46258 46260 46261 46262 46270 46275 46280 46285 46288 46320 46500 46505 46600 46601 46604 46606 46607 46608 46610 46611 46612 46614 46615 46700 46705 46706 46707 46710 46712 46715 46716 46730 46735 46740 46742 COLONOSCOPY FLEXIBLE WITH BAND LIGATION(S) UNLISTED PROCEDURE COLON LAPAROSCOPY, SURGICAL, PROCTOPEXY LAPAROSCOPY, SURGICAL, PROCTOPEXY; WITH SIGMOID RE DISEASE MODIFYING PHARMACOTHERAPY DISCUSSED TX PSEUDOBULBAR AFFECT SIALORRHEA/ALS SYMP UNLISTED LAPAROSCOPY PROCEDURE, RECTUM PROCTOPLASTY; STENOSIS PROCTOPLASTY; PROLAPSE, MUCOUS MEMBRANE OPTIONS NONINVASIVE RESP SUPPORT DISCUSSED W/PT NUTRITIONAL SUPPORT OFFERED PERIRECTAL INJECTION, SCLEROSING SOLUTION, PROLAPS PT OFFERED REFERRAL SPEECH LANGUAGE PATHOLOGIST PT OFFERED ASSISTANCE PLANNING END LIFE ISSUES PROCTOPEXY, PROLAPSE; ABDOMINAL APPROACH PROCTOPEXY, PROLAPSE; PERINEAL APPROACH PT RECEIVED INHALATIONAL ANESTHETIC AGENT PROCTOPEXY COMBINED W/SIGMOID RESECTION, ABDOMINAL PT DID NOT RECEIVE INHALATIONAL ANESTHETIC AGENT REPAIR, RECTOCELE (SEP PROC) EXPLORATION, REPAIR, AND PRESACRAL DRAINAGE, RECTA EXPLORATION, REPAIR, AND PRESACRAL DRAINAGE, RECTA PT SHOWS 3+RISK FACTORS POST-OP NAUSEA+VOMMITING PT DOES NOTSHOW3+RISK FACTORS POST-OPNAUSEA/VOMM PT RCEVD 2 PROPHYLACTIC RX AGENTS PRE+INTRA-OP 1BODY TEMP MEAS>=35.5CW/IN30-15 MINSAFTER ANESTH ANESTH DID NOT INVOLVE GENERAL/NEURAXIAL ANESTH PATIENT HAS A CORONARY ARTERY STENT PATIENT DOES NOT HAVE A CORONARY ARTERY STENT PT RECVD ASPIRIN W/IN 24 HRS PRIOR ANESTH START CLOSURE, RECTOVESICAL FISTULA CLOSURE, RECTOVESICAL FISTULA; W/COLOSTOMY CLOSURE, RECTOURETHRAL FISTULA CLOSURE, RECTOURETHRAL FISTULA; W/COLOSTOMY REDUCTION, PROCIDENTIA (SEP PROC) UNDER ANESTHESIA DILATION, ANAL SPHINCTER (SEP PROC) UNDER ANESTHES DILATION, RECTAL STRICTURE (SEP PROC) UNDER ANESTH REMOVAL, FECAL IMPACTION/FB (SEP PROC) UNDER ANEST ANORECTAL EXAM, SURGICAL; DIAGNOSTIC UNLISTED PROC, RECTUM PLACEMENT, SETON REMOVAL, ANAL SETON, OTHER MARKER INCISION AND DRAINAGE, ISCHIORECTAL AND/OR PERIREC INCISION AND DRAINAGE, INTRAMURAL/INTRAMUSCULAR/SU INCISION AND DRAINAGE, PERIANAL ABSCESS, SUPERFICI INCISION AND DRAINAGE, ISCHIORECTAL/INTRAMURAL ABS INCISION, ANAL SEPTUM (INFANT) SPHINCTEROTOMY, ANAL, DIVISION, SPHINCTER (SEP PRO INCISION, THROMBOSED HEMORRHOID, EXT FISSURECTOMY, W/WO SPHINCTEROTOMY EXCISION SINGLE EXTERNAL PAPILLA OR TAG ANUS HEMORRHOIDECTOMY INTERNAL RUBBER BAND LIGATIONS EXCISION, EXT HEMORRHOID TAGS AND/OR MULTIPLE PAPI HEMORRHOIDECTOMY XTRNL 2+ COLUMN/GROUP HEMORRHOIDECTOMY NTRNL & XTRNL 1 COLUMN/GROUP HEMORRHOIDECTOMY, INT AND EXT, SIMPLE; W/FISSURECT HRHC 1 COL/GRP W/FSTULECTMY INCL FSSRECTOMY HEMORRHOIDECTOMY INT & XTRNL 2+ COLUMN/GROUP HRHC CPLX/X10SV W/FISSURECTOMY HRHC 2+ COL/GRP W/FSTULECTMY INCL FSSRECTMY SURGICAL TREATMENT, ANAL FISTULA (FISTULECTOMY/FIS SURGICAL TREATMENT, ANAL FISTULA (FISTULECTOMY/FIS SURGICAL TREATMENT, ANAL FISTULA (FISTULECTOMY/FIS SURGICAL TREATMENT, ANAL FISTULA (FISTULECTOMY/FIS CLOSURE, ANAL FISTULA W/RECTAL ADVANCEMENT FLAP ENUCLEATION/EXCISION, EXT THROMBOTIC HEMORRHOID INJECTION, SCLEROSING SOLUTION, HEMORRHOIDS CHEMODENERVATION OF INTERNAL ANAL SPHINCTER ANOSCOPY DX W/COLLJ SPEC BR/WA SPX WHEN PRFRMD ANOSCOPY DX W/HRA &CHEM AGNTS ENHANCEMENT ANOSCOPY; W/DILATION ANOSCOPY; W/BX, SINGLE/MULTIPLE ANOSCOPY DX W/HRA &CHEM AGNTS ENHANCEMENT W/BX ANOSCOPY; W/REMOVAL, FB ANOSCOPY; W/REMOVAL, SINGLE LESION, HOT FORCEPS/CA ANOSCOPY; W/REMOVAL, SINGLE TUMOR, POLYP/OTHER LES ANOSCOPY; W/REMOVAL, MULTIPLE LESIONS, HOT FORCEPS ANOSCOPY; W/CONTROL, BLEEDING ANOSCOPY; W/ABLATION, LESION, NOT REMOVED BY HOT F ANOPLASTY, PLASTIC OPERATION, STRICTURE; ADULT ANOPLASTY, PLASTIC OPERATION, STRICTURE; INFANT REPAIR OF ANAL FISTULA W/FIBRIN GLUE RPR ANORECTAL FISTULA W/ PLUG REPAIR OF ILEOANAL POUCH FISTULA/SINUS; TRANSPERIN REPAIR OF ILEOANAL POUCH FISTULA/SINUS; COMBINED T REPAIR, LOW IMPERFORATE ANUS; W/ANOPERINEAL FISTUL REPAIR, LOW IMPERFORATE ANUS; W/TRANSPOSITION, ANO REPAIR, HIGH IMPERF ANUS W/O FISTULA; PERINEAL/SAC REPAIR, HIGH IMPERF ANUS W/O FISTULA; TRANSABDOMIN REPAIR, HIGH IMPERF ANUS W/RECTO-URETHRAL/VAGINAL REPAIR, HIGH IMPERF ANUS W/RECTO-URETHRAL/VAGINAL 1/1/2015 1/1/2015 1/1/2006 1/1/2006 1/1/2013 1/1/2013 1/1/2006 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2004 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 46744 46746 46748 46750 46751 46753 46754 46760 46761 46762 46900 46910 46916 46917 46922 46924 46930 46940 46942 46945 46946 46947 46999 47000 47001 47010 47015 47100 47120 47122 47125 47130 47133 47135 47136 47140 47141 47142 47143 47144 47145 47146 47147 47300 47350 47360 47361 47362 47370 47371 47379 47380 47381 47382 47383 47399 47400 47420 47425 47460 47480 47490 47500 47505 47510 47511 47525 47530 47531 47532 47533 47534 47535 47536 47537 47538 47539 47540 47541 47542 47543 47544 47550 47552 47553 47554 47555 47556 47560 47561 47562 REPAIR, CLOACAL ANOMALY, ANORECTOVAGINOPLASTY AND REPAIR, CLOACAL ANOMALY, ANORECTOVAGINOPLASTY, URE REPAR, CLOACAL ANOMLY, ANORECTOVAGINO/URETHROPLSTY SPHINCTEROPLASTY, ANAL, INCONTINENCE/PROLAPSE; ADU SPHINCTEROPLASTY, ANAL, INCONTINENCE/PROLAPSE; CHI GRAFT, RECTAL INCONTINENCE AND/OR PROLAPSE REMOVAL, THIERSCH WIRE/SUTURE, ANAL CANAL SPHINCTEROPLASTY, ANAL, INCONTINENCE, ADULT; MUSCL SPHINCTEROPLASTY, ANAL, INCONTINENCE, ADULT; LEVAT SPHINCTEROPLASTY, ANAL, INCONTINENCE, ADULT; IMPLA DESTRUCTION, ANAL LESION(S), SIMPLE; CHEMICAL DESTRUCTION, ANAL LESION(S), SIMPLE; ELECTRODESICC DESTRUCTION, ANAL LESION(S), SIMPLE; CRYOSURGERY DESTRUCTION, ANAL LESION(S), SIMPLE; LASER SURGERY DESTRUCTION, ANAL LESION(S), SIMPLE; SURGICAL EXCI DESTRUCTION, ANAL LESION(S), EXTENSIVE DESTRUCTION INTERNAL HEMORRHOID THERMAL ENERGY CURETTAGE/CAUTERY, ANAL FISSURE W/DILATION SPHINCT CURETTAGE/CAUTERY, ANAL FISSURE W/DILATION SPHINCT HRHC NTRNL LIG OTH THAN RBBR BAND 1 COL/GRP HRHC NTRNL LIG OTH THAN RBBR BAND 2+ COL/GRP HEMORRHOIDEPEXY BY STAPLING UNLISTED PROC, ANUS BX, LIVER, NEEDLE; PERCUTANEOUS BX, LIVER, NEEDLE; INDICATED PURPOSE, W/OTHER MAJO HEPATOTOMY OPEN DRAINAGE ABSCESS/CYST 1/2 STAGES LAPAROTOMY, W/ASPIRATION AND/OR INJECTION, HEPATIC BX, LIVER, WEDGE HEPATECTOMY, RESECTION, LIVER; PARTIAL LOBECTOMY HEPATECTOMY, RESECTION, LIVER; TRISEGMENTECTOMY HEPATECTOMY, RESECTION, LIVER; TOTAL LEFT LOBECTOM HEPATECTOMY, RESECTION, LIVER; TOTAL RIGHT LOBECTO DONOR HEPATECTOMY, W/PREP ADN MAINTENANCE, ALLOGRA LIVER ALLOTRANSPLANTATION; ORTHOTOPIC, PARTIAL/WHO LIVER ALLOTRANSPLANTATION; HETEROTOPIC, PARTIAL/WH DONOR HEPATECTOMY, W/PREP AND MAINTENANCE, ALLOGRA DONOR HEPATECTOMY, W/PREP AND MAINTENANCE, ALLOGRA DONOR HEPATECTOMY, W/PREP AND MAINTENANCE, ALLOGRA PREP DONOR, WHOLE LIVER GRAFT, WITHOUT TRISEGEMENT PREP DONOR, WHOLE LIVER GRAFT, WITH TRIGEMENT SPLI PREP DONOR, WHOLE LIVER GRAFT, WITH LOVE SPLIT INT PREP DONOR, LIVER GRAFT, VENOUS ANASTOMOSIS PREP DONOR, LIVER GRAFT, ARTERIAL ANASTOMOSIS, EAC MARSUPIALIZATION, CYST/ABSCESS, LIVER MANAGEMENT, LIVER HEMORRHAGE; SIMPLE SUTURE, LIVER MANAGEMENT, LIVER HEMORRHAGE; COMPLEX SUTURE, LIVE MANAGEMENT, LIVER HEMORRHAGE; EXPLORATION, HEPATIC MANAGEMENT, LIVER HEMORRHAGE; RE-EXPLORATION, HEPA LAPAROSCOPY, SURGICAL, ABLATION MORE THAN 1 LIVER LAPAROSCOPY, SURGICAL, ABLATION MORE THAN 1 LIVER UNLISTED LAPAROSCOPIC PROCEDURE, LIVER ABLATION, OPEN, OVER 1 LIVER TUMOR(S); RADIOFREQUE ABLATION, OPEN, OVER 1 LIVER TUMOR(S); CRYOSURGICA ABLATION, OPEN, OVER 1 LIVER TUMOR(S), PERCUTANEOU ABLATION 1/> LIVER TUMOR PERQ CRYOABLATION UNLISTED PROC, LIVER HEPATICOTOMY/HEPATICOSTOMY W/EXPLORATION/DRAINAGE/ CHOLEDOCHOTOMY/OSTOMY W/EXPLORE/DRAIN/REMOVAL CALC CHOLEDOCHOTOMY/OSTOMY W/EXPLORE/DRAIN/REMOVAL CALC TRANSDUODENAL SPHINCTEROTOMY/SPHINCTEROPLASTY, W/W CHOLECSTOT/CHOLECSTOST W/EXPL DRG/RMVL ST1 SPX CHOLECSTOST PRQ W/IMG GID INJECTION PROC, PERCUTANEOUS TRANSHEPATIC CHOLANGI INJECTION PROC, CHOLANGIOGRAPHY THROUGH EXISTING C INTRODUCTION, PERCUTANEOUS TRANSHEPATIC CATHETER, INTRODUCTION, PERCUTANEOUS TRANSHEPATIC STENT, INT CHANGE, PERCUTANEOUS BILIARY DRAINAGE CATHETER REVISION AND/OR REINSERTION, TRANSHEPATIC TUBE INJECTION PROCEDURE FOR CHOLANGIOGRAPHY, PERCUTANEOUS, COMPLETE DIAGNOSTIC P INJECTION PROCEDURE FOR CHOLANGIOGRAPHY, PERCUTANEOUS, COMPLETE DIAGNOSTIC P PLACEMENT OF BILIARY DRAINAGE CATHETER, PERCUTANEOUS, INCLUDING DIAGNOSTIC CHO PLACEMENT OF BILIARY DRAINAGE CATHETER, PERCUTANEOUS, INCLUDING DIAGNOSTIC CHO CONVERSION OF EXTERNAL BILIARY DRAINAGE CATHETER TO INTERNAL-EXTERNAL BILIARY DR EXCHANGE OF BILIARY DRAINAGE CATHETER (EG, EXTERNAL, INTERNAL-EXTERNAL, OR CONVE REMOVAL OF BILIARY DRAINAGE CATHETER, PERCUTANEOUS, REQUIRING FLUOROSCOPIC GU PLACEMENT OF STENT(S) INTO A BILE DUCT, PERCUTANEOUS, INCLUDING DIAGNOSTIC CHOLA PLACEMENT OF STENT(S) INTO A BILE DUCT, PERCUTANEOUS, INCLDNG DIAGNSTC CHOLANGIO PLACEMENT OF STENT(S) INTO A BILE DUCT, PERCUTANEOUS, INCLDNG DIAG CHOLANGIOGRA PLACEMENT OF ACCESS THROUGH THE BILIARY TREE AND INTO SMALL BOWEL TO ASSIST WIT BALLOON DILATION OF BILIARY DUCT(S) OR OF AMPULLA (SPHINCTEROPLASTY), PERCUTANEO ENDOLUMINAL BIOPSY(IES) OF BILIARY TREE, PERCUTANEOUS, ANY METHOD(S) (EG, BRUSH, F REMOVAL OF CALCULI/DEBRIS FROM BILIARY DUCT(S) AND/OR GALLBLADDER, PERCUTANEOU BILIARY ENDOSCOPY, INTRAOPERATIVE(CHOLEDOCHOSCOPY) BILIARY ENDO PRQ T-TUBE DX W/COLLECT SPEC BRUSH BILIARY ENDOSCOPY, PERCUTANEOUS VIA T-TUBE/OTHER T BILIARY ENDOSCOPY, PERCUTANEOUS VIA T-TUBE/OTHER T BILIARY ENDOSCOPY, PERCUTANEOUS; W/DILATION BILIAR BILIARY ENDOSCOPY, PERCUTANEOUS; W/DILATION BILIAR LAPAROSCOPY, SURGICAL; W/GUIDED TRANSHEPATIC CHOLA LAPAROSCOPY, SURGICAL; W/GUIDED TRANSHEPATIC CHOLA LAPAROSCOPY, SURGICAL; CHOLECYSTECTOMY 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 47563 47564 47570 47579 47600 47605 47610 47612 47620 47630 47700 47701 47711 47712 47715 47720 47721 47740 47741 47760 47765 47780 47785 47800 47801 47802 47900 47999 48000 48001 48020 48100 48102 48105 48120 48140 48145 48146 48148 48150 48152 48153 48154 48155 48160 48400 48500 48510 48520 48540 48545 48547 48548 48550 48551 48552 48554 48556 48999 49000 49002 49010 49020 49040 49060 49062 49082 49083 49084 49180 49185 49203 49204 49205 49215 49220 49250 49255 49320 49321 49322 49323 49324 49325 49326 49327 49329 49400 49402 49405 49406 LAPAROSCOPY, SURGICAL; CHOLECYSTECTOMY W/CHOLANGIO LAPAROSCOPY,SURGICAL; CHOLECYSTECTOMY W/EXPLORATIO LAPAROSCOPY, SURGICAL; CHOLECYSTOENTEROSTOMY UNLISTED PROC, LAPAROSCOPY, BILIARY TRACT CHOLECYSTECTOMY CHOLECYSTECTOMY; W/CHOLANGIOGRAPHY CHOLECYSTECTOMY W/EXPLORATION, COMMON DUCT CHOLECYSTECTOMY W/EXPLORATION, COMMON DUCT; W/CHOL CHOLECYSTECTOMY W/EXPLORATION, COMMON DUCT; W/TRAN BILIARY DUCT STONE EXTRACTION, PERCUTANEOUS, VIA T EXPLORATION, CONGENITAL ATRESIA, BILE DUCTS, W/O R PORTOENTEROSTOMY EXCISION, BILE DUCT TUMOR, W/WO PRIMARY REPAIR, BI EXCISION, BILE DUCT TUMOR, W/WO PRIMARY REPAIR, BI EXCISION, CHOLEDOCHAL CYST CHOLECYSTOENTEROSTOMY; DIRECT CHOLECYSTOENTEROSTOMY; W/GASTROENTEROSTOMY CHOLECYSTOENTEROSTOMY; ROUX-EN-Y CHOLECYSTOENTEROSTOMY; ROUX-EN-Y W/GASTROENTEROSTO ANASTOMOSIS, EXTRAHEPATIC BILIARY DUCTS AND GI TRA ANASTOMOSIS, INTRAHEPATIC DUCTS AND GI TRACT ANASTOMOSIS, ROUX-EN-Y, EXTRAHEPATIC BILIARY DUCTS ANASTOMOSIS, ROUX-EN-Y, INTRAHEPATIC BILIARY DUCTS RECONSTRUCTION, PLASTIC, EXTRAHEPATIC BILIARY DUCT PLACEMENT, CHOLEDOCHAL STENT U-TUBE HEPATICOENTEROSTOMY SUTURE, EXTRAHEPATIC BILIARY DUCT, PRE-EXISTING IN UNLISTED PROC, BILIARY TRACT PLACEMENT, DRAINS, PERIPANCREATIC, ACUTE PANCREATI PLACEMENT, DRAINS, PERIPANCREATIC, ACUTE PANCREATI REMOVAL, PANCREATIC CALCULUS BX, PANCREAS, OPEN BX, PANCREAS, PERCUTANEOUS NEEDLE Resect/debride pancreas EXCISION, LESION, PANCREAS PANCREATECTOMY, DISTAL SUBTOTAL, W/WO SPLENECTOMY; PANCREATECTOMY, DISTAL SUBTOTAL, W/WO SPLENECTOMY; PANCREATECTOMY, DISTAL, NEAR-TOTAL W/PRESERVATION, EXCISION, AMPULLA, VATER PANCREATECTOMY (WHIPPLE); W/PANCREATOJEJUNOSTOMY PANCREATECTOMY (WHIPPLE); W/O PANCREATOJEJUNOSTOMY PANCREATECTOMY (PYLORUS SPARING, WHIPPLE); W/PANCR PANCREATECTOMY (PYLORUS SPARING, WHIPPLE); W/O PAN PANCREATECTOMY, TOTAL PANCREATECTOMY, TOTAL/SUBTOTAL W/AUTOLOGOUS TRANSP INJECTION PROC, INTRAOPERATIVE PANCREATOGRAPHY MARSUPIALIZATION, CYST, PANCREAS EXTERNAL DRAINAGE PSEUDOCYST OF PANCREAS OPEN INT ANASTOMOSIS, PANCREATIC CYST TO GI TRACT; DIRE INT ANASTOMOSIS, PANCREATIC CYST TO GI TRACT; ROUX PANCREATORRHAPHY, INJURY DUODENAL EXCLUSION W/GASTROJEJUNOSTOMY, PANCREATIC Fuse pancreas and bowel DONOR PANCREATECTOMY, W/PREP AND MAINTENANCE, CADA PREP DONOR, PANCREAS ALLOGRAFT PREP DONOR, PANCREAS ALLOGRAFT, VENOUS ANASTOMOSIS TRANSPLANTATION, PANCREATIC ALLOGRAFT REMOVAL, TRANSPLANTED PANCREATIC ALLOGRAFT UNLISTED PROC, PANCREAS EXPLORATORY LAPAROTOMY, EXPLORATORY CELIOTOMY W/WO REOPENING, RECENT LAPAROTOMY EXPLORATION, RETROPERITONEAL AREA W/WO BX(S) (SEP DRAINAGE PERITON ABSCESS/LOCAL PERITONITIS OPEN DRAINAGE SUBDIAPHRAGMATIC/SUBPHREN ABSCESS OPEN DRAINAGE OF RETROPERITONEAL ABSCESS OPEN DRAINAGE, EXTRAPERITONEAL LYMPHOCELE TO PERITONEAL ABDOM PARACENTESIS DX/THER W/O IMAGING GUIDANCE ABDOM PARACENTESIS DX/THER W IMAGING GUIDANCE PERITONEAL LAVAGE W/WO IMAGING GUIDANCE BX, ABDOMINAL/RETROPERITONEAL MASS, PERCUTANEOUS N SCLEROTHERAPY OF A FLUID COLLECTION (EG, LYMPHOCELE, CYST, OR SEROMA), PERCUTANE EXCISION/DESTRUCTION OPEN ABDOMINAL TUMORS 5 CM EXC/DESTRUCTION OPEN ABDMNL TUMORS 5.1-10.0 CM EXC/DESTRUCTION OPEN ABDOMINAL TUMORS >10.0 CM EXCISION, PRESACRAL/SACROCOCCYGEAL TUMOR STAGING CELIOTOMY, HODGKINS DISEASE/LYMPHOMA UMBILECTOMY, OMPHALECTOMY, EXCISION, UMBILICUS (SE OMENTECTOMY, EPIPLOECTOMY, RESECTION, OMENTUM (SEP LAPAROSCOPY, ABDOMEN, PERITONEUM AND OMENTUM, DX, LAPAROSCOPY, SURGICAL; W/BX (SINGLE/MULTIPLE) LAPAROSCOPY, SURGICAL; W/CAVITY/CYST ASPIRATION LAPAROSCOPY, SURGICAL; W/LYMPHOCELE DRAINAGE TO PE LAPS INSERTION TUNNELED INTRAPERITONEAL CATHETER Lap revision perm ip cath Lap w/omentopexy add-on LAPS W/INSERTION NTRSTL DEV W/IMG GID 1+ UNLISTED PROC, LAPAROSCOPY, ABDOMEN, PERITONEUM AN INJECTION, AIR/CONTRAST INTO PERITONEAL CAVITY (SE Remove foreign body, adbomen IMAGE-GUIDE FLUID COLLXN DRAINAGE CATH VISC PERQ IMG-GUIDE FLUID COLLXN DRAINAG CATH PERITON PERQ 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2016 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2011 1/1/2004 1/1/2004 1/1/2007 1/1/2014 1/1/2014 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 49407 49411 49412 49418 49419 49421 49422 49423 49424 49425 49426 49427 49428 49429 49435 49436 49440 49441 49442 49446 49450 49451 49452 49460 49465 49491 49492 49495 49496 49500 49501 49505 49507 49520 49521 49525 49540 49550 49553 49555 49557 49560 49561 49565 49566 49568 49570 49572 49580 49582 49585 49587 49590 49600 49605 49606 49610 49611 49650 49651 49652 49653 49654 49655 49656 49657 49659 49900 49904 49905 49906 49999 50010 50020 50040 50045 5005F 50060 50065 50070 50075 50080 50081 50100 5010F 50120 50125 50130 50135 5015F 50200 IMAGE FLUID COLLXN DRAINAG CATH TRANSREC/VAGINAL PLMT NTRSTL DEV PRQ IABDL IPELVC &/ RPER 1/MLT PLMT INTRSTL DEV OPN W/IMG GID 1+ INSJ INTRAPERITONEAL CATHETER W/IMG GID INSERTION TUNNEL INTRAPERITONEAL CATH SUBQ PORT INSERTION TUNNEL INTRAPERITONEAL CATH DIAL OPEN REMOVAL TUNNELED INTRAPERITONEAL CATHETER EXCHANGE ABSCESS/CYST DRAINAGE CATHETER, RADIOLOGI CONTRAST INJECTION, ASSESSMENT, ABSCESS/CYST VIA D INSERTION, PERITONEAL-VENOUS SHUNT REVISION, PERITONEAL-VENOUS SHUNT INJECTION PROC, EVAL, PREVIOUSLY PLACED PERITONEAL LIGATION, PERITONEAL-VENOUS SHUNT REMOVAL, PERITONEAL-VENOUS SHUNT Insert subq exten to ip cath Embedded ip cath exit-site INSERT GASTROSTOMY TUBE PERCUTANEOUS UND FLUOROSCO INSERT DUODENOSTOMY/JEJUNOSTOMY TUBE PERQ UND FLUO INSERT CECOSTOMY/OTHER COLONIC TUBE PERCUTANEOUS CONVERT GASTROSTOMY-GASTRO-JEJUNOSTOMY TUBE PERQ REPLACE GASTROSTOMY/CECOSTOMY TUBE PERCUTANEOUS REPLACE DUODENOSTOMY/JEJUNOSTOMY TUBE PERQ UND FLU REPLACEMENT GASTRO-JEJUNOSTOMY TUBE PERCUTANEOUS MECH REMVL OBSTRCTVE MATERL GASTROSTOMY, DUODENOS CONTRAST INJECTION PERQ RADIOLOGIC EVAL GI TUBE REPAIR, INIT INGUINAL HERNIA, PRETERM INFANT (BIRT REPAIR, INIT INGUINAL HERNIA, PRETERM INFANT (BIRT REPAIR, INIT INGUINAL HERNIA, FULL/PRETERM INFANT REPAIR, INIT INGUINAL HERNIA, FULL/PRETERM INFANT REPAIR, INITIAL INGUINAL HERNIA, AGE 6 MONTHS TO U REPAIR, INITIAL INGUINAL HERNIA, AGE 6 MONTHS TO U REPAIR, INITIAL INGUINAL HERNIA, OVER AGE 5; REDUC REPAIR, INITIAL INGUINAL HERNIA, OVER AGE 5; INCAR REPAIR, RECURRENT INGUINAL HERNIA, ANY AGE; REDUCI REPAIR, RECURRENT INGUINAL HERNIA, ANY AGE; INCARC REPAIR, INGUINAL HERNIA, SLIDING, ANY AGE REPAIR, LUMBAR HERNIA REPAIR, INITIAL FEMORAL HERNIA, ANY AGE; REDUCIBLE REPAIR, INITIAL FEMORAL HERNIA, ANY AGE; INCARCERA REPAIR, RECURRENT FEMORAL HERNIA; REDUCIBLE REPAIR, RECURRENT FEMORAL HERNIA; INCARCERATED/STR REPAIR, INITIAL INCISIONAL/VENTRAL HERNIA; REDUCIB REPAIR, INITIAL INCISIONAL/VENTRAL HERNIA; INCARCE REPAIR, RECURRENT INCISIONAL/VENTRAL HERNIA; REDUC REPAIR, RECURRENT INCISIONAL/VENTRAL HERNIA; INCAR IMPLANTATION, MESH/PROSTHESIS, INCISIONAL/VENTRAL REPAIR, EPIGASTRIC HERNIA; REDUCIBLE (SEP PROC) REPAIR, EPIGASTRIC HERNIA; INCARCERATED/STRANGULAT REPAIR, UMBILICAL HERNIA, AGE UNDER 5; REDUCIBLE REPAIR, UMBILICAL HERNIA, AGE UNDER 5; INCARCERATE REPAIR, UMBILICAL HERNIA, OVER AGE 5; REDUCIBLE REPAIR, UMBILICAL HERNIA, OVER AGE 5; INCARCERATED REPAIR, SPIGELIAN HERNIA REPAIR, SMALL OMPHALOCELE, W/PRIMARY CLOSURE REPAIR, LARGE OMPHALOCELE/GASTROSCHISIS; W/WO PROS REPAIR, LARGE OMPHALOCELE/GASTROSCHISIS; W/REMOVAL REPAIR, OMPHALOCELE; 1ST STAGE REPAIR, OMPHALOCELE; 2ND STAGE LAPAROSCOPY, SURGICAL; REPAIR, INGUINAL HERNIA, IN LAPAROSCOPY, SURGICAL; REPAIR, INGUINAL HERNIA, RE LAPS REPAIR HERNIA EXCEPT INCAL/INGUN REDUCIBLE LAP RPR HRNA XCPT INCAL/INGUN NCRC8/STRANGULATED LAPAROSCOPY REPAIR INCISIONAL HERNIA REDUCIBLE LAPS RPR INCISIONAL HERNIA NCRC8/STRANGULATED LAPS RPR RECURRENT INCISIONAL HERNIA REDUCIBLE LAPS RPR RECURRENT INCAL HRNA NCRC8/STRANGULATED UNLISTED PROC, LAPAROSCOPY, HERNIOPLASTY/HERNIORRH SUTURE, SECONDARY, ABDOMINAL WALL, EVISCERATION/DE OMENTAL FLAP, EXTRA-ABDOMINAL OMENTAL FLAP, INTRA-ABDOMINAL (ADDL PROC) FREE OMENTAL FLAP W/MICROVASCULAR ANASTOMOSIS UNLISTED PROC, ABDOMEN, PERITONEUM AND OMENTUM RENAL EXPLORATION, NOT NECESSITATING OTHER SPECIFI DRAINAGE PERIRENAL/RENAL ABSCESS OPEN NEPHROSTOMY, NEPHROTOMY W/DRAINAGE NEPHROTOMY, W/EXPLORATION Pt counsld on exam for moles NEPHROLITHOTOMY; REMOVAL, CALCULUS NEPHROLITHOTOMY; SECONDARY SURGICAL OPERATION, CAL NEPHROLITHOTOMY; COMPLICATED, CONGENITAL KIDNEY AB NEPHROLITHOTOMY; REMOVAL, LARGE STAGHORN CALCULUS PERCUTANEOUS NEPHROSTOLITHOTOMY/PYELOSTOLITHOTOMY; PERCUTANEOUS NEPHROSTOLITHOTOMY/PYELOSTOLITHOTOMY; TRANSECTION/REPOSITIONING, ABERRANT RENAL VESSELS DILATED MACULAR/FUNDUS XM COMMUNJ TX PHYS/QHP PYELOTOMY; W/EXPLORATION PYELOTOMY; W/DRAINAGE, PYELOSTOMY PYELOTOMY; W/REMOVAL, CALCULUS PYELOTOMY; COMPLICATED Doc fx & test/txmnt for op RENAL BX; PERCUTANEOUS, TROCAR/NEEDLE 1/1/2014 1/1/2010 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 50205 5020F 50220 50225 50230 50234 50236 50240 50250 50280 50290 50300 50320 50323 50325 50327 50328 50329 50340 50360 50365 50370 50380 50382 50384 50385 50386 50387 50389 50390 50391 50392 50393 50394 50395 50396 50398 50400 50405 50430 50431 50432 50433 50434 50435 50500 5050F 50520 50525 50526 50540 50541 50542 50543 50544 50545 50546 50547 50548 50549 50551 50553 50555 50557 50561 50562 50570 50572 50574 50575 50576 50580 50590 50592 50593 50600 50605 50606 5060F 50610 50620 5062F 50630 50650 50660 50684 50686 50688 50690 50693 50694 RENAL BX; SURGICAL EXPOSURE, KIDNEY TX SUMM RPRT COMMUN PHYS&PT 1 MO COMPLETE NEPHRECTOMY, W/PARTIAL URETERECTOMY, ANY OPEN APPR NEPHRECTOMY, W/PARTIAL URETERECTOMY, ANY OPEN APPR NEPHRECTOMY, W/PARTIAL URETERECTOMY, OPEN, W/RIB R NEPHRECTOMY, W/TOTAL URETERECTOMY AND BLADDER CUFF NEPHRECTOMY, W/TOTAL URETERECTOMY AND BLADDER CUFF NEPHRECTOMY, PARTIAL OPEN ABLATION RENAL MASS CRYOSURG ULTRASOUND EXCISION/UNROOFING, CYST(S), KIDNEY EXCISION, PERINEPHRIC CYST DONOR NEPHRECTOMY; CADAVER DONOR, UNILAT/BILAT W/P DONOR NEPHRECTOMY, OPEN, LIVING DONOR W/O ALLOGRAF BACKBENCH STANDARD PREP; CADAVER DONOR RENAL ALLOG BACKBENCH STANDARD PREP; LIVING DONOR RENAL ALLOGR BACKBENCH RECONSTRUCTION; VENOUS ANASTOMOSIS BACKBENCH RECONSTRUCTION; ARTERIAL ANASTOMOSIS, EA BACKBENCH RECONSTRUCTION; URETERAL ANASTOMOSIS, EA RECIPIENT NEPHRECTOMY (SEP PROC) RENAL ALLOTRANSPLANTATION, IMPLANTATION, GRAFT; W/ RENAL ALLOTRANSPLANTATION, IMPLANTATION, GRAFT; W/ REMOVAL, TRANSPLANTED RENAL ALLOGRAFT RENAL AUTOTRANSPLANTATION, REIMPLANTATION, KIDNEY REMOVAL AND REPLACEMENT OF INTERNALLY DWELLING URE REMOVAL OF INTERNALLY DWELLING URETERA STENT REMOVE & REPLACE INT DWELL URETERAL STENT TRURL REMOVE INT DWELL URETERAL STENT TRANSURETHRAL REMOVAL AND REPLACEMENT OF EXTERNALLY ACCESSIBLE T REMOVAL OF NEPHROSTOMY TUBE, REQUIRING FLUOROSCOPI ASPIRATION AND/OR INJECTION, RENAL CYST/PELVIS, NE INSTILLATION OF THERAPEUTIC AGENT INTO RENAL PELVI INTRODUCTION, INTRACATHETER/CATHETER, RENAL PELVIS INTRODUCTION URETERAL CATHETER/STENT, THROUGH RENA INJECTION PROCEDURE, PYELOGRAPHY, THROUGH NEPHROST INTRODUCTION, GUIDE INTO RENAL PELVIS AND/OR URETE MANOMETRIC STUDIES THROUGH NEPHROSTOMY/PYELOSTOMY CHANGE, NEPHROSTOMY/PYELOSTOMY TUBE PYELOPLASTY, (FOLEY Y-PYELOPLASTY), PLASTIC OPERAT PYELOPLASTY, (FOLEY Y-PYELOPLASTY), PLASTIC OPERAT INJECTION PROCEDURE FOR ANTEGRADE NEPHROSTOGRAM AND/OR URETEROGRAM, COMPL INJECTION PROCEDURE FOR ANTEGRADE NEPHROSTOGRAM AND/OR URETEROGRAM, COMPL PLACEMENT OF NEPHROSTOMY CATHETER, PERCUTANEOUS, INCLUDING DIAGNOSTIC NEPHRO PLACEMENT OF NEPHROURETERAL CATHETER, PERCUTANEOUS, INCLUDING DIAGNOSTIC NEP CONVERT NEPHROSTOMY CATHETER TO NEPHROURETERAL CATHETER, PERCUTANEOUS, INCL EXCHANGE NEPHROSTOMY CATHETER, PERCUTANEOUS, INCLUDING DIAGNOSTIC NEPHROSTO NEPHRORRHAPHY, SUTURE, KIDNEY WOUND/INJURY PLAN 2 MAIN DR. BY 1 MONTH CLOSURE, NEPHROCUTANEOUS/PYELOCUTANEOUS FISTULA CLOSURE, NEPHROVISCERAL FISTULA W/VISCERAL REPAIR; CLOSURE, NEPHROVISCERAL FISTULA W/VISCERAL REPAIR; SYMPHYSIOTOMY, HORSESHOE KIDNEY W/WO PYELOPLASTY, LAPAROSCOPY, SURGICAL; ABLATION OF RENAL CYSTS LAPS ABLTJ RNL MASS LES W/INTRAOP US LAPAROSCOPY, SURGICAL; PARTIAL NEPHRECTOMY LAPAROSCOPY, SURGICAL; PYELOPLASTY LAPAROSCOPY, SURGICAL; RADICAL NEPHRECTOMY LAPAROSCOPY, SURGICAL; NEPHRECTOMY W/PARTIAL URETE LAPAROSCOPY, SURGICAL; DONOR NEPHRECTOMY, LIVING D LAPAROSCOPY, SURGICAL; NEPHRECTOMY W/TOTAL URETERE UNLISTED LAPAROSCOPY PROCEDURE, RENAL RENAL ENDOSCOPY THROUGH NEPHROSTOMY/PYELOSTOMY RENAL ENDOSCOPY THROUGH NEPHROSTOMY/PYELOSTOMY; W/ RENAL ENDOSCOPY THROUGH NEPHROSTOMY/PYELOSTOMY; W/ RENAL ENDOSCOPY THROUGH NEPHROSTOMY/PYELOSTOMY; W/ RENAL ENDOSCOPY THROUGH NEPHROSTOMY/PYELOSTOMY; W/ RENAL ENDOSCOPY THROUGH NEPHROSTOMY/PYELOSTOMY; W/ RENAL ENDOSCOPY THROUGH NEPHROTOMY/PYELOTOMY RENAL ENDOSCOPY THROUGH NEPHROTOMY/PYELOTOMY; W/UR RENAL ENDOSCOPY THROUGH NEPHROTOMY/PYELOTOMY; W/BX RENAL ENDOSCOPY THROUGH NEPHROTOMY/PYELOTOMY; W/EN RENAL ENDOSCOPY THROUGH NEPHROTOMY/PYELOTOMY; W/FU RENAL ENDOSCOPY THROUGH NEPHROTOMY/PYELOTOMY; W/RE LITHOTRIPSY, EXTRACORPOREAL SHOCK WAVE ABLATION, ONE OR MORE RENAL TUMORS, PERCUTANEOUS, ABLATION RENAL TUMOR UNILATERAL PERQ CRYOTHERAPY URETEROTOMY W/EXPLORATION/DRAINAGE (SEP PROC) URETEROTOMY, INSERTION, INDWELLING STENT, ALL TYPE ENDOLUMINAL BIOPSY OF URETER AND/OR RENAL PELVIS, NON-ENDOSCOPIC, INCLUDING IMA FNDNGS MAMMO 2PT W/IN 3 DAYS URETEROLITHOTOMY; UPPER ONE-THIRD, URETER URETEROLITHOTOMY; MIDDLE ONE-THIRD, URETER DOC F2FMAMMO FNDNG IN 3 DAYS URETEROLITHOTOMY; LOWER ONE-THIRD, URETER URETERECTOMY, W/BLADDER CUFF (SEP PROC) URETERECTOMY, TOTAL, ECTOPIC URETER, COMBINATION A INJECTION PROC, URETEROGRAPHY/URETEROPYELOGRAPHY T MANOMETRIC STUDIES THROUGH URETEROSTOMY/INDWELLING CHANGE, URETEROSTOMY TUBE INJECTION PROC, VISUALIZATION, ILEAL CONDUIT AND/O PLACEMENT OF URETERAL STENT, PERCUTANEOUS, INCLUDING DIAGNOSTIC NEPHROSTOGRA PLACEMENT OF URETERAL STENT, PERCUTANEOUS, INCLUDING DIAGNOSTIC NEPHROSTOGRA 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2005 1/1/2005 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2008 1/1/2008 1/1/2006 1/1/2006 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2006 1/1/2008 1/1/2004 1/1/2004 1/1/2016 1/1/2008 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 50695 50700 50705 50706 50715 50722 50725 50727 50728 50740 50750 50760 50770 50780 50782 50783 50785 50800 50810 50815 50820 50825 50830 50840 50845 50860 50900 50920 50930 50940 50945 50947 50948 50949 50951 50953 50955 50957 50961 50970 50972 50974 50976 50980 5100F 51020 51030 51040 51045 51050 51060 51065 51080 51100 51101 51102 51500 51520 51525 51530 51535 51550 51555 51565 51570 51575 51580 51585 51590 51595 51596 51597 51600 51605 51610 51700 51701 51702 51703 51705 51710 51715 51720 51725 51726 51727 51728 51729 51736 51741 51784 PLACEMENT OF URETERAL STENT, PERCUTANEOUS, INCLUDING DIAGNOSTIC NEPHROSTOGRA URETEROPLASTY, PLASTIC OPERATION ON URETER URETERAL EMBOLIZATION OR OCCLUSION, INCLUDING IMAGING GUIDANCE (EG, ULTRASOUN BALLOON DILATION, URETERAL STRICTURE, INCLUDING IMAGING GUIDANCE (EG, ULTRASOUN URETEROLYSIS, W/WO REPOSITIONING, URETER, RETROPER URETEROLYSIS, OVARIAN VEIN SYNDROME URETEROLYSIS, RETROCAVAL URETER, W/REANASTOMOSIS, REVISION, URINARY-CUTANEOUS ANASTOMOSIS (ANY TYPE REVISION, URINARY-CUTANEOUS ANASTOMOSIS (ANY TYPE URETEROPYELOSTOMY, ANASTOMOSIS, URETER AND RENAL P URETEROCALYCOSTOMY, ANASTOMOSIS, URETER TO RENAL C URETEROURETEROSTOMY TRANSURETEROURETEROSTOMY, ANASTOMOSIS, URETER TO C URETERONEOCYSTOSTOMY; ANASTOMOSIS, SINGLE URETER T URETERONEOCYSTOSTOMY; ANASTOMOSIS, DUPLICATED URET URETERONEOCYSTOSTOMY; W/EXTENSIVE URETERAL TAILORI URETERONEOCYSTOSTOMY; W/VESICO-PSOAS HITCH/BLADDER URETEROENTEROSTOMY, DIRECT ANASTOMOSIS, URETER TO URETEROSIGMOIDOSTOMY, W/CREATION, SIGMOID BLADDER, URETEROCOLON CONDUIT, W/BOWEL ANASTOMOSIS URETEROILEAL CONDUIT (ILEAL BLADDER), W/BOWEL ANAS CONTINENT DIVERSION, W/BOWEL ANASTOMOSIS, ANY SEGM URINARY UNDIVERSION REPLACEMENT, ALL/PART, URETER, BOWEL SEGMENT, W/BO CUTANEOUS APPENDICO-VESICOSTOMY URETEROSTOMY, TRANSPLANTATION, URETER TO SKIN URETERORRHAPHY, SUTURE, URETER (SEP PROC) CLOSURE, URETEROCUTANEOUS FISTULA CLOSURE, URETEROVISCERAL FISTULA (W/VISCERAL REPAI DELIGATION, URETER LAPAROSCOPY, SURGICAL; URETEROLITHOTOMY LAPAROSCOPY, SURGICAL; URETERONEOCYSTOSTOMY W/CYST LAPAROSCOPY, SURGICAL; URETERONEOCYSTOSTOMY W/O CY UNLISTED LAPAROSCOPY PROCEDURE, URETER URETERAL ENDOSCOPY THROUGH URETEROSTOMY URETERAL ENDOSCOPY THROUGH URETEROSTOMY; W/URETERA URETERAL ENDOSCOPY THROUGH URETEROSTOMY; W/BX URETERAL ENDOSCOPY THROUGH URETEROSTOMY; W/FULGURA URETERAL ENDOSCOPY THROUGH URETEROSTOMY; W/REMOVAL URETERAL ENDOSCOPY THROUGH URETEROTOMY, W/WO IRRIG URETERAL ENDOSCOPY THROUGH URETEROTOMY; W/URETERAL URETERAL ENDOSCOPY THROUGH URETEROTOMY; W/BX URETERAL ENDOSCOPY THROUGH URETEROTOMY; W/FULGURAT URETERAL ENDOSCOPY THROUGH URETEROTOMY; W/REMOVAL RSK FX REF W/N 24 HRS X-RAY CYSTOTOMY/CYSTOSTOMY; W/FULGURATION AND/OR INSERTI CYSTOTOMY/CYSTOSTOMY; W/CRYOSURGICAL DESTRUCTION, CYSTOSTOMY, CYSTOTOMY W/DRAINAGE CYSTOTOMY, W/INSERTION, URETERAL CATHETER/STENT (S CYSTOLITHOTOMY, CYSTOTOMY W/REMOVAL, CALCULUS, W/O TRANSVESICAL URETEROLITHOTOMY CYSTOTOMY W/STONE BASKET EXTRACTION/ULTRASONIC AND DRAINAGE, PERIVESICAL/PREVESICAL SPACE ABSCESS ASPIRATION BLADDER BY NEEDLE ASPIRATION BLADDER TROCAR/INTRACATHETER ASPIRATION BLADDER INSERT SUPRAPUBIC CATHETER EXCISION, URACHAL CYST/SINUS, W/WO UMBILICAL HERNI CYSTOTOMY; SIMPLE EXCISION, VESICAL NECK (SEP PROC CYSTOTOMY; EXCISION, BLADDER DIVERTICULUM, SINGLE/ CYSTOTOMY; EXCISION, BLADDER TUMOR CYSTOTOMY, EXCISION/INCISION/REPAIR, URETEROCELE CYSTECTOMY, PARTIAL; SIMPLE CYSTECTOMY, PARTIAL; COMPLICATED CYSTECTOMY, PARTIAL; W/REIMPLANTATION, URETER(S) I CYSTECTOMY, COMPLETE; (SEP PROC) CYSTECTOMY, COMPLETE; W/BILAT PELVIC LYMPHADENECTO CYSTECTOMY, COMPLETE, W/URETEROSIGMOIDOSTOMY/URETE CYSTECTOMY, COMPLETE, W/URETEROSIGMOIDOSTOMY/URETE CYSTECTOMY, COMPLETE, W/URETEROILEAL CONDUIT/SIGMO CYSTECTOMY, COMPLETE, W/URETEROILEAL CONDUIT/SIGMO CYSTECTOMY, COMPLETE, W/CONTINENT DIVERSION, OPEN, PELVIC EXENTERATION, COMPLETE, VESICAL/PROSTATIC/U INJECTION PROC, CYSTOGRAPHY/VOIDING URETHROCYSTOGR INJECTION PROC AND PLACEMENT, CHAIN, CONTRAST AND/ INJECTION PROC, RETROGRADE URETHROCYSTOGRAPHY BLADDER IRRIGATION, SIMPLE, LAVAGE AND/OR INSTILLA INSERTION, NON-INDWELLING BLADDER CATHETER INSERTION, TEMPORARY INDWELLING BLADDER CATHETER; INSERTION, TEMPORARY INDWELLING BLADDER CATHETER; CHANGE, CYSTOSTOMY TUBE; SIMPLE CHANGE, CYSTOSTOMY TUBE; COMPLICATED ENDOSCOPIC INJECTION, IMPLANT MATL INTO SUBMUCOSAL BLADDER INSTILLATION, ANTICARCINOGENIC AGENT (W/DE SIMPLE CYSTOMETROGRAM BLADDER PRESSURE MEASUREMENT DURING FILLING COMPLEX CYSTOMETROGRAM URETHRAL PRESS PROFILE COMPLEX CYSTOMETROGRAM VOIDING PRESSURE STUDIES COMPLX CYSTOMETRO W/VOID PRESS&URETHRAL PROFILE SIMPLE UROFLOWMETRY COMPLEX UROFLOWMETRY ELECTROMYOGRAPHY STUDIES, ANAL/URETHRAL SPHINCTER, 1/1/2016 1/1/2004 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 51785 51792 51797 51798 51800 51820 51840 51841 51845 51860 51865 51880 51900 51920 51925 51940 51960 51980 51990 51992 51999 52000 52001 52005 52007 5200F 52010 52204 52214 52224 52234 52235 52240 52250 52260 52265 52270 52275 52276 52277 52281 52282 52283 52285 52287 52290 52300 52301 52305 52310 52315 52317 52318 52320 52325 52327 52330 52332 52334 52341 52342 52343 52344 52345 52346 52351 52352 52353 52354 52355 52356 52400 52402 52441 52442 52450 52500 5250F 52601 52630 52640 52647 52648 52649 52700 53000 53010 53020 53025 53040 53060 NEEDLE ELECTROMYOGRAPHY STUDIES, ANAL AND/OR URETH STIMULUS EVOKED RESPONSE VOIDING PRESSURE STUDIES; INTRA-ABDOMINAL VOIDING MEASUREMENT, POST-VOIDING RESIDUAL URINE AND/OR BL CYSTOPLASTY/CYSTOURETHROPLASTY, PLASTIC OPERATION, CYSTOURETHROPLASTY W/UNILAT/BILAT URETERONEOCYSTOS ANTERIOR VESICOURETHROPEXY/URETHROPEXY; SIMPLE ANTERIOR VESICOURETHROPEXY/URETHROPEXY; COMPLICATE ABDOMINO-VAGINAL VESICAL NECK SUSPENSION, W/WO END CYSTORRHAPHY, SUTURE, BLADDER WOUND, INJURY/RUPTUR CYSTORRHAPHY, SUTURE, BLADDER WOUND, INJURY/RUPTUR CLOSURE, CYSTOSTOMY (SEP PROC) CLOSURE, VESICOVAGINAL FISTULA, ABDOMINAL APPROACH CLOSURE, VESICOUTERINE FISTULA CLOSURE, VESICOUTERINE FISTULA; W/HYSTERECTOMY CLOSURE, BLADDER EXSTROPHY ENTEROCYSTOPLASTY, W/BOWEL ANASTOMOSIS CUTANEOUS VESICOSTOMY LAPAROSCOPY, SURGICAL; URETHRAL SUSPENSION FOR STR LAPAROSCOPY, SURGICAL; SLING OPERATION FOR STRESS UNLISTED LAPAROSCOPY PROCEDURE, BLADDER CYSTOURETHROSCOPY (SEP PROC) CYSTOURETHROSCOPY W/IRRIGATION AND EVACUATION CLOT CYSTOURETHROSCOPY, W/URETERAL CATHETERIZATION CYSTOURETHROSCOPY, W/URETERAL CATHETERIZATION; W/B CONSID NEURO EVAL APPROP SURG THXPY EPIL 3YRS CYSTOURETHROSCOPY, W/EJACULATORY DUCT CATHETERIZAT CYSTOURETHROSCOPY, W/BX CYSTOURETHROSCOPY, W/FULGURATION TRIGONE/BLADDER N CYSTOURETHROSCOPY, W/FULGURATION/TREATMENT LESION( CYSTOURETHROSCOPY, W/FULGURATION AND/OR RESECTION; CYSTOURETHROSCOPY, W/FULGURATION AND/OR RESECTION; CYSTOURETHROSCOPY, W/FULGURATION AND/OR RESECTION; CYSTOURETHROSCOPY, W/RADIOACTIVE SUBSTANCE INSERTI CYSTOURETHROSCOPY, W/DILATION, BLADDER, INTERSTITI CYSTOURETHROSCOPY, W/DILATION, BLADDER, INTERSTITI CYSTOURETHROSCOPY, W/INT URETHROTOMY; FEMALE CYSTOURETHROSCOPY, W/INT URETHROTOMY; MALE CYSTOURETHROSCOPY, W/DIRECT VISION INT URETHROTOMY CYSTOURETHROSCOPY, W/RESECTION, EXT SPHINCTER CYSTOURETHROSCOPY, W/CALIBRATION AND/OR DILATION, CYSTOURETHROSCOPY, W/INSERTION, URETHRAL STENT CYSTOURETHROSCOPY, W/STEROID INJECTION INTO STRICT CYSTOURETHROSCOPY, TREATMENT, FEMALE URETHRAL SYND CYSTOURETHROSCOPY INJ CHEMODENERVATION BLADDER CYSTOURETHROSCOPY; W/URETERAL MEATOTOMY, UNILAT/BI CYSTOURETHROSCOPY; W/RESECTION/FULGURATION, ORTHOT CYSTOURETHROSCOPY; W/RESECTION/FULGURATION, ECTOPI CYSTOURETHROSCOPY; W/INCISION/RESECTION, ORIFICE, CYSTOURETHROSCOPY, W/REMOVAL, FB/CALCULUS/URETERAL CYSTOURETHROSCOPY, W/REMOVAL, FB/CALCULUS/URETERAL LITHOLAPAXY; SIMPLE/SMALL (LESS THAN 2.5 CM) LITHOLAPAXY; COMPLICATED/LARGE (LESS THAN 2.5 CM) CYSTOURETHROSCOPY; W/REMOVAL, URETERAL CALCULUS CYSTOURETHROSCOPY; W/FRAGMENTATION, URETERAL CALCU CYSTOURETHROSCOPY; W/SUBURETERIC INJECTION, IMPLAN CYSTOURETHROSCOPY; W/MANIPULATION, W/O REMOVAL URE CYSTOURETHROSCOPY, W/INSERTION, INDWELLING URETERA CYSTOURETHROSCOPY W/INSERTION, URETERAL GUIDE WIRE CYSTOURETHROSCOPY; W/TREATMENT URETERAL STRICTURE CYSTOURETHROSCOPY; W/TREATMENT URETEROPELVIC JUNCT CYSTOURETHROSCOPY; W/TREATMENT INTRA-RENAL STRICTU CYSTOURETHROSCOPY W/URETEROSCOPY; W/TREATMENT URET CYSTOURETHROSCOPY W/URETEROSCOPY; W/TREATMENT URET CYSTOURETHROSCOPY W/URETEROSCOPY; W/TREATMENT INTR CYSTOURETHROSCOPY W/URETEROSCOPY AND/OR PYELOSCOPY CYSTOURETHROSCOPY W/URETEROSCOPY AND/OR PYELOSCOPY CYSTOURETHROSCOPY W/URETEROSCOPY AND/OR PYELOSCOPY CYSTOURETHROSCOPY W/URETEROSCOPY AND/OR PYELOSCOPY CYSTOURETHROSCOPY W/URETEROSCOPY AND/OR PYELOSCOPY CYSTO/URETERO W/LITHOTRIPSY &INDWELL STENT INSRT CYSTOURETHROSCOPY W/INCISION/FULGURATION/RESECTION CYSTOURETERO W/CONGEN REPR CYSTO INSERTION TRANSPROSTATIC IMPLANT SINGLE CYSTO INSERTION TRANSPROSTATIC IMPLANT EA ADDL TRANSURETHRAL INCISION, PROSTATE TRANSURETHRAL RESECTION, BLADDER NECK (SEP PROC) ASTHMA DISCHARGE PLAN PRESENT TRANSURETHRAL ELECTROSURGICAL RESECTION, PROSTATE, TRANSURETHRAL RESECTION; REGROWTH, OBSTRUCTIVE TIS TRANSURETHRAL RESECTION; POSTOPERATIVE BLADDER NEC NON-CONTACT LASER COAGULATION, PROSTATE, W/CONTROL CONTACT LASER VAPOR, W/WO TRANSURETHRAL RESECTION LASER ENUCLEATION PROSTATE W MORCELLATION TRANSURETHRAL DRAINAGE, PROSTATIC ABSCESS URETHROTOMY/URETHROSTOMY, EXT (SEP PROC); PENDULOU URETHROTOMY/URETHROSTOMY, EXT (SEP PROC); PERINEAL MEATOTOMY, CUTTING, MEATUS (SEP PROC); EXCEPT INFA MEATOTOMY, CUTTING, MEATUS (SEP PROC); INFANT DRAINAGE, DEEP PERIURETHRAL ABSCESS DRAINAGE, SKENES GLAND ABSCESS/CYST 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2004 7/1/2005 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 53080 53085 53200 53210 53215 53220 53230 53235 53240 53250 53260 53265 53270 53275 53400 53405 53410 53415 53420 53425 53430 53431 53440 53442 53444 53445 53446 53447 53448 53449 53450 53460 53500 53502 53505 53510 53515 53520 53600 53601 53605 53620 53621 53660 53661 53665 53850 53852 53855 53860 53899 54000 54001 54015 54050 54055 54056 54057 54060 54065 54100 54105 54110 54111 54112 54115 54120 54125 54130 54135 54150 54160 54161 54162 54163 54164 54200 54205 54220 54230 54231 54235 54240 54250 54300 54304 54308 54312 54316 54318 54322 DRAINAGE, PERINEAL URINARY EXTRAVASATION; UNCOMPLI DRAINAGE, PERINEAL URINARY EXTRAVASATION; COMPLICA BX, URETHRA URETHRECTOMY, TOTAL, W/CYSTOSTOMY; FEMALE URETHRECTOMY, TOTAL, W/CYSTOSTOMY; MALE EXCISION/FULGURATION, CARCINOMA, URETHRA EXCISION, URETHRAL DIVERTICULUM (SEP PROC); FEMALE EXCISION, URETHRAL DIVERTICULUM (SEP PROC); MALE MARSUPIALIZATION, URETHRAL DIVERTICULUM, MALE/FEMA EXCISION, BULBOURETHRAL GLAND EXCISION/FULGURATION; URETHRAL POLYP(S), DISTAL UR EXCISION/FULGURATION; URETHRAL CARUNCLE EXCISION/FULGURATION; SKENES GLANDS EXCISION/FULGURATION; URETHRAL PROLAPSE URETHROPLASTY; 1ST STAGE, FISTULA/DIVERTICULUM/STR URETHROPLASTY; 2ND STAGE (FORMATION, URETHRA), W/U URETHROPLASTY, 1-STAGE RECONSTRUCTION, MALE ANTERI URETHROPLASTY, TRANSPUBIC/PERINEAL, 1-STAGE, REPAI URETHROPLASTY, 2-STAGE, RECONSTR/REPAIR, PROSTATIC URETHROPLASTY, 2-STAGE, RECONSTR/REPAIR, PROSTATIC URETHROPLASTY, RECONSTRUCTION, FEMALE URETHRA URETHROPLASTY, W/TUBULARIZATION POSTERIOR URETHRA SLING OPERATION, CORRECTION, MALE URINARY INCONTIN REMOVAL, REVISION OF SLING FOR MALE URINARY INCONT INSERTION TANDEM CUFF (DUAL) INSERTION, INFLATABLE URETHRA/BLADDER NECK SPHINCT REMOVAL, INFLATABLE URETHRAL/BLADDER NECK SPHINCTE REMOVAL AND REPLACEMENT, INFLATABLE SPHINCTER W/PU REMOV AND REPLACE INFLATABLE SPHINCTER W/PUMP/RESE REPAIR, INFLATABLE URETHRAL/BLADDER NECK SPHINCTER URETHROMEATOPLASTY, W/MUCOSAL ADVANCEMENT URETHROMEATOPLASTY, W/PARTIAL EXCISION, DISTAL URE URETHROLYSIS, TRANSVAGINAL, SECONDARY, OPEN, W/CYS URETHRORRHAPHY, SUTURE, URETHRAL WOUND/INJURY; FEM URETHRORRHAPHY, SUTURE, URETHRAL WOUND/INJURY; PEN URETHRORRHAPHY, SUTURE, URETHRAL WOUND/INJURY; PER URETHRORRHAPHY, SUTURE, URETHRAL WOUND/INJURY; PRO CLOSURE, URETHROSTOMY/URETHROCUTANEOUS FISTULA, MA DILATION, URETHRAL STRICTURE, PASSAGE, SOUND/URETH DILATION, URETHRAL STRICTURE, PASSAGE, SOUND/URETH DILATION, URETHRAL STRICTURE/VESICAL NECK, MALE, G DILATION, URETHRAL STRICTURE, PASSAGE, FILIFORM AN DILATION, URETHRAL STRICTURE, PASSAGE, FILIFORM AN DILATION, FEMALE URETHRA W/SUPPOSITORY AND/OR INST DILATION, FEMALE URETHRA W/SUPPOSITORY AND/OR INST DILATION, FEMALE URETHRA, GENERAL/CONDUCTION (SPIN TRANSURETHRAL DESTRUCTION, PROSTATE TISSUE; MICROW TRANSURETHRAL DESTRUCTION, PROSTATE TISSUE; RADIOF INSERT TEMP PROSTATIC URETH STENT W/MEASUREMENT TRURL RF FEMALE BLADDER NECK STRS URIN INCONT UNLISTED PROC, URINARY SYSTEM SLITTING, PREPUCE, DORSAL/LATERAL (SEP PROC); NEWB SLITTING, PREPUCE, DORSAL/LATERAL (SEP PROC); EXCE INCISION AND DRAINAGE, PENIS, DEEP DESTRUCTION, PENILE LESION, SIMPLE; CHEMICAL DESTRUCTION, PENILE LESION, SIMPLE; ELECTRODESICCA DESTRUCTION, PENILE LESION, SIMPLE; CRYOSURGERY DESTRUCTION, PENILE LESION, SIMPLE; LASER SURGERY DESTRUCTION, PENILE LESION, SIMPLE; SURGICAL EXCIS DESTRUCTION, PENILE LESION, EXTENSIVE BX OF PENIS; (SEP PROC) BX, PENIS; DEEP STRUCTURES EXCISION, PENILE PLAQUE (PEYRONIE DISEASE); EXCISION, PENILE PLAQUE (PEYRONIE DISEASE); W/GRAF EXCISION, PENILE PLAQUE (PEYRONIE DISEASE); W/GRAF REMOVAL FB, DEEP PENILE TISSUE AMPUTATION, PENIS; PARTIAL AMPUTATION, PENIS; COMPLETE AMPUTATION, PENIS, RADICAL; W/BILAT INGUINOFEMORAL AMPUTATION, PENIS, RADICAL; W/BILAT PELVIC LYMPHAD CIRCUMCISION, USING CLAMP/OTHER DEVICE; NEWBORN CIRCUMCISION, SURGICAL EXCISION OTHER THAN CLAMP/D CIRCUMCISION, SURGICAL EXCISION OTHER THAN CLAMP/D LYSIS/EXCISION, PENILE POSTCIRCUMCISION ADHESIONS REPAIR, INCOMPLETE CIRCUMCISION FRENULOTOMY, PENIS INJECTION PROC, PEYRONIE DISEASE INJECTION PROC, PEYRONIE DISEASE; W/SURGICAL EXPOS IRRIGATION, CORPORA CAVERNOSA, PRIAPISM INJECTION PROC, CORPORA CAVERNOSOGRAPHY DYNAMIC CAVERNOSOMETRY W/INJECTION VASOACTIVE DRUG INJECTION, CORPORA CAVERNOSA W/PHARMACOLOGIC AGENT PENILE PLETHYSMOGRAPHY NOCTURNAL PENILE TUMESCENCE AND/OR RIGIDITY TEST PLASTIC OPERATION, PENIS, STRAIGHTENING, CHORDEE, PLASTIC OPERATION, PENIS, CORRECTION, CHORDEE/1ST URETHROPLASTY, 2ND STAGE HYPOSPADIAS REPAIR (W/URI URETHROPLASTY, 2ND STAGE HYPOSPADIAS REPAIR (W/URI URETHROPLASTY, 2ND STAGE HYPOSPADIAS REPAIR W/FREE URETHROPLASTY, 3RD STAGE HYPOSPADIAS REPAIR, RELEA 1 STAGE DISTAL HYPOSPADIAS REPAIR; W/SIMPLE MEATAL 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 54324 54326 54328 54332 54336 54340 54344 54348 54352 54360 54380 54385 54390 54400 54401 54405 54406 54408 54410 54411 54415 54416 54417 54420 54430 54435 54437 54438 54440 54450 54500 54505 54512 54520 54522 54530 54535 54550 54560 54600 54620 54640 54650 54660 54670 54680 54690 54692 54699 54700 54800 54830 54840 54860 54861 54865 54900 54901 55000 55040 55041 55060 55100 55110 55120 55150 55175 55180 55200 55250 55300 55400 55450 55500 55520 55530 55535 55540 55550 55559 55600 55605 55650 55680 55700 55705 55706 55720 55725 55801 55810 1 STAGE DISTAL HYPOSPADIAS REPAIR; W/URETHROPLASTY 1 STAGE DISTAL HYPOSPADIAS REPAIR; W/URETHROPLASTY 1 STAGE DISTAL HYPOSPADIAS REPAIR; W/EXTENSIVE DIS 1 STAGE PROXIMAL PENILE/PENOSCROTAL HYPOSPADIAS RE 1 STAGE PERINEAL HYPOSPADIAS REPAIR W/EXTENSIVE DI REPAIR, HYPOSPADIAS COMPLICATIONS; SIMPLE CLOSURE/ REPAIR, HYPOSPADIAS COMPLICATIONS; W/MOBILIZATION/ REPAIR, HYPOSPADIAS COMPLICATIONS; W/EXTENSIVE DIS REPAIR, HYPOSPADIAS CRIPPLE W/EXTENSIVE DISSECTION PLASTIC OPERATION, PENIS TO CORRECT ANGULATION PLASTIC OPERATION, PENIS, EPISPADIAS, DISTAL TO SP PLASTIC OPERATION, PENIS, EPISPADIAS, DISTAL TO SP PLASTIC OPERATION, PENIS, EPISPADIAS, DISTAL TO SP INSERTION, PENILE PROSTHESIS; NON-INFLATABLE (SEMI INSERTION, PENILE PROSTHESIS; INFLATABLE (SELF-CON INSERTION, (MULTI-COMPONENT) INFLATABLE PENILE PRO REMOVAL, MULTI-COMPONENT INFLATABLE PENILE PROSTHE REPAIR COMPONENT(S) MULTI-COMPONENT, INFLATABLE PE REMOVAL AND REPLACEMENT, MULTI-COMPONENT INFLATABL REMOVAL AND REPLACEMENT, MULTI-COMPONENT INFLATABL REMOVAL, NON-INFLATABLE (SEMI-RIGID)/INFLATABLE (S REMOVAL AND REPLACEMENT, NON-INFLATABLE (SEMI-RIGI REMOVAL AND REPLACE, NON-INFLATABLE/INFLATABLE PEN CORPORA CAVERNOSA-SAPHENOUS VEIN SHUNT (PRIAPISM O CORPORA CAVERNOSA-CORPUS SPONGIOSUM SHUNT (PRIAPIS CORPORA CAVERNOSA-GLANS PENIS FISTULIZATION, PRIAP REPAIR OF TRAUMATIC CORPOREAL TEAR(S) REPLANTATION, PENIS, COMPLETE AMPUTATION INCLUDING URETHRAL REPAIR PLASTIC OPERATION, PENIS, INJURY FORESKIN MANIPULATION W/LYSIS, PREPUTIAL ADHESIONS BX, TESTIS, NEEDLE (SEP PROC) BX, TESTIS, INCISIONAL (SEP PROC) EXCISION, EXTRAPARENCHYMAL LESION, TESTIS ORCHIECTOMY, SIMPLE, W/WO PROSTHESIS, SCROTAL/INGU ORCHIECTOMY, PARTIAL ORCHIECTOMY, RADICAL, TUMOR; INGUINAL APPROACH ORCHIECTOMY, RADICAL, TUMOR; W/ABDOMINAL EXPLORATI EXPLORATION, UNDESCENDED TESTIS (INGUINAL/SCROTAL EXPLORATION, UNDESCENDED TESTIS W/ABDOMINAL EXPLOR REDUCTION, TORSION, TESTIS, SURGICAL, W/WO FIXATIO FIXATION, CONTRALATERAL TESTIS (SEP PROC) ORCHIOPEXY, INGUINAL APPROACH, W/WO HERNIA REPAIR ORCHIOPEXY, ABDOMINAL APPROACH, FOR INTRA-ABDOMINA INSERTION, TESTICULAR PROSTHESIS (SEP PROC) SUTURE/REPAIR, TESTICULAR INJURY TRANSPLANTATION, TESTIS(ES) TO THIGH (FOR SCROTAL LAPAROSCOPY, SURGICAL; ORCHIECTOMY LAPAROSCOPY, SURGICAL; ORCHIOPEXY, INTRA-ABDOMINAL UNLISTED PROC, LAPAROSCOPY, TESTIS INCISION AND DRAINAGE, EPIDIDYMIS, TESTIS AND/OR S BX, EPIDIDYMIS, NEEDLE EXCISION, LOCAL LESION, EPIDIDYMIS EXCISION, SPERMATOCELE, W/WO EPIDIDYMECTOMY EPIDIDYMECTOMY; UNILAT EPIDIDYMECTOMY; BILAT Explore epididymis EPIDIDYMOVASOSTOMY, ANASTOMOSIS, EPIDIDYMIS TO VAS EPIDIDYMOVASOSTOMY, ANASTOMOSIS, EPIDIDYMIS TO VAS PUNCTURE ASPIRATION, HYDROCELE, TUNICA VAGINALIS, EXCISION, HYDROCELE; UNILAT EXCISION, HYDROCELE; BILAT REPAIR, TUNICA VAGINALIS HYDROCELE (BOTTLE TYPE) DRAINAGE, SCROTAL WALL ABSCESS SCROTAL EXPLORATION REMOVAL, FB IN SCROTUM RESECTION, SCROTUM SCROTOPLASTY; SIMPLE SCROTOPLASTY; COMPLICATED VASOTOMY, CANNULIZATION, W/WO INCISION, VAS, UNILA VASECTOMY, UNILAT/BILAT, W/POSTOPERATIVE SEMEN EXA VASOTOMY, VASOGRAMS, SEMINAL VESICULOGRAMS/EPIDIDY VASOVASOSTOMY, VASOVASORRHAPHY LIGATION (PERCUTANEOUS), VAS DEFERENS, UNILAT/BILA EXCISION, HYDROCELE, SPERMATIC CORD, UNILAT (SEP P EXCISION, LESION, SPERMATIC CORD (SEP PROC) EXCISION, VARICOCELE/LIGATION, SPERMATIC VEINS, VA EXCISION, VARICOCELE/LIGATION, SPERMATIC VEINS, VA EXCISION, VARICOCELE/LIGATION, SPERMATIC VEINS, VA LAPAROSCOPY, SURGICAL; W/LIGATION, SPERMATIC VEINS UNLISTED PROC, LAPAROSCOPY, SPERMATIC CORD VESICULOTOMY VESICULOTOMY; COMPLICATED VESICULECTOMY, ANY APPROACH EXCISION, MULLERIAN DUCT CYST BX, PROSTATE; NEEDLE/PUNCH, SINGLE/MULTIPLE, ANY A BX, PROSTATE; INCISIONAL, ANY APPROACH BX PROSTATE STRTCTC SATURATION SAMPLING IMG GID PROSTATOTOMY, EXT DRAINAGE, PROSTATIC ABSCESS, ANY PROSTATOTOMY, EXT DRAINAGE, PROSTATIC ABSCESS, ANY PROSTATECTOMY, PERINEAL, SUBTOTAL PROSTATECTOMY, PERINEAL RADICAL 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N Y N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 55812 55815 55821 55831 55840 55842 55845 55860 55862 55865 55866 55870 55873 55875 55876 55899 55920 55970 55980 56405 56420 56440 56441 56442 56501 56515 56605 56606 56620 56625 56630 56631 56632 56633 56634 56637 56640 56700 56740 56800 56805 56810 56820 56821 57000 57010 57020 57022 57023 57061 57065 57100 57105 57106 57107 57109 57110 57111 57112 57120 57130 57135 57150 57155 57156 57160 57170 57180 57200 57210 57220 57230 57240 57250 57260 57265 57267 57268 57270 57280 57282 57283 57284 57285 57287 57288 57289 57291 57292 57295 57296 PROSTATECTOMY, PERINEAL RADICAL; W/LYMPH NODE BX(S PROSTATECTOMY, PERINEAL RADICAL; W/BILAT PELVIC LY PROSTATECTOMY; SUPRAPUBIC, SUBTOTAL, 1/2 STAGES PROSTATECTOMY; RETROPUBIC, SUBTOTAL PROSTATECTOMY, RETROPUBIC RADICAL, W/WO NERVE SPAR PROSTATECTOMY, RETROPUBIC RADICAL, W/WO NERVE SPAR PROSTATECTOMY, RETROPUBIC RADICAL, W/WO NERVE SPAR EXPOSURE, PROSTATE, ANY APPROACH, RADIATION INSERT EXPOSURE, PROSTATE, ANY APPROACH, RADIATION INSERT EXPOSURE, PROSTATE, ANY APPROACH, RADIATION INSERT LAPS PRSTECT RETROPUBIC RAD W/NRV SPARING ROBOT ELECTROEJACULATION ABLATION, CRYOSURGICAL, PROSTATE Transperi needle place, pros PLACE INTERSTITIAL DEV RADIATION TX PROSTATE 1+ UNLISTED PROC, MALE GENITAL SYSTEM PLACEMENT NEEDLE PELVIC ORGAN RADIOELEMENT APPL INTERSEX SURGERY; MALE TO FEMALE INTERSEX SURGERY; FEMALE TO MALE INCISION AND DRAINAGE, VULVA/PERINEAL ABSCESS INCISION AND DRAINAGE, BARTHOLINS GLAND ABSCESS MARSUPIALIZATION, BARTHOLINS GLAND CYST LYSIS, LABIAL ADHESIONS Hymenotomy DESTRUCTION, LESION(S), VULVA; SIMPLE DESTRUCTION, LESION(S), VULVA; EXTENSIVE BX, VULVA/PERINEUM (SEP PROC); 1 LESION BX, VULVA/PERINEUM (SEP PROC); ADDL LESION VULVECTOMY SIMPLE; PARTIAL VULVECTOMY SIMPLE; COMPLETE VULVECTOMY, RADICAL, PARTIAL VULVECTOMY, RADICAL, PARTIAL; W/UNILAT INGUINOFEMO VULVECTOMY, RADICAL, PARTIAL; W/BILAT INGUINOFEMOR VULVECTOMY, RADICAL, COMPLETE VULVECTOMY, RADICAL, COMPLETE; W/UNILAT INGUINOFEM VULVECTOMY, RADICAL, COMPLETE; W/BILAT INGUINOFEMO VULVECTOMY, RADICAL, COMPLETE, W/INGUINOFEMORAL, I PARTIAL HYMENECTOMY/REVISION, HYMENAL RING EXCISION, BARTHOLINS GLAND/CYST PLASTIC REPAIR, INTROITUS CLITOROPLASTY, INTERSEX STATE PERINEOPLASTY, REPAIR, PERINEUM, NONOBSTETRICAL (S COLPOSCOPY, VULVA COLPOSCOPY, VULVA; W/BIOPSY(S) COLPOTOMY; W/EXPLORATION COLPOTOMY; W/DRAINAGE, PELVIC ABSCESS COLPOCENTESIS (SEP PROC) INCISION AND DRAINAGE, VAGINAL HEMATOMA; OBSTETRIC INCISION AND DRAINAGE, VAGINAL HEMATOMA; NON-OBSTE DESTRUCTION, VAGINAL LESION(S); SIMPLE DESTRUCTION, VAGINAL LESION(S); EXTENSIVE BX, VAGINAL MUCOSA; SIMPLE (SEP PROC) BX, VAGINAL MUCOSA; EXTENSIVE, REQUIRING SUTURE (W VAGINECTOMY, PARTIAL REMOVAL, VAGINAL WALL; VAGINECTOMY, PARTIAL REMOVAL, VAGINAL WALL; W/REMO VAGINECTOMY, PARTIAL REMOVAL, VAGINAL WALL; W/REMO VAGINECTOMY, COMPLETE REMOVAL, VAGINAL WALL VAGINECTOMY, COMPLETE REMOVAL, VAGINAL WALL; W/REM VAGINECTOMY, COMPLETE REMOVAL, WALL; W/REMOVAL, PA COLPOCLEISIS (LE FORT TYPE) EXCISION, VAGINAL SEPTUM EXCISION, VAGINAL CYST/TUMOR IRRIGATION/TREATMENT, VAGINAL INFECTION INSJ UTERINE TANDEM&/VAG OVOIDS INSJ VAGINAL RADIATION DEVICE FITTING AND INSERTION, PESSARY/OTHER INTRAVAGINAL DIAPHRAGM/CERVICAL CAP FITTING W/INSTRUCTIONS INTRO HEMOSTATIC AGENT/PACK, TREATMENT, VAGINAL BL COLPORRHAPHY, SUTURE, INJURY, VAGINA (NONOBSTETRIC COLPOPERINEORRHAPHY, SUTURE, INJURY, VAGINA AND/OR PLASTIC OPERATION ON URETHRAL SPHINCTER, VAGINAL A PLASTIC REPAIR, URETHROCELE ANTERIOR COLPORRHAPHY, REPAIR, CYSTOCELE W/WO REPA POSTERIOR COLPORRHAPHY, REPAIR, RECTOCELE W/WO PER COMBINED ANTEROPOSTERIOR COLPORRHAPHY COMBINED ANTEROPOSTERIOR COLPORRHAPHY; W/ENTEROCEL INSERTION OF MESH OR OTHER PROSTHESIS FOR REPAIR O REPAIR, ENTEROCELE, VAGINAL APPROACH (SEP PROC) REPAIR, ENTEROCELE, ABDOMINAL APPROACH (SEP PROC) COLPOPEXY, ABDOMINAL APPROACH SACROSPINOUS LIGAMENT FIXATION, PROLAPSE, VAGINA COLPOPEXY, VAGINAL, INTRA-PERITNEAL APPROACH PARAVAGINAL DEFECT REPAIR (W/REPAIR, CYSTOCELE, ST PARAVAGINAL DEFECT REPAIR VAGINAL APPROACH REMOVAL/REVISION, SLING, STRESS INCONTINENCE SLING OPERATION, STRESS INCONTINENCE PEREYRA PROC, W/ANTERIOR COLPORRHAPHY CONSTRUCTION, ARTIFICIAL VAGINA; W/O GRAFT CONSTRUCTION, ARTIFICIAL VAGINA; W/GRAFT REVISION OF PROSTHETIC VAGINAL GRAFT, VAGINAL APP Revise vag graft, open abd 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2007 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 57300 57305 57307 57308 57310 57311 57320 57330 57335 57400 57410 57415 57420 57421 57423 57425 57426 57452 57454 57455 57456 57460 57461 57500 57505 57510 57511 57513 57520 57522 57530 57531 57540 57545 57550 57555 57556 57558 57700 57720 57800 58100 58110 58120 58140 58145 58146 58150 58152 58180 58200 58210 58240 58260 58262 58263 58267 58270 58275 58280 58285 58290 58291 58292 58293 58294 58300 58301 58321 58322 58323 58340 58345 58346 58350 58353 58356 58400 58410 58520 58540 58541 58542 58543 58544 58545 58546 58548 58550 58552 58553 CLOSURE, RECTOVAGINAL FISTULA; VAGINAL/TRANSANAL A CLOSURE, RECTOVAGINAL FISTULA; ABDOMINAL APPROACH CLOSURE, RECTOVAGINAL FISTULA; ABDOMINAL APPROACH, CLOSURE, RECTOVAGINAL FISTULA; TRANSPERINEAL APPRO CLOSURE, URETHROVAGINAL FISTULA CLOSURE, URETHROVAGINAL FISTULA; W/BULBOCAVERNOSUS CLOSURE, VESICOVAGINAL FISTULA; VAGINAL APPROACH CLOSURE, VESICOVAGINAL FISTULA; TRANSVESICAL AND V VAGINOPLASTY, INTERSEX STATE DILATION, VAGINA UNDER ANESTHESIA PELVIC EXAM UNDER ANESTHESIA REMOVAL, IMPACTED VAGINAL FB (SEP PROC) UNDER ANES COLPOSCOPY, ENTIRE VAGINA, W/CERVIX IF PRESENT COLPOSCOPY, ENTIRE VAGINA, W/CERVIX IF PRESENT; W/ PARAVAGINAL DEFECT REPAIR LAPAROSCOPIC APPROACH LAPAROSCOPY, SURGICAL, COLPOPEXY REVISION PROSTHETIC VAGINAL GRAFT LAPAROSCOPIC COLPOSCOPY, CERVIX W/UPPER ADJACENT VAGINA COLPOSCOPY, CERVIX W/UPPER ADJACENT VAGINA; W/BIOP COLPOSCOPY, CERVIX W/UPPER ADJACENT VAGINA; W/BIOP COLPOSCOPY, CERVIX W/UPPER ADJACENT VAGINA; W/ENDO COLPOSCOPY, CERVIX W/UPPER ADJACENT VAGINA; W/LOOP COLPOSCOPY, CERVIX W/UPPER ADJACENT VAGINA; W/LOOP BX/EXCISION, CERVIX LESION W/WO FULGURATION (SEP P ENDOCERVICAL CURETTAGE (NOT DONE AS PART OF A DILA CAUTERIZATION, CERVIX; ELECTRO/THERMAL CAUTERIZATION, CERVIX; CRYOCAUTERY, INITIAL/REPEAT CAUTERIZATION, CERVIX; LASER ABLATION CONIZATION, CERVIX W/WO FULGURATION, W/WO D AND C/ CONIZATION, CERVIX W/WO FULGURATION, W/WO D AND C/ TRACHELECTOMY (CERVICECTOMY), AMPUTATION, CERVIX ( TRACHELECTOMY, RADICAL W/BILAT PELVIC LYMPHADENECT EXCISION, CERVICAL STUMP, ABDOMINAL APPROACH EXCISION, CERVICAL STUMP, ABDOMINAL APPROACH; W/PE EXCISION, CERVICAL STUMP, VAGINAL APPROACH EXCISION, CERVICAL STUMP, VAGINAL APPROACH; W/ANTE EXCISION, CERVICAL STUMP, VAGINAL APPROACH; W/REPA D&C of cervical stump CERCLAGE, UTERINE CERVIX, NONOBSTETRICAL TRACHELORRHAPHY, PLASTIC REPAIR, UTERINE CERVIX, V DILATION, CERVICAL CANAL, INSTRUMENTAL (SEP PROC) ENDOMETRIAL BX W/WO ENDOCERVICAL BX, W/O DILATION, ENDOMETRIAL SAMPLING PERFORMED IN CONJUNCTION WITH DILATION AND CURETTAGE, DX AND/OR THERAPEUTIC (NON MYOMECTOMY 1-4 MYOMA(S), W/TOTAL WEIGHT 250GMS OR MYOMECTOMY 1-4 MYOMA(S), W/TOTAL WEIGHT 250 GMS OR MYOMECTOMY 5 OR MORE INTRAMURAL MYOMAS AND/OR TOTA TOTAL ABDOMINAL HYSTERECTOMY W/WO REMOVAL TUBE(S)/ TOTAL ABDOMINAL HYSTERECTOMY W/WO REMOVAL TUBE(S)/ SUPRACERVICAL ABDOMINAL HYSTERECTOMY, W/WO REMOVAL TOTAL ABDOMINAL HYSTERECTOMY, W/PARTIAL VAGINECT, RADICAL ABDOMINAL HYSTERECTOMY W/BILAT PELVIC LYMP PELVIC EXENTERATION, GYNECOLOGIC MALIGNANCY VAGINAL HYSTERECTOMY, UTERUS 250 GMS OR LESS VAGINAL HYSTERECTOMY FOR UTERUS 250 GRAMS OR LESS; VAGINAL HYSTERECTOMY, UTERUS 250 GMS OR LESS; W/RE VAGINAL HYSTERECTOMY, UTERUS 250 GMS OR LESS; W/CO VAGINAL HYSTERECTOMY, UTERUS 250 GMS OR LESS; W/RE VAGINAL HYSTERECTOMY, W/TOTAL/PARTIAL VAGINECTOMY VAGINAL HYSTERECTOMY; W/TOTAL/PARTIAL VAGINECTOMY; VAGINAL HYSTERECTOMY; RADICAL VAGINAL HYSTERECTOMY, UTERUS GREATER THAN 250 GMS; VAGINAL HYSTERECTOMY, UTERUS GREATER THAN 250 GMS; VAGINAL HYSTERECTOMY, UTERUS GREATER THAN 250 GMS; VAGINAL HYSTERECTOMY, UTERUS GREATER THAN 250 GMS; VAGINAL HYSTERECTOMY, UTERUS GREATER THAN 250 GMS; INSERTION, INTRAUTERINE DEVICE REMOVAL, INTRAUTERINE DEVICE ARTIFICIAL INSEMINATION; INTRA-CERVICAL ARTIFICIAL INSEMINATION; INTRA-UTERINE SPERM WASHING, ARTIFICIAL INSEMINATION CATHETERIZATION AND INTROD, SALINE INFUSION SONOHY TRANSCERVICAL INTRODUCTION, FALLOPIAN TUBE CATHETE INSERTION, HEYMAN CAPSULES, CLINICAL BRACHYTHERAPY CHROMOTUBATION, OVIDUCT, W/MATLS ABLATION, ENDOMETRIAL, THERMAL, W/O HYSTEROSCOPIC ENDOMETRIAL CRYOABLATION WITH ULTRASONIC GUIDANCE, UTERINE SUSPENSION, W/WO SHORTENING ROUND/SACROUTE UTERINE SUSPENSION W/WO SHORTENING ROUND/SACROUTER HYSTERORRHAPHY, REPAIR, RUPTURED UTERUS (NONOBSTET HYSTEROPLASTY, REPAIR, UTERINE ANOMALY Lsh, uterus 250 g or less Lsh w/t/o ut 250 g or less Lsh uterus above 250 g Lsh w/t/o uterus above 250 g LAPAROSCOPY, SURG, MYOMECTOMY; 1-4 INTRAMURAL MYOM LAPAROSCOPY, SURG, MYOMECTOMY; 5 OR LESS INTRAMURA Lap radical hyst LAPAROSCOPY, SURG, W/VAGINAL HYSTERECTOMY, UTERUS LAPAROSCOPY, SURGICAL, MYOMECTOMY, EXCISION; W/REM LAPAROSCOPY, SURG, W/VAGINAL HYSTERECTOMY, UTERUS 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 POS 22 or 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 58554 58555 58558 58559 58560 58561 58562 58563 58565 58570 58571 58572 58573 58578 58579 58600 58605 58611 58615 58660 58661 58662 58670 58671 58672 58673 58679 58700 58720 58740 58750 58752 58760 58770 58800 58805 58820 58822 58825 58900 58920 58925 58940 58943 58950 58951 58952 58953 58954 58956 58957 58958 58960 58970 58974 58976 58999 59000 59001 59012 59015 59020 59025 59030 59050 59051 59070 59072 59074 59076 59100 59120 59121 59130 59135 59136 59140 59150 59151 59160 59200 59300 59320 59325 59350 59400 59409 59410 59412 59414 59425 LAPAROSCOPY, SURG, W/VAGINAL HYSTERECTOMY, FOR UTE HYSTEROSCOPY, DX (SEP PROC) HYSTEROSCOPY, SURGICAL; W/ENDOMETRIAL BX AND/OR PO HYSTEROSCOPY, SURGICAL; W/LYSIS INTRAUTERINE ADHES HYSTEROSCOPY, SURGICAL; W/DIVISION/RESECTION INTRA HYSTEROSCOPY, SURGICAL; W/REMOVAL LEIOMYOMATA HYSTEROSCOPY, SURGICAL, W/REMOVAL IMPACTED FB HYSTEROSCOPY, SURGICAL; W/ENDOMETRIAL ABLATION HYSTEROSCOPY, ABLATION LAPAROSCOPY W TOTAL HYSTERECTOMY UTERUS 250 G/< LAPS TOTAL HYSTERECTOMY 250 G/< W TUBE/OVARY LAPAROSCOPY TOTAL HYSTERECTOMY UTERUS > 250 G LAPAROSCOPY TOT HYSTERECTOMY > 250 G W TUBE/OVAR UNLISTED PROC, LAPAROSCOPY, UTERUS UNLISTED PROC, HYSTEROSCOPY, UTERUS LIGATION OR TRANSECTION OF FALLOPIAN TUBE(S), ABDO LIGATION/TRANSECTION, FALLOPIAN TUBE, ABD/VAGINAL LIGATION/TRANSECTION, FALLOPIAN TUBE W/C-SECTION/S OCCLUSION OF FALLOPIAN TUBE(S) BY DEVICE (EG, BAND LAPAROSCOPY, SURGICAL; W/LYSIS, ADHESIONS (SALPING LAPAROSCOPY, SURGICAL; W/REMOVAL, ADNEXAL STRUCTUR LAPAROSCOPY, SURGICAL; W/FULGURATION/EXCISION, LES LAPAROSCOPY, SURGICAL; W/FULGURATION OF OVIDUCTS W LAPAROSCOPY, SURGICAL; W/OCCLUSION, OVIDUCTS BY DE LAPAROSCOPY, SURGICAL; W/FIMBRIOPLASTY LAPAROSCOPY, SURGICAL; W/SALPINOSTOMY UNLISTED PROC, LAPAROSCOPY, OVIDUCT/OVARY SALPINGECTOMY, COMPLETE/PARTIAL, UNILAT/BILAT (SEP SALPINGO-OOPHORECTOMY, COMPLETE/PARTIAL, UNILAT/BI LYSIS, ADHESIONS (SALPINGOLYSIS, OVARIOLYSIS) TUBOTUBAL ANASTOMOSIS TUBOUTERINE IMPLANTATION FIMBRIOPLASTY SALPINGOSTOMY (SALPINGONEOSTOMY) DRAINAGE, OVARIAN CYST(S), UNILAT/BILAT, (SEP PROC DRAINAGE, OVARIAN CYST(S), UNILAT/BILAT, (SEP PROC DRAINAGE, OVARIAN ABSCESS; VAGINAL APPROACH, OPEN DRAINAGE, OVARIAN ABSCESS; ABDOMINAL APPROACH TRANSPOSITION, OVARY(S) BX, OVARY, UNILAT/BILAT (SEP PROC) WEDGE RESECTION/BISECTION, OVARY, UNILAT/BILAT OVARIAN CYSTECTOMY, UNILAT/BILAT OOPHORECTOMY, PARTIAL/TOTAL, UNILAT/BILAT OOPHORECTOMY, PARTIAL/TOTAL, OVARIAN/TUBAL/PRIMARY RESECTION, OVARIAN/TUBAL/PRIMARY MALIGNANCY W/BSO RESECTION, OVARIAN/TUBAL/PRIMARY MALIGNANCY W/BSO RESECTION, OVARIAN/TUBAL/PRIMARY MALIGNANCY W/BSO BILAT SALPINGO-OOPHORECT W/OMENTECT, TOTAL ABDOM H BILAT SALPING-OOPHOREC W/OMENTEC, TL ABD HYST AND BILATERAL SALPINGO-OOPHORESCTOMY WITH TOTAL OMENTE Resect recurrent gyn mal Resect recur gyn mal w/lym LAPAROTOMY, STAGING/RESTAGING, OVARIAN/TUBAL/PRIMA FOLLICLE PUNCTURE, OOCYTE RETRIEVAL, ANY METHOD EMBRYO TRANSFER, INTRAUTERINE GAMETE, ZYGOTE/EMBRYO INTRAFALLOPIAN TRANSFER, ANY UNLISTED PROC, FEMALE GENITAL SYSTEM (NONOBSTETRIC AMNIOCENTESIS; DIAGNOSTIC AMNIOCENTESIS; THERAPEUTIC AMNIOTIC FLUID REDUCTIO CORDOCENTESIS (INTRAUTERINE), ANY METHOD CHORIONIC VILLUS SAMPLING, ANY METHOD FETAL CONTRACTION STRESS TEST FETAL NON-STRESS TEST FETAL SCALP BLOOD SAMPLING FETAL MONITORING IN LABOR, PHYSICIAN W/WRITTEN REP FETAL MONITORING IN LABOR, PHYSICIAN W/WRITTEN REP TRANSABDOMINAL AMNIOINFUSION, W/US GUIDANCE FETAL UMBILICAL CORD OCCLUSION, W/US GUIDANCE FETAL FLUID DRAINAGE, W/US GUIDANCE FETAL SHUNT PLACEMENT, W/US GUIDANCE HYSTEROTOMY, ABDOMINAL SURGICAL TREATMENT, ECTOPIC PREGNANCY; TUBAL/OVARI SURGICAL TREATMENT, ECTOPIC PREGNANCY; TUBAL/OVARI SURGICAL TREATMENT, ECTOPIC PREGNANCY; ABDOMINAL P SURGICAL TREATMENT, ECTOPIC PREGNANCY; INTERSTITIA SURGICAL TREATMENT, ECTOPIC PREGNANCY; INTERSTITIA SURGICAL TREATMENT, ECTOPIC PREGNANCY; CERVICAL, W LAPAROSCOPIC TREATMENT, ECTOPIC PREGNANCY; W/O SAL LAPAROSCOPIC TREATMENT, ECTOPIC PREGNANCY; W/SALPI CURETTAGE, POSTPARTUM INSERTION, CERVICAL DILATOR (SEP PROC) EPISIOTOMY/VAG RPR OTH/THN ATTENDING CERCLAGE, CERVIX, DURING PREGNANCY; VAGINAL CERCLAGE, CERVIX, DURING PREGNANCY; ABDOMINAL HYSTERORRHAPHY, RUPTURED UTERUS ROUTINE OBSTETRIC CARE, ANTEPARTUM CARE, VAGINAL D VAGINAL DELIVERY ONLY (W/WO EPISIOTOMY AND/OR FORC VAGINAL DELIVERY ONLY (W/WO EPISIOTOMY AND/OR FORC EXT CEPHALIC VERSION, W/WO TOCOLYSIS DELIVERY, PLACENTA (SEP PROC) ANTEPARTUM CARE ONLY; 4 TO 6 VISITS 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 59426 59430 59510 59514 59515 59525 59610 59612 59614 59618 59620 59622 59812 59820 59821 59830 59840 59841 59850 59851 59852 59855 59856 59857 59866 59870 59871 59897 59898 59899 60000 6005F 60100 60200 60210 60212 60220 60225 60240 60252 60254 60260 60270 60271 60280 60281 60300 6030F 6040F 6045F 60500 60502 60505 60512 60520 60521 60522 60540 60545 60600 60605 60650 60659 60699 6070F 6080F 6090F 61000 61001 6100F 6101F 61020 61026 6102F 61050 61055 61070 61105 61107 61108 6110F 61120 61140 61150 61151 61154 61156 61210 61215 61250 61253 ANTEPARTUM CARE ONLY; 7 (PLUS) VISITS POSTPARTUM CARE ONLY (SEP PROC) ROUTINE OBSTETRIC CARE W/ANTEPARTUM CARE, CESAREAN CESAREAN DELIVERY ONLY CESAREAN DELIVERY ONLY; W/POSTPARTUM CARE SUBTOTAL/TOTAL HYSTERECTOMY AFTER CESAREAN DELIVER ROUTINE OBSTETRIC CARE, VAGINAL DELIVERY, W/ANTEPA VAGINAL DELIVERY ONLY, PREVIOUS CESAREAN DELIVERY VAGINAL DELIVERY ONLY, PREVIOUS CESAREAN DELIVERY; ROUTINE OB CARE, ANTE/POSTPARTUM, CESAREAN DELIVER CESAREAN DELIVERY, AFTER FAILED VAGINAL DELIVERY, CESAREAN DELIVERY, AFTER FAILED VAGINAL DELIVERY, TREATMENT, INCOMPLETE ABORTION, ANY TRIMESTER, COM TREATMENT, MISSED ABORTION, COMPLETED SURGICALLY; TREATMENT, MISSED ABORTION, COMPLETED SURGICALLY; TREATMENT, SEPTIC ABORTION, COMPLETED SURGICALLY INDUCED ABORTION, DILATION AND CURETTAGE INDUCED ABORTION, DILATION AND EVACUATION INDUCED ABORTION, INTRA-AMNIOTIC INJECTIONS W/HOSP INDUCED ABORTION, INTRA-AMNIOTIC INJECTIONS W/HOSP INDUCED ABORTION, INTRA-AMNIOTIC INJECTIONS W/HOSP INDUCED ABORTION, VAGINAL SUPPOSITORIES W/HOSPITAL INDUCED ABORTION, VAGINAL SUPPOSITORIES W/HOSPITAL INDUCED ABORTION, VAGINAL SUPPOSITORIES W/HOSPITAL MULTIFETAL PREGNANCY REDUCTION(S) UTERINE EVACUATION AND CURETTAGE, HYDATIDIFORM MO REMOVAL, CERCLAGE SUTURE UNDER ANESTHESIA (OTHER T UNLISTED FETAL INVASIVE PROCEDURE, W/US GUIDANCE UNLISTED PROC, LAPAROSCOPY, MATERNITY CARE AND DEL UNLISTED PROC, MATERNITY CARE AND DELIVERY INCISION AND DRAINAGE, THYROGLOSSAL CYST, INFECTED Care level rationale doc BX THYROID, PERCUTANEOUS CORE NEEDLE EXCISION, CYST/ADENOMA, THYROID/TRANSECTION, ISTHM PARTIAL THYROID LOBECTOMY, UNILAT; W/WO ISTHMUSECT PARTIAL THYROID LOBECTOMY, UNILAT; W/CONTRALATERAL TOTAL THYROID LOBECTOMY, UNILAT; W/WO ISTHMUSECTOM TOTAL THYROID LOBECTOMY, UNILAT; W/CONTRALATERAL S THYROIDECTOMY, TOTAL/COMPLETE THYROIDECTOMY, TOTAL/SUBTOTAL, MALIGNANCY; W/LIMIT THYROIDECTOMY, TOTAL/SUBTOTAL, MALIGNANCY; W/RADIC THYROIDECTOMY, REMOVAL REMAINING TISSUE, FOLLOWING THYROIDECTOMY, W/SUBSTERNAL THYROID; STERNAL SPLIT THYROIDECTOMY, W/SUBSTERNAL THYROID; CERVICAL APPR EXCISION, THYROGLOSSAL DUCT CYST/SINUS EXCISION, THYROGLOSSAL DUCT CYST/SINUS; RECURRENT ASPIRATION AND/OR INJECTION THYROID CYST MAX STERILE BARRIERS FOLLWD APPRO RAD DS DVCS TECHS DOCD RADXPS IN END RPRT4FLURO PXD PARATHYROIDECTOMY/EXPLORATION, PARATHYROID(S) PARATHYROIDECTOMY/EXPLORATION, PARATHYROID(S); REPARATHYROIDECTOMY/EXPLORATION, PARATHYROID(S); W/M PARATHYROID AUTOTRANSPLANTATION THYMECTOMY, PART/TOTAL; TRANSCERVICAL APPROACH (SE THYMECTOMY, PART/TOTAL; STERNAL SPLIT/TRANSTHORACI THYMECTOMY, PART/TOTAL; STERNAL SPLIT/TRANSTHORACI ADRENALECTOMY/EXPLORATION, ADRENAL GLAND, W/WO BX ADRENALECTOMY/EXPLORATION, ADRENAL GLAND, W/WO BX EXCISION, CAROTID BODY TUMOR; W/O EXCISION, CAROTI EXCISION, CAROTID BODY TUMOR; W/EXCISION, CAROTID LAPAROSCOPY, SURGICAL, W/TRANSABD PARTL/COMPLETE A UNLISTED PROC, LAPAROSCOPY, SURGICAL, ENDOCRINE SY UNLISTED PROC, ENDOCRINE SYSTEM PATIENT QUERIED COUNSELED RE AED SIDE EFFECTS PATIENT QUERIED ABOUT FALLS PT-CAREGIVER COUNSELED RE SAFETY ISSUES SUBDURAL TAP THROUGH FONTANELLE/SUTURE, INFANT, UN SUBDURAL TAP THROUGH FONTANELLE/SUTURE, INFANT, UN VERIFY CORRECT PT SITE PXD DOCUMENTED SAFTETY COUNSELING FOR DEMENTIA PROVIDED (DEM) VENTRICULAR PUNCTURE, PREVIOUS BURR HOLE; W/O INJE VENTRICULAR PUNCTURE, PREVIOUS BURR HOLE; W/INJECT SAFETY COUNSELING FOR DEMENTIA ORDERED (DEM) CISTERNAL/LATERAL (C1-C2) CERVICAL PUNCTURE; W/O I CISTERNAL/LATERAL C1-C2 PUNCTURE W/INJECTION PUNCTURE, SHUNT TUBING/RESERVOIR, ASPIRATION/INJEC TWIST DRILL HOLE, SUBDURAL/VENTRICULAR PUNCTURE TWIST DRILL HOLE, SUBDURAL/VENTRICULAR PUNCTURE; I TWIST DRILL HOLE, SUBDURAL/VENTRICULAR PUNCTURE; E COUNSELING PROVIDED REGARDING RISKS OF DRIVING BURR HOLE(S), VENTRICULAR PUNCTURE W/INJECTION BURR HOLE(S)/TREPHINE; W/BX, BRAIN/INTRACRANIAL LE BURR HOLE(S)/TREPHINE; W/DRAINAGE, BRAIN ABSCESS/C BURR HOLE(S)/TREPHINE; W/SUBSEQUENT TAPPING (ASPIR BURR HOLE(S) W/EVACUATION AND/OR DRAINAGE, HEMATOM BURR HOLE(S); W/ASPIRATION, HEMATOMA/CYST, INTRACE BURR HOLE(S); IMPLANTING VENTRICULAR CATHETER/RESE INSERTION, SUBQ RESERVOIR/PUMP/INFUSION SYSTEM, VE BUR HOLE(S)/TREPHINE, SUPRATENTORIAL, EXPLORATORY, BUR HOLE(S)/TREPHINE, INFRATENTORIAL, UNILAT/BILAT 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 4/1/2013 10/1/2010 1/1/2004 1/1/2004 7/1/2011 1/1/2012 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 61304 61305 61312 61313 61314 61315 61316 61320 61321 61322 61323 61330 61332 61333 61340 61343 61345 61450 61458 61460 61480 61500 61501 6150F 61510 61512 61514 61516 61517 61518 61519 61520 61521 61522 61524 61526 61530 61531 61533 61534 61535 61536 61537 61538 61539 61540 61541 61543 61544 61545 61546 61548 61550 61552 61556 61557 61558 61559 61563 61564 61566 61567 61570 61571 61575 61576 61580 61581 61582 61583 61584 61585 61586 61590 61591 61592 61595 61596 61597 61598 61600 61601 61605 61606 61607 61608 61610 61611 61612 61613 61615 CRANIECTOMY/CRANIOTOMY, EXPLORATORY; SUPRATENTORIA CRANIECTOMY/CRANIOTOMY, EXPLORATORY; INFRATENTORIA CRANIECTOMY/CRANIOTOMY, EVACUATION, HEMATOMA, SUPR CRANIECTOMY/CRANIOTOMY, EVACUATION, HEMATOMA, SUPR CRANIECTOMY/CRANIOTOMY, EVACUATION, HEMATOMA, INFR CRANIECTOMY/CRANIOTOMY, EVACUATION, HEMATOMA, INFR INCISION AND SUBQ PLACEMENT, CRANIAL BONE GRAFT CRANIECTOMY/CRANIOTOMY, DRAINAGE, INTRACRANIAL ABS CRANIECTOMY/CRANIOTOMY, DRAINAGE, INTRACRANIAL ABS CRANIECTOMY/CRANIOTOMY, DECOMPRES, W/DURAPLASTY, W CRANIECTOMY/CRANIOTOMY, DECOMPRES, W/WO DURAPLASTY DECOMPRESSION, ORBIT ONLY, TRANSCRANIAL APPROACH EXPLORATION, ORBIT (TRANSCRANIAL APPROACH); W/BX EXPLORATION, ORBIT (TRANSCRANIAL APPROACH); W/REMO SUBTEMPORAL CRANIAL DECOMPRESSION (PSEUDOTUMOR CER CRANIECTOMY, SUBOCCIPITAL W/CERVICAL LAMINECTOMY, OTHER CRANIAL DECOMPRESSION, POSTERIOR FOSSA CRANIECTOMY, SUBTEMPORAL, SECTION/COMPRESSION/DECO CRANIECTOMY, SUBOCCIPITAL; EXPLORATION/DECOMPRESSI CRANIECTOMY, SUBOCCIPITAL; SECTION, OVER 1 CRANIAL CRANIECTOMY, SUBOCCIPITAL; MESENCEPHALIC TRACTOTOM CRANIECTOMY; W/EXCISION, TUMOR/OTHER BONE LESION, CRANIECTOMY; OSTEOMYELITIS PT NOT RCVNG 1ST COURSE OF ANTI-TNF THERAPY CRANIECTOMY, TREPHINATION, BONE FLAP CRANIOTOMY; E CRANIECTOMY, TREPHINATION, BONE FLAP CRANIOTOMY; E CRANIECTOMY, TREPHINATION, BONE FLAP CRANIOTOMY; E CRANIECTOMY, TREPHINATION, BONE FLAP CRANIOTOMY; E IMPLANTATION, BRAIN INTRACAVITARY CHEMOTHERAPY AGE CRANIECTOMY, EXCISION TUMOR, INFRATENTORIAL/POSTER CRANIECTOMY, EXCISION TUMOR, INFRATENTORIAL/POSTER CRANIECTOMY, EXCISION TUMOR, INFRATENTORIAL/POSTER CRANIECTOMY, EXCISION TUMOR, INFRATENTORIAL/POSTER CRANIECTOMY, INFRATENTORIAL/POSTERIOR FOSSA; EXCIS CRANIECTOMY, INFRATENTORIAL/POSTERIOR FOSSA; EXCIS CRANIECTOMY, CEREBELLOPONTINE ANGLE TUMOR CRANIECTOMY, CEREBELLOPONTINE ANGLE TUMOR; MIDDLE/ SUBDURAL IMPLANTATION, STRIP ELECTRODES, LONG TERM CRANIOTOMY W/ELEVATION, BONE FLAP; IMPLANTATION, E CRANIOTOMY W/ELEVATION, BONE FLAP; EXCISION, EPILE CRANIOTOMY W/ELEVATION, BONE FLAP;REMOVAL, EPIDUR/ CRANIOTOMY W/ELEVATION, BONE FLAP; EXCISION, CEREB CRANIOTOMY W/ELEVATION, BONE FLAP; LOBECTOMY, TEMP CRANIOTOMY W/ELEVATION, BONE FLAP; LOBECTOMY, TEMP CRANIOTOMY W/ELEVATION, BONE FLAP; LOBECTOMY EXCEP CRANIOTOMY W/ELEVATION, BONE FLAP; LOBECTOMY EXCEP CRANIOTOMY W/ELEVATION, BONE FLAP; TRANSECTION, CO CRANIOTOMY W/ELEVATION, BONE FLAP; PARTIAL/SUBTOTA CRANIOTOMY W/ELEVATION, BONE FLAP; EXCISION/COAGUL CRANIOTOMY W/ELEVATION, BONE FLAP; EXCISION, CRANI CRANIOTOMY, HYPOPHYSECTOMY/EXCISION, PITUITARY TUM HYPOPHYSECTOMY/EXCISION, PITUITARY TUMOR, TRANSNAS CRANIECTOMY, CRANIOSYNOSTOSIS; SINGLE CRANIAL SUTU CRANIECTOMY, CRANIOSYNOSTOSIS; MULTIPLE CRANIAL SU CRANIOTOMY, CRANIOSYNOSTOSIS; FRONTAL/PARIETAL BON CRANIOTOMY, CRANIOSYNOSTOSIS; BIFRONTAL BONE FLAP EXTENSIVE CRANIECTOMY, MULTIPLE CRANIAL SUTURE CRA EXTENSIVE CRANIECTOMY, MULTIPLE CRANIAL SUTURE CRA EXCISION, BENIGN TUMOR, CRANIAL BONE; W/O OPTIC NE EXCISION, BENIGN TUMOR, CRANIAL BONE; W/OPTIC NERV CRANIOTOMY W/ELEVATION, BONE FLAP; SELECTIVE AMYGD CRANIOTOMY W/ELEVATION, BONE FLAP; MULTIPLE SUBPIA CRANIECTOMY/CRANIOTOMY; W/EXCISION, FB, BRAIN CRANIECTOMY/CRANIOTOMY; W/TREATMENT, PENETRATING W TRANSORAL APPROACH TO SKULL BASE, BX/DECOMPRESSION TRANSORAL APPROACH TO SKULL BASE, BX/DECOMPRES/EXC CRANIOFACIAL APPROACH TO ANTERIOR CRANIAL FOSSA; E CRANIOFACIAL APPROACH TO ANTERIOR CRANIAL FOSSA; E CRANIOFACIAL APPROACH TO ANTERIOR CRANIAL FOSSA; E CRANIOFACIAL APPROACH TO ANT CRANIAL FOSSA; INTRAD ORBITOCRANIAL APPROACH TO ANTERIOR CRANIAL FOSSA; ORBITOCRANIAL APPROACH TO ANTERIOR CRANIAL FOSSA; BICORONAL TRANSZYGOMATIC AND/OR LEFORT I APPROACH, INFRATEMPORAL PRE-AURICULAR APPROACH TO MIDDLE CRA INFRATEMPORAL POST-AURICULAR APPROACH TO MIDDLE CR ORBITOCRANIAL ZYGOMATIC APPROACH TO MIDDLE CRANIAL TRANSTEMPORAL APPROACH TO POSTERIOR CRANIAL FOSSA/ TRANSCOCHLEAR APPROACH TO POSTERIOR CRANIAL FOSSA/ TRANSCONDYLAR APPROACH TO POSTERIOR CRANIAL FOSSA/ TRANSPETROSAL APPROACH TO POSTERIOR CRANIAL FOSSA/ RESECT/EXCISE, LESION, BASE, ANTERIOR CRANIAL FOSS RESECT/EXCISE, LESION, BASE, ANTERIOR CRANIAL FOSS RESECT/EXCISE, LESION, INFRATEMPORAL FOSSA/PARAPHA RESECT/EXCISE, LESION, INFRATEMPORAL FOSSA/PARAPHA RESECT/EXCISE, LESION, PARASELLAR AREA/CAVERNOUS S RESECT/EXCISE, LESION, PARASELLAR AREA/CAVERNOUS S TRNSXJ/LIG CAROTID ARTERY SINUS W/RPR ANAST/GRFT TRANSECTION/LIGATION, CAROTID ARTERY IN PETROUS CA TRANSECTION/LIGATION, CAROTID ARTERY IN PETROUS CA OBLITERATION, CAROTID ANEURYSM/AVM/CAROTID-CAVERNO RESECT/EXCISE, LESION, BASE POSTERIOR CRANIAL FOSS 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 61616 61618 61619 61623 61624 61626 61630 61635 61640 61641 61642 61645 61650 61651 61680 61682 61684 61686 61690 61692 61697 61698 61700 61702 61703 61705 61708 61710 61711 61720 61735 61750 61751 61760 61770 61781 61782 61783 61790 61791 61796 61797 61798 61799 61800 61850 61860 61863 61864 61867 61868 61870 61880 61885 61886 61888 62000 62005 62010 62100 62115 62117 62120 62121 62140 62141 62142 62143 62145 62146 62147 62148 62160 62161 62162 62163 62164 62165 62180 62190 62192 62194 62200 62201 62220 62223 62225 62230 62252 62256 62258 RESECT/EXCISE, LESION, BASE POSTERIOR CRANIAL FOSS SECONDARY REPAIR, CSF LEAK/CRANIAL FOSSA; FREE TIS SECONDARY REPAIR, CSF LEAK/CRANIAL FOSSA; LOCAL/RE ENDOVASC TEMP OCCLUSION, HEAD/NCK W/VESSEL CATH, B TRANSCATHETER PERM OCCLUSION/EMBOLIZATION, PERCUTA TRANSCATHETER PERM OCCLUSION/EMBOLIZATION, PERCUTA BALLOON ANGIOPLASTY, INTRACRANIAL, PERCUTANEOUS TRANSCATHETER PLACEMENT OF INTRAVASCULAR STENT, IN BALLOON DILATATION OF INTRA VASOSPASM PERCUTANEOUS BALLOON DILATATION OF INTRA VASOSPASM PERCUTANEOUS BALLOON DILATATION OF INTRA VASOSPASM PERCUTANEOUS PERCUTANEOUS ARTERIAL TRANSLUMINAL MECHANICAL THROMBECTOMY AND/OR INFUSION ENDOVASCULAR INTRACRANIAL PROLONGED ADMINISTRATION OF PHARMACOLOGIC AGENT( ENDOVASCULAR INTRACRANIAL PROLONGED ADMINISTRATION OF PHARMACOLOGIC AGENT( SURGERY, INTRACRANIAL ARTERIOVENOUS MALFORMATION; SURGERY, INTRACRANIAL ARTERIOVENOUS MALFORMATION; SURGERY, INTRACRANIAL ARTERIOVENOUS MALFORMATION; SURGERY, INTRACRANIAL ARTERIOVENOUS MALFORMATION; SURGERY, INTRACRANIAL ARTERIOVENOUS MALFORMATION; SURGERY, INTRACRANIAL ARTERIOVENOUS MALFORMATION; SURGERY, INTRACRANIAL ANEURYSM, COMPLEX, INTRACRAN SURGERY, INTRACRANIAL ANEURYSM, COMPLEX, INTRACRAN SURGERY, INTRACRANIAL ANEURYSM, SIMPLE, INTRACRANI SURGERY, SIMPLE INTRACRANIAL ANEURYSM, INTRACRANIA SURGERY, INTRACRANIAL ANEURYSM, CERVICAL APPROACH, SURGERY, ANEURYSM, VASCULAR MALFORMATION; OCCLUSIO SURGERY, ANEURYSM, VASCULAR MALFORMATION; INTRACRA SURGERY, ANEURYSM, VASCULAR MALFORMATION; INTRA-AR ANASTOMOSIS, ARTERIAL, EXTRACRANIAL-INTRACRANIAL A CREATION, LESION, STEREOTACTIC W/BURR HOLE(S), SIN CREATION, LESION, STEREOTACTIC W/BURR HOLE(S), SIN STEREOTACTIC BX, ASPIRATION/EXCISION, W/BURR HOLE( STEREOTACTIC BX/ASPIRATION/EXCISION, W/BURR HOLE(S STEREOTACTIC IMPLANTATION, DEPTH ELECTRODES, CEREB STEREOTACTIC LOCALIZATION, W/INSERTION, CATHETER/P STRTCTC CPTR ASSTD PX IDRL CRNL STRTCTC CPTR ASSTD PX XDRL CRNL STRTCTC CPTR ASSTD PX SPINAL CREATION, LESION, STEREOTACTIC, PERCUTANEOUS, NEUR CREATION, LESION, STEREOTACTIC, PERCUTANEOUS, NEUR STEREOTACTIC RADIOSURGERY 1 SIMPLE CRANIAL LES STRTCTC RADIOSURGERY EA ADDL CRANIAL LES SIMPLE STEREOTACTIC RADIOSURGERY 1 COMPLEX CRANIAL LES STRTCTC RADIOSURGERY EA ADDL CRANIAL LES COMPLEX APPL STRTCTC HEADFRAME STEREOTACTIC RADIOSURGERY TWIST DRILL/BURR HOLE(S), IMPLANTATION, NEUROSTIMU CRANIECTOMY/CRANIOTOMY, IMPLANTATION, NEUROSTIMULA STEREOTACTIC IMPLANT NEUROSTIM ELECTRODE ARRAY, SU STEREOTACTIC IMPLANT NEUROSTIM ELECTRODE ARRAY, SU STEREOTACTIC IMPLANT NEUROSTIM ELECTRODE ARRAY, SU STEREOTACTIC IMPLANT NEUROSTIM ELECTRODE ARRAY, SU CRNEC IMPLTJ NSTIM ELTRD CEREBELLAR CORTICAL REVISION/REMOVAL, INTRACRANIAL NEUROSTIMULATOR ELE SUBQ PLACEMENT CRANIAL NEUROSTIMULATOR PULSE GENER SUBQ PLACEMENT CRANIAL NEUROSTIMULATOR PULSE GENER REVISION/REMOVAL, CRANIAL NEUROSTIMULATOR PULSE GE ELEVATION, DEPRESSED SKULL FX; SIMPLE, EXTRADURAL ELEVATION, DEPRESSED SKULL FX; COMPOUND/COMMINUTED ELEVATION, DEPRESSED SKULL FX; W/REPAIR, DURA AND/ CRANIOTOMY, REPAIR, DURAL/CSF LEAK, W/SURGERY, RHI REDUCTION, CRANIOMEGALIC SKULL; NO BONE GRAFTS/CRA REDUCTION, CRANIOMEGALIC SKULL; W/CRANIOTOMY/RECON REPAIR, ENCEPHALOCELE, SKULL VAULT, W/CRANIOPLASTY CRANIOTOMY, REPAIR, ENCEPHALOCELE, SKULL BASE CRANIOPLASTY, SKULL DEFECT; UP TO 5 CM DIAMETER CRANIOPLASTY, SKULL DEFECT; GREATER THAN 5 CM DIAM REMOVAL, BONE FLAP/PROSTHETIC PLATE, SKULL REPLACEMENT, BONE FLAP/PROSTHETIC PLATE, SKULL CRANIOPLASTY, SKULL DEFECT W/REPARATIVE BRAIN SURG CRANIOPLASTY W/AUTOGRAFT (INCLUDES OBTAINING BONE CRANIOPLASTY W/AUTOGRAFT (INCLUDES OBTAINING BONE INCISION AND RETRIEVAL SUBQ CRANIAL BONE GRAFT FOR NEUROENDOSCOPY, INTRACRANIAL, PLACE/REPLACE VENTRI NEUROENDOSCOPY, INTRACRANIAL; W/O DISSECT ADHESION NEUROENDSCOPY, INTRACRANIAL; W/FENESTRATION/EXCISE NEUROENDOSCOPY, INTRACRANIAL; W/RETRIEVAL, FB NEUROENDOSCOPY, INTRACRANIAL; W/EXCISE BRAIN TUMOR NEUROENDOSCOPY, INTRACRANIAL; W/EXCISE, PITUITARY VENTRICULOCISTERNOSTOMY (TORKILDSEN TYPE OPERATION CREATION, SHUNT; SUBARACHNOID/SUBDURAL-ATRIAL, -JU CREATION, SHUNT; SUBARACHNOID/SUBDURAL-PERITONEAL, REPLACEMENT/IRRIGATION, SUBARACHNOID/SUBDURAL CATH VENTRICULOCISTERNOSTOMY, 3RD VENTRICLE VENTRICULOCISTERNOSTOMY, 3RD VENTRICLE; STEREOTACT CREATION, SHUNT; VENTRICULO-ATRIAL, -JUGULAR, -AUR CREATION, SHUNT; VENTRICULO-PERITONEAL, -PLEURAL, REPLACEMENT/IRRIGATION, VENTRICULAR CATHETER REPLACEMENT/REVISION, CSF SHUNT, OBSTRUCTED VALVE/ REPROGRAMMING, PROGRAMMABLE CSF SHUNT REMOVAL, COMPLETE CSF SHUNT SYSTEM; W/O REPLACEMEN REMOVAL, COMPLETE CSF SHUNT SYSTEM; W/REPLACEMENT 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 62263 62264 62267 62268 62269 62270 62272 62273 62280 62281 62282 62284 62287 62290 62291 62292 62294 62302 62303 62304 62305 62310 62311 62318 62319 62350 62351 62355 62360 62361 62362 62365 62367 62368 62369 62370 63001 63003 63005 63011 63012 63015 63016 63017 63020 63030 63035 63040 63042 63043 63044 63045 63046 63047 63048 63050 63051 63055 63056 63057 63064 63066 63075 63076 63077 63078 63081 63082 63085 63086 63087 63088 63090 63091 63101 63102 63103 63170 63172 63173 63180 63182 63185 63190 63191 63194 63195 63196 63197 63198 63199 LYSIS, PERQ, EPIDURAL ADHESIONS, SOLUTION INJECTIO LYSIS, PERQ, EPIDURAL ADHESIONS, SOLUTION INJECTIO PRQ ASPIR PULPOSUS/INTERVERTEBRAL DISC/PVRT TISS PERCUTANEOUS ASPIRATION, SPINAL CORD CYST/SYRINX BX, SPINAL CORD, PERCUTANEOUS NEEDLE SPINAL PUNCTURE, LUMBAR, DX SPINAL PUNCTURE, THERAPEUTIC, DRAINAGE, SPINAL FLU INJECTION, EPIDURAL, BLOOD/CLOT PATCH INJECTION/INFUSION NEUROLYTIC SUBSTANCE, W/WO THER INJECTION/INFUSION NEUROLYTIC SUBSTANCE, W/WO THER INJECTION/INFUSION NEUROLYTIC SUBSTANCE, W/WO THER INJECTION PROCEDURE MYELOGRAPHY/CT LUMBAR DCMPRN PERQ NUCLEUS PULPOSUS 1/> LEVELS LUMBAR INJECTION, DISKOGRAPHY, EACH LEVEL; LUMBAR INJECTION, DISKOGRAPHY, EACH LEVEL; CERVICAL/THORA INJECTION, CHEMONUCLEOLYSIS, W/DISKOGRAPHY, LUMBAR INJECTION, ARTERIAL, OCCLUSION, ARTERIOVENOUS MALF MYELOGRAPHY VIA LUMBAR INJECTION RS&I CERVICAL MYELOGRAPHY VIA LUMBAR INJECTION RS&I THORACIC MYELOGRAPHY VIA LUMBAR INJECT RS&I LUMBOSACRAL MYELOGRAPHY VIA LUMBAR INJECTION RS&I 2+ REGIONS NJX DX/THER SBST EPIDURAL/SUBRACH CERV/THORACIC NJX DX/THER SBST EPIDURAL/SUBARACH LUMBAR/SACRAL NJXS INFUS/BOLUS DX/SBST EDRL/SUBARACH CRV/THRC NJX INFUS/BOLUS DX/SBST EDRL/SUBARACH LUM/SACRAL IMPLANT/REVISN/REPOSITION INTRATHECAL/EPIDURAL CAT IMPLANT/REVISN/REPOSITION INTRATHECAL/EPIDURAL CAT REMOVAL, PREVIOUSLY IMPLANTED INTRATHECAL/EPIDURAL IMPLANTATION/REPLACE, DEVICE, INTRATHECAL/EPIDURAL IMPLANTATION/REPLACE, DEVICE, INTRATHECAL/EPIDURAL IMPLANTATION/REPLACE, DEVICE, INTRATHECAL/EPIDURAL REMOVAL, SUBQ RESERVOIR/PUMP ELECT ANLYS IMPLT ITHCL/EDRL PMP W/O REPRG/REFIL ELECTRONIC ANALYSIS, PROGRAMMABLE PUMP; W/REPROGRA ELECT ANLYS IMPLT ITHCL/EDRL PMP W/REPRG&REFILL ELEC ANLYS IMPLT ITHCL/EDRL PMP W/REPR PHYS/QHP LAMINECTOMY, W/O FACETECTOMY/FORAMINOTOMY/DISKECTO LAMINECTOMY, W/O FACETECTOMY/FORAMINOTOMY/DISKECTO LAMINECTOMY, W/O FACETECTOMY/FORAMINOTOMY/DISKECTO LAMINECTOMY, W/O FACETECTOMY/FORAMINOTOMY/DISKECTO LAMINECTOMY, W/REMOVAL, ABNORMAL FACETS, LUMBAR LAMINECTOMY, W/O FACETECTOMY/FORAMINOTOMY/DISKECTO LAMINECTOMY, W/O FACETECTOMY/FORAMINOTOMY/DISKECTO LAMINECTOMY W/O FACETECTOMY/FORAMINOTOMY/DISKECTOM LAMNOTMY INCL W/DCMPRSN NRV ROOT 1 INTRSPC CERVC LAMNOTMY INCL W/DCMPRSN NRV ROOT 1 INTRSPC LUMBR LAMNOTMY W/DCMPRSN NRV EACH ADDL CRVCL/LMBR LAMINOTOMY W/PARTL FACETECTMY/FORAMNOTMY/HERNIATED LAMINOTOMY W/PARTL FACETECTOMY/FORAMINOTOMY/HERNIA LAMINOTMY W/PARTL FACETECT/FORAMNOTMY/HERN DISKECT LAMINOTMY W/PARTL FACETECT/FORAMNOTMY/HERN DISKECT LAMINECTOMY, FACETECTOMY AND FORAMINOTOMY, 1 SEGME LAMINECTOMY, FACETECTOMY AND FORAMINOTOMY, 1 SEGME LAMINECTOMY, FACETECTOMY AND FORAMINOTOMY, 1 SEGME LAMINECTOMY, FACETECTOMY AND FORAMINOTOMY; ADDL SE LAMINOPLASTY, CERVICAL, WITH DECOMPRESSION , 2 OR LAMINOPLASTY, CERVICAL, WITH RECONSTRUCTION OF THE TRANSPEDICULAR APPROACH, 1 SEGMENT; THORACIC TRANSPEDICULAR APPROACH, 1 SEGMENT; LUMBAR (TRANSF TRANSPEDICULAR APPROACH, ADDL SEGMENT; THORACIC/LU COSTOVERTEBRAL APPROACH, THORACIC; 1 SEGMENT COSTOVERTEBRAL APPROACH, THORACIC; ADDL SEGMENT DISKECTOMY, ANTERIOR; CERVICAL, 1 INTERSPACE DISKECTOMY, ANTERIOR; CERVICAL, ADDL INTERSPACE DISKECTOMY, ANTERIOR; THORACIC, 1 INTERSPACE DISKECTOMY, ANTERIOR; THORACIC, ADDL INTERSPACE VERTEBRAL CORPECTOMY, ANTERIOR; CERVICAL, 1 SEGMEN VERTEBRAL CORPECTOMY, ANTERIOR; CERVICAL, ADDL SEG VERTEBRAL CORPECTOMY, TRANSTHORACIC; THORACIC, 1 S VERTEBRAL CORPECTOMY, TRANSTHORACIC; THORACIC, ADD VERTEBRAL CORPECTOMY, THORACOLUMBAR, LOWER THORACI VERTEBRAL CORPECTOMY, THORACOLUMBAR, LOWER THORACI VERTEBRAL CORPECTOMY, TRANSPERITONEAL/RETROPERITON VERTEBRAL CORPECTOMY, TRANS/RETROPERITONEAL, LOWER VERTEBRAL CORPECTOMY, LAT EXTRACAVITARY W/DECOMPRE VERTEBRAL CORPECTOMY, LAT EXTRACAVITARY W/DECOMPRE VERTEBRAL CORPECTOMY, LAT EXTRACAVITARY W/DECOMPRE LAMINECTOMY W/MYELOTOMY, CERVICAL, THORACIC/THORAC LAMINECTOMY W/DRAINAGE, INTRAMEDULLARY CYST/SYRINX LAMINECTOMY W/DRAINAGE, INTRAMEDULLARY CYST/SYRINX LAMINECTOMY/SECTION, DENTATE LIGAMENTS, CERVICAL; LAMINECTOMY/SECTION, DENTATE LIGAMENTS, CERVICAL; LAMINECTOMY, W/RHIZOTOMY; 1/2 SEGMENTS LAMINECTOMY, W/RHIZOTOMY; OVER 2 SEGMENTS LAMINECTOMY, W/SECTION, SPINAL ACCESSORY NERVE LAMINECTOMY, W/CORDOTOMY, W/SECTION, ONE SPINOTHAL LAMINECTOMY, W/CORDOTOMY, W/SECTION, ONE SPINOTHAL LAMINECTOMY, W/CORDOTOMY, W/SECTION, BOTH SPINOTHA LAMINECTOMY, W/CORDOTOMY, W/SECTION, BOTH SPINOTHA LAMINECTOMY, W/CORDOTOMY, W/SECTION, BOTH SPINOTHA LAMINECTOMY, W/CORDOTOMY, W/SECTION, BOTH SPINOTHA 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 63200 63250 63251 63252 63265 63266 63267 63268 63270 63271 63272 63273 63275 63276 63277 63278 63280 63281 63282 63283 63285 63286 63287 63290 63295 63300 63301 63302 63303 63304 63305 63306 63307 63308 63600 63610 63615 63620 63621 63650 63655 63661 63662 63663 63664 63685 63688 63700 63702 63704 63706 63707 63709 63710 63740 63741 63744 63746 64400 64402 64405 64408 64410 64412 64413 64415 64416 64417 64418 64420 64421 64425 64430 64435 64445 64446 64447 64448 64449 64450 64455 64461 64462 64463 64479 64480 64483 64484 64486 64487 64488 LAMINECTOMY, W/RELEASE, TETHERED SPINAL CORD, LUMB LAMINECTOMY, EXCISION/OCCLUSION, AVM, SPINAL CORD; LAMINECTOMY, EXCISION/OCCLUSION, AVM, SPINAL CORD; LAMINECTOMY, EXCISION/OCCLUSION, AVM, SPINAL CORD; LAMINECTOMY, EXCISION, NON-NEOPLASTIC LESION, EXTR LAMINECTOMY, EXCISION, NON-NEOPLASTIC LESION, EXTR LAMINECTOMY, EXCISION, NON-NEOPLASTIC LESION, EXTR LAMINECTOMY, EXCISION, NON-NEOPLASTIC LESION, EXTR LAMINECTOMY, EXCISION, INTRASPINAL LESION OTHER TH LAMINECTOMY, EXCISION, INTRASPINAL LESION OTHER TH LAMINECTOMY, EXCISION, INTRASPINAL LESION OTHER TH LAMINECTOMY, EXCISION, INTRASPINAL LESION OTHER TH LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; EX LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; EX LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; EX LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; EX LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; IN LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; IN LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; IN LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; IN LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; IN LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; IN LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; IN LAMINECTOMY, BX/EXCISION, INTRASPINAL NEOPLASM; EX OSTEOPLASTIC RECONSTRUCTION OF DORSAL SPINE ELEMEN VERTEBRAL CORPECTOMY, 1 SEGMENT; EXTRADURAL, CERVI VERTEBRAL CORPECTOMY, 1 SEGMENT; EXTRADURAL, THORA VERTEBRAL CORPECTOMY, 1 SEGMENT; EXTRADURAL, THORA VERTEBRAL CORPECTOMY, 1 SEGMENT; EXTRADURAL, LUMBA VERTEBRAL CORPECTOMY, 1 SEGMENT; INTRADURAL, CERVI VERTEBRAL CORPECTOMY, 1 SEGMENT; INTRADURAL, THORA VERTEBRAL CORPECTOMY, 1 SEGMENT; INTRADURAL, THORA VERTEBRAL CORPECTOMY, 1 SEGMENT; INTRADURAL, LUMBA VERTEBRAL CORPECTOMY, ADDL SEGMENT CREATION, LESION, SPINAL CORD, STEREOTACTIC, PERCU STEREOTACTIC STIMULATION, SPINAL CORD, PERCUTANEOU STEREOTACTIC BX, ASPIRATION/EXCISION, LESION, SPIN STEREOTACTIC RADIOSURGERY 1 SPINAL LESION STEREOTACTIC RADIOSURGERY EA ADDL SPINAL LESION PERCUTANEOUS IMPLANTATION, NEUROSTIMULATOR ELECTRO LAMINECTOMY, IMPLANTATION, NEUROSTIMULATOR ELECTRO RMVL SPINAL NSTIM ELTRD PRQ ARRAY INCL FLUOR RMVL SPINAL NSTIM ELTRD PLATE/PADDLE INCL FLUOR REVJ INCL RPLCMT NSTIM ELTRD PRQ RA INCL FLUOR REVJ INCL RPLCMT NSTIM ELTRD PLT/PDLE INCL FLUOR INCISION/PLACEMENT, SPINAL NEUROSTIMULATOR PULSE G REVISION/REMOVAL, IMPLANTED SPINAL NEUROSTIMULATOR REPAIR, MENINGOCELE; LESS THAN 5 CM DIAMETER REPAIR, MENINGOCELE; LARGER THAN 5 CM DIAMETER REPAIR, MYELOMENINGOCELE; LESS THAN 5 CM DIAMETER REPAIR, MYELOMENINGOCELE; GREATER 5 CM DIAMETER REPAIR, DURAL/CSF LEAK, NOT REQUIRING LAMINECTOMY REPAIR, DURAL/CSF LEAK, PSEUDOMENINGOCELE, W/LAMIN DURAL GRAFT, SPINAL CREATION, SHUNT, LUMBAR/SUBARACHNOID-PERITONEAL/PL CREATION, SHUNT, LUMBAR/SUBARACHNOID-PERITONEAL/PL REPLACEMENT, IRRIGATION/REVISION, LUMBOSUBARACHNOI REMOVAL, ENTIRE LUMBOSUBARACHNOID SHUNT SYSTEM W/O INJECTION, ANESTHETIC AGENT; TRIGEMINAL NERVE, ANY INJECTION, ANESTHETIC AGENT; FACIAL NERVE INJECTION, ANESTHETIC AGENT; GREATER OCCIPITAL NER INJECTION, ANESTHETIC AGENT; VAGUS NERVE INJECTION, ANESTHETIC AGENT; PHRENIC NERVE INJECTION, ANESTHETIC AGENT; SPINAL ACCESSORY NERV INJECTION, ANESTHETIC AGENT; CERVICAL PLEXUS INJECTION, ANESTHETIC AGENT; BRACHIAL PLEXUS, SING INJECTION, ANESTHETIC AGENT; BRACHIAL PLEXUS, CONT INJECTION, ANESTHETIC AGENT; AXILLARY NERVE INJECTION, ANESTHETIC AGENT; SUPRASCAPULAR NERVE INJECTION, ANESTHETIC AGENT; INTERCOSTAL NERVE, SI INJECTION, ANESTHETIC AGENT; INTERCOSTAL NERVES, M INJECTION, ANESTHETIC AGENT; ILIOINGUINAL, ILIOHYP INJECTION, ANESTHETIC AGENT; PUDENDAL NERVE INJECTION, ANESTHETIC AGENT; PARACERVICAL (UTERINE INJECTION, ANESTHETIC AGENT; SCIATIC NERVE, SINGLE INJECTION, ANESTHETIC AGENT; SCIATIC NERVE, CONT C INJECTION, ANESTHETIC AGENT; FEMORAL NERVE, SINGLE INJECTION, ANESTHETIC AGENT; FEMORAL NERVE, CONT C INJECTION, ANESTHETIC AGENT; LUMBAR PLEXUS, POSTER INJECTION, ANESTHETIC AGENT; OTHER PERIPHERAL NERV NJX ANES&/STEROID PLANTAR COMMON DIGITAL NERVE PARAVERTEBRAL BLOCK (PVB) (PARASPINOUS BLOCK), THORACIC; SINGLE INJECTION SITE (INC PARAVERTEBRAL BLOCK (PVB) (PARASPINOUS BLOCK), THORACIC; SECOND AND ANY ADDITION PARAVERTEBRAL BLOCK (PVB) (PARASPINOUS BLOCK), THORACIC; CONTINUOUS INFUSION BY NJX ANES&/STRD W/IMG TFRML EDRL CRV/THRC 1 LVL NJX ANES&/STRD W/IMG TFRML EDRL CRV/THRC EA LVL NJX ANES&/STRD W/IMG TFRML EDRL LMBR/SAC 1 LVL NJX ANES&/STRD W/IMG TFRML EDRL LMBR/SAC EA LVL TAP BLOCK UNILATERAL BY INJECTION(S) TAP BLOCK UNILATERAL BY CONTINUOUS INFUSION(S) TAP BLOCK BILATERAL BY INJECTION(S) 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2015 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 64489 64490 64491 64492 64493 64494 64495 64505 64508 64510 64517 64520 64530 64550 64553 64555 64561 64565 64566 64568 64569 64570 64575 64580 64581 64585 64590 64595 64600 64605 64610 64611 64612 64615 64616 64617 64620 64630 64632 64633 64634 64635 64636 64640 64642 64643 64644 64645 64646 64647 64650 64653 64680 64681 64702 64704 64708 64712 64713 64714 64716 64718 64719 64721 64722 64726 64727 64732 64734 64736 64738 64740 64742 64744 64746 64755 64760 64763 64766 64771 64772 64774 64776 64778 64782 64783 64784 64786 64787 64788 64790 TAP BLOCK BILATERAL BY CONTINUOUS INFUSION(S) NJX DX/THER AGT PVRT FACET JT CRV/THRC 1 LEVEL NJX DX/THER AGT PVRT FACET JT CRV/THRC 2ND LEVEL NJX DX/THER AGT PVRT FACET JT CRV/THRC 3+ LEVEL NJX DX/THER AGT PVRT FACET JT LMBR/SAC 1 LEVEL NJX DX/THER AGT PVRT FACET JT LMBR/SAC 2ND LEVEL NJX DX/THER AGT PVRT FACET JT LMBR/SAC 3+ LEVEL INJECTION, ANESTHETIC AGENT; SPHENOPALATINE GANGLI INJECTION, ANESTHETIC AGENT; CAROTID SINUS (SEP PR INJECTION, ANESTHETIC AGENT; STELLATE GANGLION (CE INJECTION, ANESTHETIC AGENT; SUPERIOR HYPOGASTRIC INJECTION, ANESTHETIC AGENT; LUMBAR/THORACIC (PARA INJECTION, ANESTHETIC AGENT; CELIAC PLEXUS, W/WO R APPLICATION, SURFACE (TRANSCUTANEOUS) NEUROSTIMULA PRQ IMPLTJ NEUROSTIMULATOR ELTRD CRANIAL NERVE PRQ IMPLTJ NEUROSTIMULATOR ELTRD PERIPHERAL NRV PRQ IMPLTJ NEUROSTIM ELTRD SACRAL NRVE W/IMAGING PRQ IMPLTJ NSTIM ELTRD NEUROMUSCULAR POST TIB NEUROSTIMULATION PRQ NEEDLE ELECTRODE INC IMPLTJ CRNL NRV NSTIM ELTRDS & PULSE GENER REVISION/REPLMT NSTIM CRNL ELTRDS REMOVAL CRNL NRV NSTIM ELTRDS & PULSE GENERATOR INC IMPLTJ PERIPH NERVE NEUROSTIMULATOR ELTRD INC IMPLTJ NSTIM ELTRD NEUROMUSCULAR INCISION FOR IMPLANTATION OF NEUROSTIMULATOR ELECT REVJ/RMVL PERIPHERAL NEUROSTIMULATOR ELECTRODE INCISION AND SUBQ PLACEMENT, PERIPHERAL NEUROSTIMU REVISION/REMOVAL, PERIPHERAL NEUROSTIMULATOR PULSE DESTRUCTION, NEUROLYTIC, TRIGEMINAL NERVE; SUPRAOR DESTRUCTION, NEUROLYTIC, TRIGEMINAL NERVE; 2ND AND DESTRUCTION, NEUROLYTIC, TRIGEMINAL NERVE; 2ND AND CHEMODENERV PAROTID&SUBMANDIBL SALIVARY GLNDS BI CHEMODNRVTJ MUSC MUSC INNERVATED FACIAL NRV UNIL CHEMODERVATE FACIAL/TRIGEM/CERV MUSC MIGRAINE CHEMODENERVATION MUSCLE NECK UNILAT FOR DYSTONIA CHEMODENERVATION MUSCLE LARYNX UNILAT W/EMG DESTRUCTION, NEUROLYTIC, INTERCOSTAL NERVE DESTRUCTION, NEUROLYTIC; PUDENDAL NERVE DSTRJ NEUROLYTIC PLANTAR COMMON DIGITAL NERVE DSTR NROLYTC AGNT PARVERTEB FCT SNGL CRVCL/THORA DSTR NROLYTC AGNT PARVERTEB FCT ADDL CRVCL/THORA DSTR NROLYTC AGNT PARVERTEB FCT SNGL LMBR/SACRAL DSTR NROLYTC AGNT PARVERTEB FCT ADDL LMBR/SACRAL DESTRUCTION, NEUROLYTIC; OTHER PERIPHERAL NERVE/BR CHEMODENERVATION ONE EXTREMITY 1-4 MUSCLE CHEMODENERVATION 1 EXTREMITY EA ADDL 1-4 MUSCLE CHEMODENERVATION 1 EXTREMITY 5 OR MORE MUSCLES CHEMODENERVATION 1 EXTREMITY EA ADDL 5/> MUSCLES CHEMODENERVATION OF TRUNK MUSCLE 1-5 MUSCLES CHEMODENERVATION OF TRUNK 6 OR MORE MUSCLES CHEMODENERVATION OF ECCRINE GLANDS; BOTH AXILLAE CHEMODENERVATION OF ECCRINE GLANDS; OTHER AREA; PE DESTRUCTION, NEUROLYTIC AGENT, W/WO RADIOLOGIC MON DESTRUCTION, NEUROLYTIC AGENT, W/WO RADIOLOGIC MON NEUROPLASTY; DIGITAL, ONE/BOTH, SAME DIGIT NEUROPLASTY; NERVE, HAND/FOOT NEURP MAJOR PRPH NRV ARM/LEG OPN OTH/THN SPEC NEURP MAJOR PRPH NRV OPN ARM/LEG SCIATIC NRV NEURP MAJOR PRPH NRV OPN ARM/LEG BRACH PLEXUS NEURP MAJOR PRPH NRV OPN ARM/LEG LMBR PLEXUS NEUROPLASTY AND/OR TRANSPOSITION; CRANIAL NERVE (S NEUROPLASTY AND/OR TRANSPOSITION; ULNAR NERVE AT E NEUROPLASTY AND/OR TRANSPOSITION; ULNAR NERVE AT W NEUROPLASTY AND/OR TRANSPOSITION; MEDIAN NERVE AT DECOMPRESSION; UNSPECIFIED NERVE(S) (SPECIFY) DECOMPRESSION; PLANTAR DIGITAL NERVE INT NEUROLYSIS, W/MICROSCOPE TRANSECTION/AVULSION; SUPRAORBITAL NERVE TRANSECTION/AVULSION; INFRAORBITAL NERVE TRANSECTION/AVULSION; MENTAL NERVE TRANSECTION/AVULSION; INFERIOR ALVEOLAR NERVE, OST TRANSECTION/AVULSION; LINGUAL NERVE TRANSECTION/AVULSION; FACIAL NERVE, DIFFERENTIAL/C TRANSECTION/AVULSION; GREATER OCCIPITAL NERVE TRANSECTION/AVULSION; PHRENIC NERVE TRANSECTION/AVULSION; VAGUS NERVES, PROXIMAL STOMA TRANSECTION/AVULSION; VAGUS NERVE (VAGOTOMY), ABDO TRANSECTION/AVULSION, OBTURATOR NERVE, EXTRAPELVIC TRANSECTION/AVULSION, OBTURATOR NERVE, INTRAPELVIC TRANSECTION/AVULSION, OTHER CRANIAL NERVE, EXTRADU TRANSECTION/AVULSION, OTHER SPINAL NERVE, EXTRADUR EXCISION, NEUROMA; CUTANEOUS NERVE, SURGICALLY IDE EXCISION, NEUROMA; DIGITAL NERVE, ONE/BOTH, SAME D EXCISION, NEUROMA; DIGITAL NERVE, ADDL DIGIT EXCISION, NEUROMA; HAND/FOOT, EXCEPT DIGITAL NERVE EXCISION, NEUROMA; HAND/FOOT, ADDL NERVE, EXCEPT S EXCISION, NEUROMA; MAJOR PERIPHERAL NERVE, EXCEPT EXCISION, NEUROMA; SCIATIC NERVE IMPLANTATION, NERVE END INTO BONE/MUSCLE EXCISION, NEUROFIBROMA/NEUROLEMMOMA; CUTANEOUS NER EXCISION, NEUROFIBROMA/NEUROLEMMOMA; MAJOR PERIPHE 1/1/2015 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2013 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2009 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 64792 64795 64802 64804 64809 64818 64820 64821 64822 64823 64831 64832 64834 64835 64836 64837 64840 64856 64857 64858 64859 64861 64862 64864 64865 64866 64868 64872 64874 64876 64885 64886 64890 64891 64892 64893 64895 64896 64897 64898 64901 64902 64905 64907 64910 64911 64999 65091 65093 65101 65103 65105 65110 65112 65114 65125 65130 65135 65140 65150 65155 65175 65205 65210 65220 65222 65235 65260 65265 65270 65272 65273 65275 65280 65285 65286 65290 65400 65410 65420 65426 65430 65435 65436 65450 65600 65710 65730 65750 65755 65756 EXCISION, NEUROFIBROMA/NEUROLEMMOMA; EXTENSIVE (W/ BX, NERVE SYMPATHECTOMY, CERVICAL SYMPATHECTOMY, CERVICOTHORACIC SYMPATHECTOMY, THORACOLUMBAR SYMPATHECTOMY, LUMBAR SYMPATHECTOMY; DIGITAL ARTERIES, EACH DIGIT SYMPATHECTOMY; RADIAL ARTERY SYMPATHECTOMY; ULNAR ARTERY SYMPATHECTOMY; SUPERFICIAL PALMAR ARCH SUTURE, DIGITAL NERVE, HAND/FOOT; ONE NERVE SUTURE, DIGITAL NERVE, HAND/FOOT; ADDL DIGITAL NER SUTURE, 1 NERVE, HAND/FOOT; COMMON SENSORY NERVE SUTURE, 1 NERVE, HAND/FOOT; MEDIAN MOTOR THENAR SUTURE, 1 NERVE, HAND/FOOT; ULNAR MOTOR SUTURE, ADDL NERVE, HAND/FOOT SUTURE, POSTERIOR TIBIAL NERVE SUTURE, MAJOR PERIPHERAL NERVE, ARM/LEG, EXCEPT SC SUTURE, MAJOR PERIPHERAL NERVE, ARM/LEG, EXCEPT SC SUTURE, SCIATIC NERVE SUTURE, ADDL MAJOR PERIPHERAL NERVE SUTURE; BRACHIAL PLEXUS SUTURE; LUMBAR PLEXUS SUTURE, FACIAL NERVE; EXTRACRANIAL SUTURE, FACIAL NERVE; INFRATEMPORAL, W/WO GRAFTING ANASTOMOSIS; FACIAL-SPINAL ACCESSORY ANASTOMOSIS; FACIAL-HYPOGLOSSAL SUTURE, NERVE; W/SECONDARY/DELAYED SUTURE SUTURE, NERVE; W/EXTENSIVE MOBILIZATION/TRANSPOSIT SUTURE, NERVE; W/SHORTENING, BONE, EXTREMITY NERVE GRAFT (INCLUDES OBTAINING GRAFT), HEAD/NECK; NERVE GRAFT (INCLUDES OBTAINING GRAFT), HEAD/NECK; NERVE GRAFT (INCLUDES OBTAINING GRAFT), SINGLE STR NERVE GRAFT (INCLUDES OBTAINING GRAFT), SINGLE STR NERVE GRAFT (INCLUDES OBTAINING GRAFT), SINGLE STR NERVE GRAFT (INCLUDES OBTAINING GRAFT), SINGLE STR NERVE GRAFT (INCLUDES OBTAINING GRAFT), MULTIPLE S NERVE GRAFT (INCLUDES OBTAINING GRAFT), MULTIPLE S NERVE GRAFT (INCLUDES OBTAINING GRAFT), MULTIPLE S NERVE GRAFT (INCLUDES OBTAINING GRAFT), MULTIPLE S NERVE GRAFT (INCLUDES OBTAINING GRAFT), ADDL NERVE NERVE GRAFT (INCLUDES OBTAINING GRAFT), ADDL NERVE NERVE PEDICLE TRANSFER; 1ST STAGE NERVE PEDICLE TRANSFER; 2ND STAGE Nerve repair w/allograft Neurorraphy w/vein autograft UNLISTED PROC, NERVOUS SYSTEM EVISCERATION, OCULAR CONTENTS; W/O IMPLANT EVISCERATION, OCULAR CONTENTS; W/IMPLANT ENUCLEATION, EYE; W/O IMPLANT ENUCLEATION, EYE; W/IMPLANT, MUSCLES NOT ATTACHED ENUCLEATION, EYE; W/IMPLANT, MUSCLES ATTACHED TO I EXENTERATION, ORBIT (DOES NOT INCLUDE SKIN GRAFT), EXENTERATION, ORBIT (DOES NOT INCLUDE SKIN GRAFT); EXENTERATION, ORBIT (DOES NOT INCLUDE SKIN GRAFT); MODIFICATION, OCULAR IMPLANT W/PLACEMENT/REPLACEME INSERTION, OCULAR IMPLANT SECONDARY; AFTER EVISCER INSERTION, OCULAR IMPLANT SECONDARY; AFTER ENUCLEA INSERTION, OCULAR IMPLANT SECONDARY; AFTER ENUCLEA REINSERTION, OCULAR IMPLANT; W/WO CONJUNCTIVAL GRA REINSERTION, OCULAR IMPLANT; W/REINFORCEMENT AND/O REMOVAL, OCULAR IMPLANT REMOVAL, FB, EXT EYE; CONJUNCTIVAL SUPERFICIAL REMOVAL, FB, EXT EYE; CONJUNCTIVAL EMBEDDED/SUBCON REMOVAL, FB, EXT EYE; CORNEAL, W/O SLIT LAMP REMOVAL, FB, EXT EYE; CORNEAL, W/SLIT LAMP REMOVAL, FB, INTRAOCULAR; ANTERIOR CHAMBER/LENS REMOVAL, FB, INTRAOCULAR; POSTERIOR SEGMENT, MAGNE REMOVAL, FB, INTRAOCULAR; POSTERIOR SEGMENT, NONMA REPAIR, LACERATION; CONJUNCTIVA, W/WO LACERATED SC REPAIR, LACERATION; CONJUNCTIVA, MOBILIZATION AND REPAIR, LACERATION; CONJUNCTIVA, MOBILIZATION AND REPAIR, LACERATION; CORNEA, NONPERFORATING, W/WO R REPAIR, LACERATION; CORNEA AND/OR SCLERA, PERFORAT REPAIR, LACERATION; CORNEA AND/OR SCLERA, PERFORAT REPAIR, LACERATION; APPLICATION, TISSUE GLUE, WOUN REPAIR, WOUND, EXTRAOCULAR MUSCLE, TENDON AND/OR T EXCISION, LESION, CORNEA (KERATECTOMY, LAMELLAR, P BX, CORNEA EXCISION/TRANSPOSITION, PTERYGIUM; W/O GRAFT EXCISION/TRANSPOSITION, PTERYGIUM; W/GRAFT SCRAPING, CORNEA, DX, SMEAR AND/OR CULTURE REMOVAL, CORNEAL EPITHELIUM; W/WO CHEMOCAUTERIZATI REMOVAL, CORNEAL EPITHELIUM; W/APPLICATION, CHELAT DESTRUCTION, LESION, CORNEA, CRYOTHERAPY, PHOTOCOA MULTIPLE PUNCTURES, ANTERIOR CORNEA KERATOPLASTY (CORNEAL TRANSPLANT); LAMELLAR KERATOPLASTY (CORNEAL TRANSPLANT); PENETRATING (EX KERATOPLASTY (CORNEAL TRANSPLANT); PENETRATING (IN KERATOPLASTY (CORNEAL TRANSPLANT); PENETRATING (IN KERATOPLASTY ENDOTHELIAL 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 65757 65760 65765 65767 65770 65771 65772 65775 65778 65779 65780 65781 65782 65785 65800 65810 65815 65820 65850 65855 65860 65865 65870 65875 65880 65900 65920 65930 66020 66030 66130 66150 66155 66160 66170 66172 66174 66175 66179 66180 66183 66184 66185 66220 66225 66250 66500 66505 66600 66605 66625 66630 66635 66680 66682 66700 66710 66711 66720 66740 66761 66762 66770 66820 66821 66825 66830 66840 66850 66852 66920 66930 66940 66982 66983 66984 66985 66986 66990 66999 67005 67010 67015 67025 67027 67028 67030 67031 67036 67039 67040 BACKBENCH PREPJ CORNEAL ENDOTHELIAL ALLOGRAFT KERATOMILEUSIS KERATOPHAKIA EPIKERATOPLASTY KERATOPROSTHESIS RADIAL KERATOTOMY CORNEAL RELAXING INCISION, CORRECTION, SURGICALLY CORNEAL WEDGE RESECTION, CORRECTION, SURGICALLY IN PLACE AMNIOTIC MEMBRA OCULAR SURFACE W/O SUTURES PLACE AMNIOTIC MEMBRANE OCULAR SURFACE SUTURED OCULAR SURFACE RECONSTRUCTION AMNIOTIC MEMBRANE OCULAR SURFACE RECONSTRUCTION; LIMBAL STEM CELL AL OCULAR SURFACE RECONSTRUCTION; LIMBAL CONJUNCTIVAL IMPLANTATION OF INTRASTROMAL CORNEAL RING SEGMENTS PARACENTSIS ANT CHAMB EYE ASPIR AQUEOUS SPX PARACENTESIS, EYE, ANTERIOR CHAMBER (SEP PROC); W/ PARACENTESIS, EYE, ANTERIOR CHAMBER (SEP PROC); W/ GONIOTOMY TRABECULOTOMY AB EXTERNO TRABECULOPLASTY BY LASER SURGERY, 1 OR MORE SESSIO SEVERING ADHESIONS, ANTERIOR SEGMENT, LASER TECHNI SEVERING ADHESIONS, ANTERIOR SEGMENT, EYE, INCISIO SEVERING ADHESIONS, ANTERIOR SEGMENT, EYE, INCISIO SEVERING ADHESIONS, ANTERIOR SEGMENT, EYE, INCISIO SEVERING ADHESIONS, ANTERIOR SEGMENT, EYE, INCISIO REMOVAL, EPITHELIAL DOWNGROWTH, ANTERIOR CHAMBER, REMOVAL, IMPLANTED MATERIAL, ANTERIOR SEGMENT, EYE REMOVAL, BLOOD CLOT, ANTERIOR SEGMENT, EYE INJECTION, ANTERIOR CHAMBER, EYE (SEP PROC); AIR/L INJECTION, ANTERIOR CHAMBER, EYE (SEP PROC); MEDIC EXCISION, LESION, SCLERA FISTULIZATION, SCLERA, GLAUCOMA; TREPHINATION W/IR FISTULIZATION, SCLERA, GLAUCOMA; THERMOCAUTERIZATI FISTULIZATION, SCLERA, GLAUCOMA; SCLERECTOMY W/PUN FISTULIZATION, SCLERA, GLAUCOMA; TRABECULECTOMY AB FISTULIZATION, SCLERA, GLAUCOMA; TRABECULECTOMY AB TRLUML DILAT AQUEOUS CANAL W/O DEV/STNT TRLUML DILAT AQUEOUS CANAL W/DEV/STNT AQUEOUS SHUNT EXTRAOCULAR RESERVOIR W/O GRAFT AQUEOUS SHUNT EXTRAOC EQUAT PLATE RSVR W/GRAFT INSERT ANTER DRAINAGE DEV W/O EXTRAOC RESERVOIR REVJ SHUNT EXTRAOCULAR RESERVOIR W/O GRAFT REVJ AQUEOUS SHUNT EXTRAOCULAR RESERVOIR W/GRAFT REPAIR, SCLERAL STAPHYLOMA; W/O GRAFT REPAIR, SCLERAL STAPHYLOMA; W/GRAFT REVISION/REPAIR, OPERATIVE WOUND, ANTERIOR SEGMENT IRIDOTOMY, STAB INCISION (SEP PROC); EXCEPT TRANSF IRIDOTOMY, STAB INCISION (SEP PROC); W/TRANSFIXION IRIDECTOMY, W/CORNEOSCLERAL/CORNEAL SECTION; REMOV IRIDECTOMY, W/CORNEOSCLERAL/CORNEAL SECTION; W/CYC IRIDECTOMY, W/CORNEOSCLERAL/CORNEAL SECTION; PERIP IRIDECTOMY, W/CORNEOSCLERAL/CORNEAL SECTION; SECTO IRIDECTOMY, W/CORNEOSCLERAL/CORNEAL SECTION; OPTIC REPAIR, IRIS, CILIARY BODY (AS, IRIDODIALYSIS) SUTURE, IRIS, CILIARY BODY (SEP PROC) W/SUTURE RET CILIARY BODY DESTRUCTION; DIATHERMY CILIARY BODY DESTRUCTION; CYCLOPHOTOCOAGULATION DESTRUCTION, CILIARY BODY CILIARY BODY DESTRUCTION; CRYOTHERAPY CILIARY BODY DESTRUCTION; CYCLODIALYSIS IRIDOTOMY/IRDEC LASER SURG PER SESSION IRIDOPLASTY, PHOTOCOAGULATION (OVER 1 SESSIONS) DESTRUCTION, CYST/LESION IRIS/CILIARY BODY (NONEXC DISCISSION, SECONDARY MEMBRANOUS CATARACT; STAB IN DISCISSION, SECONDARY MEMBRANOUS CATARACT; LASER ( REPOSITIONING, INTRAOCULAR LENS PROSTHESIS, REQUIR REMOVAL, SECONDARY MEMBRANOUS CATARACT W/CORNEO-SC REMOVAL, LENS MATERIAL; ASPIRATION TECHNIQUE, OVER REMOVAL, LENS MATERIAL; PHACOFRAGMENTATION, W/ASPI REMOVAL, LENS MATERIAL; PARS PLANA APPROACH, W/WO REMOVAL, LENS MATERIAL; INTRACAPSULAR REMOVAL, LENS MATERIAL; INTRACAPSULAR, DISLOCATED REMOVAL, LENS MATERIAL; EXTRACAPSULAR (OTHER THAN EXTRACAPSULAR CATARACT REMOVAL W/INSERTION, LENS P INTRACAPSULAR CATARACT EXTRACTION W/INSERTION, LEN EXTRACAPSULAR CATARACT REMOVAL W/INSERTION, LENS P INSERTION, INTRAOCULAR LENS PROSTHESIS (SECONDARY EXCHANGE, INTRAOCULAR LENS OPHTHALMIC ENDOSCOPE USE UNLISTED PROC, ANTERIOR SEGMENT, EYE REMOVAL, VITREOUS, ANTERIOR APPROACH; PARTIAL REMO REMOVAL, VITREOUS, ANTERIOR APPROACH; SUBTOTAL REM ASPIRATION/RELEASE, VITREOUS/SUBRETINAL/CHOROIDAL INJECTION, VITREOUS SUBSTITUTE, PARS PLANA/LIMBAL IMPLANT, INTRAVITREAL DRUG DELIVERY SYSTEM W/REMOV INTRAVITREAL INJECTION, A PHARMACOLOGIC AGENT (SEP DISCISSION, VITREOUS STRANDS (W/O REMOVAL), PARS P SEVERING, VITREOUS STRANDS/FACE ADHESIONS/SHEETS/M VITRECTOMY, MECHANICAL, PARS PLANA APPROACH VITRECTOMY, MECHANICAL, PARS PLANA APPROACH; W/FOC VITRECTOMY, MECHANICAL, PARS PLANA APPROACH; W/END 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2015 1/1/2004 1/1/2014 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 67041 67042 67043 67101 67105 67107 67108 67110 67112 67113 67115 67120 67121 67141 67145 67208 67210 67218 67220 67221 67225 67227 67228 67229 67250 67255 67299 67311 67312 67314 67316 67318 67320 67331 67332 67334 67335 67340 67343 67345 67346 67399 67400 67405 67412 67413 67414 67415 67420 67430 67440 67445 67450 67500 67505 67515 67550 67560 67570 67599 67700 67710 67715 67800 67801 67805 67808 67810 67820 67825 67830 67835 67840 67850 67875 67880 67882 67900 67901 67902 67903 67904 67906 67908 67909 67911 67912 67914 67915 67916 67917 VITRECTOMY PARS PLANA REMOVE PRERETINAL MEMBRANE VITRECTOMY PARS PLANA REMOVE INT MEMB RETINA VITRECTOMY PARS PLANA REMOVE SUBRETINAL MEMBRANE REPAIR, RETINAL DETACHMENT, 1PLUS SESSIONS; CRYOTH REPAIR, RETINAL DETACHMENT, 1PLUS SESSIONS; PHOTOC REPAIR, RETINAL DETACHMENT; SCLERAL BUCKLING, W/WO REPAIR, RETINAL DETACHMENT; W/VITRECTOMY, ANY METH REPAIR, RETINAL DETACHMENT; INJECTION, AIR/OTHER G REPAIR, RETINAL DETACHMENT; SCLERAL BUCKLING/VITRE RPR COMPLEX RETINA DETACH VITRECTOMY & MEMB PEEL RELEASE, ENCIRCLING MATL (POSTERIOR SEGMENT) REMOVAL, IMPLANTED MATL, POSTERIOR SEGMENT; EXTRAO REMOVAL, IMPLANTED MATL, POSTERIOR SEGMENT; INTRAO PROPHYLAXIS, RETINAL DETACHMENT 1PLUS SESSIONS; CR PROPHYLAXIS, RETINAL DETACHMENT 1PLUS SESSIONS; PH DESTRUCTION, LOCALIZED RETINAL LESION, 1PLUS SESSI DESTRUCTION OF LOCALIZED LESION OF RETINA, 1 OR MO DESTRUCTION, LOCALIZED RETINAL LESION, 1PLUS SESSI DESTRUCTION, LOCALIZED LESION, CHOROID; PHOTOCOAGU DESTRUCTION, LOCALIZED LESION, CHOROID; PHOTODYNAM DESTRUCTION, LOCALIZED LESION, CHOROID; PHOTODYNAM DESTRUCTION, EXTENSIVE/PROGRESSIVE RETINOPATHY, 1P DESTRUCTION, EXTENSIVE/PROGRESSIVE RETINOPATHY, 1P TRMT EXTENSVE RETINOPATHY 1+ SESS PRETERM-1YR SCLERAL REINFORCEMENT (SEP PROC); W/O GRAFT SCLERAL REINFORCEMENT (SEP PROC); W/GRAFT UNLISTED PROC, POSTERIOR SEGMENT STRABISMUS SURGERY, RECESSION/RESECTION PROC; 1 HO STRABISMUS SURGERY, RECESSION/RESECTION PROC; 2 HO STRABISMUS SURGERY, RECESSION/RESECTION; 1 VERTICA STRABISMUS SURGERY, RECESSION/RESECTION; 2 PLUS VE STRABISMUS SURGERY, ANY PROC, SUPERIOR OBLIQUE MUS TRANSPOSITION PROC, ANY EXTRAOCULAR MUSCLE STRABISMUS SURGERY, PRIOR EYE SURGERY/INJURY NOT I STRABISMUS SURGERY, PRIOR SCARRING, EXTRAOCULAR MU STRABISMUS SURGERY, POSTERIOR FIXATION SUTURE, W/W PLACEMENT, ADJUSTABLE SUTURES DURING STRABISMUS SU STRABISMUS SURGERY, EXPLORATION AND/OR REPAIR, DET RELEASE, EXTENSIVE SCAR TISSUE W/O DETACHING EXTRA CHEMODENERVATION, EXTRAOCULAR MUSCLE Biopsy, eye muscle UNLISTED PROC, OCULAR MUSCLE ORBITOTOMY W/O BONE FLAP; EXPLORATION, W/WO BX ORBITOTOMY W/O BONE FLAP; W/DRAINAGE ONLY ORBITOTOMY W/O BONE FLAP; W/REMOVAL, LESION ORBITOTOMY W/O BONE FLAP; W/REMOVAL, FB ORBITOTOMY W/O BONE FLAP; W/REMOVAL, BONE, DECOMPR FINE NEEDLE ASPIRATION, ORBITAL CONTENTS ORBITOTOMY W/BONE FLAP/WINDOW, LATERAL APPROACH; W ORBITOTOMY W/BONE FLAP/WINDOW, LATERAL APPROACH; W ORBITOTOMY W/BONE FLAP/WINDOW, LATERAL APPROACH; W ORBITOTOMY W/BONE FLAP/WINDOW, LATERAL APPROACH; W ORBITOTOMY W/BONE FLAP/WINDOW, LATERAL APPROACH; E RETROBULBAR INJECTION; MEDICATION (SEP PROC, MEDIC RETROBULBAR INJECTION; ALCOHOL INJECTION, THERAPEUTIC AGENT INTO TENONS CAPSULE ORBITAL IMPLANT (OUTSIDE MUSCLE CONE); INSERTION ORBITAL IMPLANT (OUTSIDE MUSCLE CONE); REMOVAL/REV OPTIC NERVE DECOMPRESSION UNLISTED PROC, ORBIT BLEPHAROTOMY, DRAINAGE OF ABSCESS, EYELID SEVERING, TARSORRHAPHY CANTHOTOMY (SEP PROC) EXCISION, CHALAZION; SINGLE EXCISION OF CHALAZION; MULTIPLE, SAME LID EXCISION OF CHALAZION; MULTIPLE, DIFF LIDS EXCISION, CHALAZION; W/ANESTHESIA/HOSPITALIZATION, INCISIONAL BIOPSY EYELID SKIN & LID MARGIN CORRECTION, TRICHIASIS; EPILATION, FORCEPS ONLY CORRECTION, TRICHIASIS; EPILATION, NON-FORCEPS CORRECTION, TRICHIASIS; INCISION, LID MARGIN CORRECTION, TRICHIASIS; INCISION, LID MARGIN, W/FR EXCISION, LESION, EYELID (EXCEPT CHALAZION) W/O CL DESTRUCTION OF LESION OF LID MARGIN (UP TO 1 CM) TEMPORARY CLOSURE, EYELIDS, SUTURE CONSTRUCTION, INTERMARGINAL ADHESIONS, MEDIAN TARS CONSTRUCTION, INTERMARGINAL ADHESIONS/MEDIAN TARSO REPAIR, BROW PTOSIS, (SUPRACILIARY/MID-FOREHEAD/CO REPAIR, BLEPHAROPTOSIS; FRONTALIS MUSCLE TECHNIQUE REPAIR, BLEPHAROPTOSIS; FRONTALIS MUSCLE W/FASCIAL REPAIR, BLEPHAROPTOSIS; (TARSO) LEVATOR RESECTION/ REPAIR, BLEPHAROPTOSIS; (TARSO) LEVATOR RESECTION/ REPAIR, BLEPHAROPTOSIS; SUPERIOR RECTUS W/FASCIAL REPAIR, BLEPHAROPTOSIS; CONJUNCTIVO-TARSO-MULLERS REDUCTION, OVERCORRECTION, PTOSIS CORRECTION, LID RETRACTION CORRECTION, LAGOPHTHALMOS, W/IMPLANT, UPPER EYELID REPAIR, ECTROPION; SUTURE REPAIR, ECTROPION; THERMOCAUTERIZATION REPAIR, ECTROPION; EXCISION TARSAL WEDGE REPAIR, ECTROPION; EXTENSIVE 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 67921 67922 67923 67924 67930 67935 67938 67950 67961 67966 67971 67973 67974 67975 67999 68020 68040 68100 68110 68115 68130 68135 68200 68320 68325 68326 68328 68330 68335 68340 68360 68362 68371 68399 68400 68420 68440 68500 68505 68510 68520 68525 68530 68540 68550 68700 68705 68720 68745 68750 68760 68761 68770 68801 68810 68811 68815 68816 68840 68850 68899 69000 69005 69020 69090 69100 69105 69110 69120 69140 69145 69150 69155 69200 69205 69209 69210 69220 69222 69300 69310 69320 69399 69420 69421 69424 69433 69436 69440 69450 69501 REPAIR, ENTROPION; SUTURE REPAIR, ENTROPION; THERMOCAUTERIZATION REPAIR, ENTROPION; EXCISION TARSAL WEDGE REPAIR, ENTROPION; EXTENSIVE SUTURE, RECENT WOUND, EYELID; PARTIAL THICKNESS SUTURE, RECENT WOUND, EYELID; FULL THICKNESS REMOVAL, EMBEDDED FB, EYELID CANTHOPLASTY (RECONSTRUCTION, CANTHUS) EXCISION/REPAIR, EYELID; UP T0 ONE QUARTER, LID MA EXCISION/REPAIR, EYELID; OVER ONE QUARTER, LID MAR RECONSTRUCTION, EYELID, FULL THICKNESS; UP TO TWO RECONSTRUCTION, EYELID, FULL THICKNESS; TOTAL LID, RECONSTRUCTION, EYELID, FULL THICKNESS; TOTAL LID, RECONSTRUCTION, EYELID, FULL THICKNESS; 2ND STAGE UNLISTED PROC, EYELIDS INCISION, CONJUNCTIVA, DRAINAGE, CYST EXPRESSION, CONJUNCTIVAL FOLLICLES BX, CONJUNCTIVA EXCISION OF LESION, CONJUNCTIVA; UP TO 1 CM EXCISION, LESION, CONJUNCTIVA; OVER 1 CM EXCISION, LESION, CONJUNCTIVA; W/ADJACENT SCLERA DESTRUCTION, LESION, CONJUNCTIVA SUBCONJUNCTIVAL INJECTION CONJUNCTIVOPLASTY; W/CONJUNCTIVAL GRAFT/EXTENSIVE CONJUNCTIVOPLASTY; W/BUCCAL MUCOUS MEMBRANE GRAFT CONJUNCTIVOPLASTY, RECONSTRUCTION CUL-DE-SAC; W/CO CONJUNCTIVOPLASTY, RECONSTRUCTION CUL-DE-SAC; W/BU REPAIR, SYMBLEPHARON; CONJUNCTIVOPLASTY, W/O GRAFT REPAIR, SYMBLEPHARON; W/FREE GRAFT CONJUNCTIVA/BUC REPAIR, SYMBLEPHARON; DIVISION, SYMBLEPHARON CONJUNCTIVAL FLAP; BRIDGE/PARTIAL (SEP PROC) CONJUNCTIVAL FLAP; TOTAL, GUNDERSON THIN FLAP/PURS HARVESTING CONJUNCTIVAL ALLOGRAFT, LIVING DONOR UNLISTED PROC, CONJUNCTIVA INCISION, DRAINAGE, LACRIMAL GLAND INCISION, DRAINAGE, LACRIMAL SAC (DACRYOCYSTOTOMY/ SNIP INCISION, LACRIMAL PUNCTUM EXCISION, LACRIMAL GLAND (DACRYOADENECTOMY), EXCEP EXCISION, LACRIMAL GLAND (DACRYOADENECTOMY), EXCEP BX, LACRIMAL GLAND EXCISION, LACRIMAL SAC (DACRYOCYSTECTOMY) BX, LACRIMAL SAC REMOVAL OF FOREIGN BODY OR DACRYOLITH, LACRIMAL PA EXCISION, LACRIMAL GLAND TUMOR; FRONTAL APPROACH EXCISION, LACRIMAL GLAND TUMOR; INVOLVING OSTEOTOM PLASTIC REPAIR, CANALICULI CORRECTION, EVERTED PUNCTUM, CAUTERY DACRYOCYSTORHINOSTOMY (FISTULIZATION, LACRIMAL SAC CONJUNCTIVORHINOSTOMY; W/O TUBE CONJUNCTIVORHINOSTOMY; W/INSERTION, TUBE/STENT CLOSURE, LACRIMAL PUNCTUM; THERMOCAUTERIZATION/LIG CLOSURE, LACRIMAL PUNCTUM; PLUG, EACH CLOSURE, LACRIMAL FISTULA (SEP PROC) DILATION, LACRIMAL PUNCTUM, W/WO IRRIGATION PROBING, NASOLACRIMAL DUCT, W/WO IRRIGATION PROBING, NASOLACRIMAL DUCT, W/WO IRRIGATION; REQUI PROBING, NASOLACRIMAL DUCT, W/WO IRRIGATION; W/INS PROBE NASOLACRIMAL DUCT WITH CATHETER DILATION PROBING, LACRIMAL CANALICULI, W/WO IRRIGATION INJECTION, CONTRAST MEDIUM, DACRYOCYSTOGRAPHY UNLISTED PROC, LACRIMAL SYSTEM DRAINAGE EXT EAR, ABSCESS/HEMATOMA; SIMPLE DRAINAGE EXT EAR, ABSCESS/HEMATOMA; COMPLICATED DRAINAGE EXT AUDITORY CANAL, ABSCESS EAR PIERCING BX EXT EAR BX EXT AUDITORY CANAL EXCISION EXT EAR; PARTIAL, SIMPLE REPAIR EXCISION EXT EAR; COMPLETE AMPUTATION EXCISION EXOSTOSIS(ES), EXT AUDITORY CANAL EXCISION SOFT TISSUE LESION, EXT AUDITORY CANAL RADICAL EXCISION EXT AUDITORY CANAL LESION; W/O NE RADICAL EXCISION EXT AUDITORY CANAL LESION; W/NECK REMOVAL FB, EXT AUDITORY CANAL; W/O GENERAL ANESTH REMOVAL FB, EXT AUDITORY CANAL; W/GENERAL ANESTHES REMOVAL IMPACTED CERUMEN USING IRRIGATION/LAVAGE, UNILATERAL REMOVAL IMPACTED CERUMEN INSTRUMENTATION UNILAT DEBRIDEMENT, MASTOIDECTOMY CAVITY, SIMPLE DEBRIDEMENT, MASTOIDECTOMY CAVITY, COMPLEX (EG, W/ OTOPLASTY, PROTRUDING EAR, W/WO SIZE REDUCTION RECONSTRUCTION, EXT AUDITORY CANAL (SEP PROC) RECONSTRUCTION, EXT AUDITORY CANAL, CONGENITAL ATR UNLISTED PROC, EXT EAR MYRINGOTOMY W/ASPIRATION AND/OR EUSTACHIAN TUBE IN MYRINGOTOMY W/ASPIRATION AND/OR EUSTACHIAN TUBE IN VENTILATING TUBE REMOVAL REQUIRING GENERAL ANESTHE TYMPANOSTOMY (REQUIRING INSERTION, VENTILATING TUB TYMPANOSTOMY (REQUIRING INSERTION, VENTILATING TUB MIDDLE EAR EXPLORATION THROUGH POSTAURICULAR/EAR C TYMPANOLYSIS, TRANSCANAL TRANSMASTOID ANTROTOMY (SIMPLE MASTOIDECTOMY) 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 69502 69505 69511 69530 69535 69540 69550 69552 69554 69601 69602 69603 69604 69605 69610 69620 69631 69632 69633 69635 69636 69637 69641 69642 69643 69644 69645 69646 69650 69660 69661 69662 69666 69667 69670 69676 69700 69710 69711 69714 69715 69717 69718 69720 69725 69740 69745 69799 69801 69805 69806 69820 69840 69905 69910 69915 69930 69949 69950 69955 69960 69970 69979 69990 70010 70015 70030 70100 7010F 70110 70120 70130 70134 70140 70150 70160 70170 70190 70200 7020F 70210 70220 70240 70250 7025F 70260 70300 70310 70320 70328 70330 MASTOIDECTOMY; COMPLETE MASTOIDECTOMY; MODIFIED RADICAL MASTOIDECTOMY; RADICAL PETROUS APICECTOMY W/RADICAL MASTOIDECTOMY RESECTION TEMPORAL BONE, EXT APPROACH EXCISION AURAL POLYP EXCISION AURAL GLOMUS TUMOR; TRANSCANAL EXCISION AURAL GLOMUS TUMOR; TRANSMASTOID EXCISION AURAL GLOMUS TUMOR; EXTENDED (EXTRATEMPOR REVISION MASTOIDECTOMY; RESULTING IN COMPLETE MAST REVISION MASTOIDECTOMY; RESULTING IN MODIFIED RADI REVISION MASTOIDECTOMY; RESULTING IN RADICAL MASTO REVISION MASTOIDECTOMY; RESULTING IN TYMPANOPLASTY REVISION MASTOIDECTOMY; W/APICECTOMY TYMPANIC MEMBRANE REPAIR, W/WO SITE PREPARATION/PE MYRINGOPLASTY (SURGERY CONFINED TO DRUMHEAD AND DO TYMPANOPLASTY W/O MASTOIDECTOMY INITIAL/REVISION; TYMPANOPLASTY W/O MASTOIDECTOMY INITIAL/REVISION; TYMPANOPLASTY W/O MASTOIDECTOMY INITIAL/REVISION; TYMPANOPLASTY W/ANTROTOMY/MASTOIDOTOMY; W/O OSSICL TYMPANOPLASTY W/ANTROTOMY/MASTOIDOTOMY; W/OSSICLE TYMPANOPLASTY W/ANTROTOMY/MASTOIDOTOMY; W/OSSICLE TYMPANOPLASTY W/MASTOIDECTOMY; W/O OSSICLE RECONST TYMPANOPLASTY W/MASTOIDECTOMY; W/OSSICLE RECONSTRU TYMPANOPLASTY W/MASTOIDECTOMY; W/O OSSICLE RECONST TYMPANOPLASTY W/MASTOIDECTOMY; W/OSSICLE RECONSTRU TYMPANOPLASTY W/MASTOIDECTOMY; W/O OSSICLE RECONST TYMPANOPLASTY W/MASTOIDECTOMY; W/OSSICLE RECONSTRU STAPES MOBILIZATION STABEDECTOMY/STAPEDOTOMY, W/WO FOREIGN MATL STABEDECTOMY/STAPEDOTOMY, W/WO FOREIGN MATL; W/FOO REVISION, STAPEDECTOMY/STAPEDOTOMY REPAIR OVAL WINDOW FISTULA REPAIR ROUND WINDOW FISTULA MASTOID OBLITERATION (SEP PROC) TYMPANIC NEURECTOMY CLOSURE POSTAURICULAR FISTULA, MASTOID (SEP PROC) IMPLANTATION/REPLACEMENT, ELECTROMAGNETIC BONE CON REMOVAL/REPAIR, ELECTROMAGNETIC BONE CONDUCTION HE IMPLANTATION, OSSEOINTEGRATED IMPLANT TEMPORAL BON IMPLANTATION, OSSEOINTEGRATED IMPLANT, TEMPORAL BO REPLACEMENT, OSSEOINTEGRATED IMPLANT, TEMPORAL BON REPLACEMENT, OSSEOINTEGRATED IMPLANT, TEMPORAL BON DECOMPRESSION FACIAL NERVE, INTRATEMPORAL; LATERAL DECOMPRESSION FACIAL NERVE, INTRATEMPORAL; W/MEDIA SUTURE FACIAL NERVE, INTRATEMPORAL; LATERAL TO GEN SUTURE FACIAL NERVE, INTRATEMPORAL; MEDIAL TO GENI UNLISTED PROC, MIDDLE EAR LABYRINTHOTOMY TRANSCANAL ENDOLYMPHATIC SAC OPERATION; W/O SHUNT ENDOLYMPHATIC SAC OPERATION; W/SHUNT FENESTRATION SEMICIRCULAR CANAL REVISION FENESTRATION OPERATION LABYRINTHECTOMY; TRANSCANAL LABYRINTHECTOMY; W/MASTOIDECTOMY VESTIBULAR NERVE SECTION, TRANSLABYRINTHINE APPROA COCHLEAR DEVICE IMPLANTATION, W/WO MASTOIDECTOMY UNLISTED PROC, INNER EAR VESTIBULAR NERVE SECTION, TRANSCRANIAL APPROACH TOTAL FACIAL NERVE DECOMPRESSION AND/OR REPAIR, (M DECOMPRESSION INT AUDITORY CANAL REMOVAL, TUMOR, TEMPORAL BONE UNLISTED PROC, TEMPORAL BONE, MIDDLE FOSSA APPROAC MICROSURGICAL TECHNIQUES, REQUIRING USE OF OPERATI MYELOGRAPHY, POSTERIOR FOSSA, RADIOLOGICAL S AND I CISTERNOGRAPHY, POSITIVE CONTRAST, RADIOLOGICAL S RADIOLOGIC EXAM, EYE, DETECTION, FB RADIOLOGIC EXAM, MANDIBLE; PARTIAL, LESS THAN4 VIE PT INFO INTO RECALL SYSTEM RADIOLOGIC EXAM, MANDIBLE; COMPLETE, MINIMUM, 4 VI RADIOLOGIC EXAM, MASTOIDS; LESS THAN 3 VIEWS PER S RADIOLOGIC EXAM, MASTOIDS; COMPLETE, MINIMUM, 3 VI RADIOLOGIC EXAM, INT AUDITORY MEATI, COMPLETE RADIOLOGIC EXAM, FACIAL BONES; LESS THAN 3 VIEWS RADIOLOGIC EXAM, FACIAL BONES; COMPLETE, MINIMUM, RADIOLOGIC EXAM, NASAL BONES, COMPLETE, MINIMUM, 3 DACRYOCYSTOGRAPHY, NASOLACRIMAL DUCT, RADIOLOGICAL RADIOLOGIC EXAM; OPTIC FORAMINA RADIOLOGIC EXAM; ORBITS, COMPLETE, MINIMUM, 4 VIEW MAMMO ASSESSMENT CAT IN DATABASE FOR RATE RADIOLOGIC EXAM, SINUSES, PARANASAL, LESS THAN3 VI RADIOLOGIC EXAM, SINUSES, PARANASAL, COMPLETE, MIN RADIOLOGIC EXAM, SELLA TURCICA RADIOLOGIC EXAM, SKULL; LESS THAN 4 VIEWS PT INFOSYS ALARM 4 NXT MAMMO RADIOLOGIC EXAM, SKULL; COMPLETE, MINIMUM 4 VIEWS RADIOLOGIC EXAM, TEETH; SINGLE VIEW RADIOLOGIC EXAM, TEETH; PARTIAL EXAM, LESS THAN FU RADIOLOGIC EXAM, TEETH; COMPLETE, FULL MOUTH RADIOLOGIC EXAM, TEMPOROMANDIBULAR JOINT, OPEN AND RADIOLOGIC EXAM, TEMPOROMANDIBULAR JOINT, OPEN AND 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 70332 70336 70350 70355 70360 70370 70371 70373 70380 70390 70450 70460 70470 70480 70481 70482 70486 70487 70488 70490 70491 70492 70496 70498 70540 70542 70543 70544 70545 70546 70547 70548 70549 70551 70552 70553 70554 70555 70557 70558 70559 71010 71015 71020 71021 71022 71023 71030 71034 71035 71100 71101 71110 71111 71120 71130 71250 71260 71270 71275 71550 71551 71552 71555 72010 72020 72040 72050 72052 72069 72070 72072 72074 72080 72081 72082 72083 72084 72090 72100 72110 72114 72120 72125 72126 72127 72128 72129 72130 72131 72132 TEMPOROMANDIBULAR JOINT ARTHROGRAPHY, RADIOLOGICAL MRI, TEMPOROMANDIBULAR JOINTS CEPHALOGRAM, ORTHODONTIC ORTHOPANTOGRAM RADIOLOGIC EXAM; NECK, SOFT TISSUE RADIOLOGIC EXAM; PHARYNX/LARYNX, W/FLUOROSCOPY AND COMPLEX DYNAMIC PHARYNGEAL AND SPEECH EVALUATION, LARYNGOGRAPHY, CONTRAST, RADIOLOGICAL S AND I RADIOLOGIC EXAM, SALIVARY GLAND, CALCULUS SIALOGRAPHY, RADIOLOGICAL S AND I CT SCAN, HEAD/BRAIN; W/O CONTRAST MATL CT SCAN, HEAD/BRAIN; W/CONTRAST MATL(S) CT SCAN, HEAD/BRAIN; W/O CONTRAST MATL, THEN W/CON CT SCAN, ORBIT/SELLA/POSTERIOR FOSSA/OUTER, MIDDLE CT SCAN, ORBIT/SELLA/POSTERIOR FOSSA/OUTER, MIDDLE CT SCAN, ORBIT/SELLA/POSTERIOR FOSSA/OUTER, MIDDLE CT SCAN, MAXILLOFACIAL AREA; W/O CONTRAST MATL CT SCAN, MAXILLOFACIAL AREA; W/CONTRAST MATL(S) CT SCAN, MAXILLOFACIAL AREA; W/O CONTRAST MATL, TH CT SCAN, SOFT TISSUE NECK; W/O CONTRAST MATL CT SCAN, SOFT TISSUE NECK; W/CONTRAST MATL(S) CT SCAN, NECK TISSUE; W/O CONTRAST MATL, THEN W/CO CT ANGIOGRAPHY, HEAD, W/O CONTRAST MATL(S), FOLLOW CT ANGIOGRAPHY, NECK, W/O CONTRAST MATL(S), FOLLOW MRI, ORBIT, FACE, AND NECK; W/O CONTRAST MATL(S) MRI, ORBIT, FACE, AND NECK; W/CONTRAST MATL(S) MRI, ORBIT, FACE, AND NECK; W/O CONTRAST MATL(S), MRA, HEAD; W/O CONTRAST MATL(S) MRA, HEAD; W/CONTRAST MATL(S) MRA, HEAD; W/O CONTRAST MATL(S), FOLLOWED BY CONTR MRA, NECK; W/O CONTRAST MATL(S) MRA, NECK; W/CONTRAST MATL(S) MRA, NECK; W/O CONTRAST MATL(S), FOLLOWED BY CONTR MRI, BRAIN; W/O CONTRAST MATL MRI, BRAIN; W/CONTRAST MATL (S) MRI, BRAIN; W/O CONTRAST MATL, THEN W/CONTRASTMATL Fmri brain by tech Fmri brain by phys/psych MRI, BRAIN, DURING INTRACRANIAL PROCEDURE; W/O CON MRI, BRAIN, DURING INTRACRANIAL PROCEDURE; W/ CONT MRI, BRAIN, DURING INTRACRANIAL PROC; W/O CONTRST RADIOLOGIC EXAM, CHEST; SINGLE VIEW, FRONTAL RADIOLOGIC EXAM, CHEST; STEREO, FRONTAL RADIOLOGIC EXAM, CHEST, 2 VIEWS, FRONTAL AND LATER RADIOLOGIC EXAM, CHEST, 2 VIEWS, FRONTAL AND LATER RADIOLOGIC EXAM, CHEST, 2 VIEWS, FRONTAL AND LATER RADIOLOGIC EXAM, CHEST, 2 VIEWS, FRONTAL AND LATER RADIOLOGIC EXAM, CHEST, COMPLETE, MINIMUM, 4 VIEWS RADIOLOGIC EXAM, CHEST, COMPLETE, MINIMUM, 4 VIEWS RADIOLOGIC EXAM, CHEST, SPECIAL VIEWS RADIOLOGIC EXAM, RIBS, UNILAT; 2 VIEWS RADIOLOGIC EXAM, RIBS, UNILAT; W/POSTEROANTERIOR C RADIOLOGIC EXAM, RIBS, BILAT; 3 VIEWS RADIOLOGIC EXAM, RIBS, BILAT; W/POSTEROANTERIOR CH RADIOLOGIC EXAM; STERNUM, 2 PLUS VIEWS RADIOLOGIC EXAM; STERNOCLAVICULAR JOINT/JOINTS, 3 CT SCAN, THORAX; W/O CONTRAST MATL CT SCAN, THORAX; W/CONTRAST MATL(S) CT SCAN, THORAX; W/O CONTRAST MATL, THEN W/CONTRAS CT ANGIOGRAPHY, CHEST, W/O CONTRAST MATL(S), FOLLO MRI, CHEST; W/O CONTRAST MATL(S) MRI, CHEST; W/CONTRAST MATL(S) MRI, CHEST; W/O CONTRAST MATL(S), FOLLOWED BY CONT MRA, CHEST (EXCLUDE MYOCARDIUM), W/WO CONTRAST MAT RADIOLOGIC EXAM, SPINE, ENTIRE, SURVEY STUDY, ANTE RADIOLOGIC EXAM, SPINE, SINGLE VIEW, SPECIFY LEVEL RADEX SPINE CERVICAL 2 OR 3 VIEWS RADEX SPINE CERVICAL 4 OR 5 VIEWS RADEX SPINE CERVICAL 6 OR MORE VIEWS RADIOLOGIC EXAM, SPINE, THORACOLUMBAR, STANDING (S RADIOLOGIC EXAM, SPINE; THORACIC, 2 VIEWS RADIOLOGIC EXAM, SPINE; THORACIC, 3 VIEWS RADIOLOGIC EXAM, SPINE; THORACIC, OVER 4 VIEWS RADIOLOGIC EXAM, SPINE; THORACOLUMBAR, 2 VIEWS RADIOLOGIC EXAMINATION, SPINE, ENTIRE THORACIC AND LUMBAR, INCLUDING SKULL, CERV RADIOLOGIC EXAMINATION, SPINE, ENTIRE THORACIC AND LUMBAR, INCLUDING SKULL, CERV RADIOLOGIC EXAMINATION, SPINE, ENTIRE THORACIC AND LUMBAR, INCLUDING SKULL, CERV RADIOLOGIC EXAMINATION, SPINE, ENTIRE THORACIC AND LUMBAR, INCLUDING SKULL, CERV RADIOLOGIC EXAM, SPINE; SCOLIOSIS STUDY, W/SUPINE RADIOLOGIC EXAM, SPINE, LUMBOSACRAL; 2 OR 3 VIEWS RADIOLOGIC EXAM, SPINE, LUMBOSACRAL; OVER 4 VIEWS RADEX SPINE LUMBSCRL COMPL W/BENDING VIEWS MIN 6 RADEX SPINE LUMBOSACRAL ONLY BENDING 2/3 VIEWS CT SCAN, CERVICAL SPINE; W/O CONTRAST MATL CT SCAN, CERVICAL SPINE; W/CONTRAST MATL CT SCAN, CERVICAL SPINE; W/O CONTRAST MATL, THEN W CT SCAN, THORACIC SPINE; W/O CONTRAST MATL CT SCAN, THORACIC SPINE; W/CONTRAST MATL CT SCAN, THORACIC SPINE; W/O CONTRAST MATL, THEN W CT SCAN, LUMBAR SPINE; W/O CONTRAST MATL CT SCAN, LUMBAR SPINE; W/CONTRAST MATL 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N Y N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N Y Y Y N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 72133 72141 72142 72146 72147 72148 72149 72156 72157 72158 72159 72170 72190 72191 72192 72193 72194 72195 72196 72197 72198 72200 72202 72220 72240 72255 72265 72270 72275 72285 72295 73000 73010 73020 73030 73040 73050 73060 73070 73080 73085 73090 73092 73100 73110 73115 73120 73130 73140 73200 73201 73202 73206 73218 73219 73220 73221 73222 73223 73225 73500 73501 73502 73503 73510 73520 73521 73522 73523 73525 73530 73540 73550 73551 73552 73560 73562 73564 73565 73580 73590 73592 73600 73610 73615 73620 73630 73650 73660 73700 73701 CT SCAN, LUMBAR SPINE; W/O CONTRAST MATL, THEN W/C MRI, CERVICAL SPINE; W/O CONTRAST MATL MRI, CERVICAL SPINE; W/CONTRAST MATL(S) MRI, THORACIC SPINE; W/O CONTRAST MATL MRI, THORACIC SPINE; W/CONTRAST MATL(S) MRI, LUMBAR SPINE; W/O CONTRAST MATL MRI, LUMBAR SPINE; W/CONTRAST MATL(S) MRI, SPINE W/O CONTRAST MATL, THEN W/CONTRAST MATL MRI, SPINE W/O CONTRAST MATL, THEN W/CONTRAST MATL MRI, SPINE W/O CONTRAST MATL, THEN W/CONTRAST MATL MRA, SPINE W/WO CONTRAST MATL(S) RADIOLOGIC EXAM, PELVIS; 1 OR 2 VIEWS RADIOLOGIC EXAM, PELVIS; COMPLETE, OVER 3 VIEWS CT ANGIOGRAPHY, PELVIS, W/O CONTRAST MATL(S), FOLL CT SCAN, PELVIS; W/O CONTRAST MATL CT SCAN, PELVIS; W/CONTRAST MATL(S) CT SCAN, PELVIS; W/O CONTRAST MATL, THEN W/CONTRAS MRI, PELVIS; W/O CONTRAST MATL(S) MRI, PELVIS; W/CONTRAST MATL(S) MRI, PELVIS; W/O CONTRAST MATL(S), FOLLOWED BY CON MRA, PELVIS, W/WO CONTRAST MATL RADIOLOGIC EXAM, SACROILIAC JOINTS; LESS THAN 3 VI RADIOLOGIC EXAM, SACROILIAC JOINTS; OVER 3 VIEWS RADIOLOGIC EXAM, SACRUM AND COCCYX, OVER 2 VIEWS MYELOGRAPHY, CERVICAL, RADIOLOGICAL S AND I MYELOGRAPHY, THORACIC, RADIOLOGICAL S AND I MYELOGRAPHY, LUMBOSACRAL, RADIOLOGICAL S AND I MYELOGRAPHY, 2 OR MORE REGIONS, RADIOLOGICAL S AND EPIDUROGRAPHY, RADIOLOGICAL S AND I DISKOGRAPHY, CERVICAL/THORACIC, RADIOLOGICAL S AND DISKOGRAPHY, LUMBAR, RADIOLOGICAL S AND I RADIOLOGIC EXAM; CLAVICLE, COMPLETE RADIOLOGIC EXAM; SCAPULA, COMPLETE RADIOLOGIC EXAM, SHOULDER; 1 VIEW RADIOLOGIC EXAM, SHOULDER; COMPLETE, 2 OR MORE VIE RADIOLOGIC EXAM, SHOULDER, ARTHROGRAPHY, RADIOLOGI RADIOLOGIC EXAM; ACROMIOCLAVICULAR JOINTS, BILAT W RADIOLOGIC EXAM; HUMERUS, OVER 2 VIEWS RADIOLOGIC EXAM, ELBOW; 2 VIEWS RADIOLOGIC EXAM, ELBOW; COMPLETE, OVER 3 VIEWS RADIOLOGIC EXAM, ELBOW, ARTHROGRAPHY, RADIOLOGICAL RADIOLOGIC EXAM; FOREARM, 2 VIEWS RADIOLOGIC EXAM; UPPER EXTREMITY, INFANT, OVER 2 V RADIOLOGIC EXAM, WRIST; 2 VIEWS RADIOLOGIC EXAM, WRIST; COMPLETE, OVER 3 VIEWS RADIOLOGIC EXAM, WRIST, ARTHROGRAPHY, RADIOLOGICAL RADIOLOGIC EXAM, HAND; 2 VIEWS RADIOLOGIC EXAM, HAND; OVER 3 VIEWS RADIOLOGIC EXAM, FINGER(S), OVER 2 VIEWS CT SCAN, UPPER EXTREMITY; W/O CONTRAST MATL CT SCAN, UPPER EXTREMITY; W/CONTRAST MATL(S) CT SCAN, UPPER EXTREMITY; W/O CONTRAST MATL, THEN CT ANGIOGRAPHY, UPPR EXTREM, W/O CONTRAST MATL(S), MRI, UPPER EXTREMITY, OTHER THAN JOINT; W/O CONTRA MRI, UPPER EXTREMITY, OTHER THAN JOINT; W/CONTRAST MRI, UPPER EXTREMITY, OTHER THAN JOINT; W/O CONTRA MRI, ANY JOINT, UPPER EXTREMITY; W/O CONTRAST MATL MRI, ANY JOINT, UPPER EXTREMITY; W/CONTRAST MATL(S MRI, ANY JOINT OF UPPER EXTREMITY; W/O CONTRAST MA MRA, UPPER EXTREMITY, W/WO CONTRAST MATL(S) RADIOLOGIC EXAM, HIP, UNILAT; 1 VIEW RADIOLOGIC EXAMINATION, HIP, UNILATERAL, WITH PELVIS WHEN PERFORMED; 1 VIEW RADIOLOGIC EXAMINATION, HIP, UNILATERAL, WITH PELVIS WHEN PERFORMED; 2-3 VIEWS RADIOLOGIC EXAMINATION, HIP, UNILATERAL, WITH PELVIS WHEN PERFORMED; MINIMUM O RADIOLOGIC EXAM, HIP, UNILAT; COMPLETE, 2 OR MORE RADIOLOGIC EXAM, HIPS, BILAT, 2 OR MORE VIEWS, W/A RADIOLOGIC EXAMINATION, HIPS, BILATERAL, WITH PELVIS WHEN PERFORMED; 2 VIEWS RADIOLOGIC EXAMINATION, HIPS, BILATERAL, WITH PELVIS WHEN PERFORMED; 3-4 VIEWS RADIOLOGIC EXAMINATION, HIPS, BILATERAL, WITH PELVIS WHEN PERFORMED; MINIMUM OF RADIOLOGIC EXAM, HIP, ARTHROGRAPHY, RADIOLOGICAL S RADIOLOGIC EXAM, HIP, DURING OPERATIVE PROC RADIOLOGIC EXAM, PELVIS AND HIPS, INFANT/CHILD, OV RADIOLOGIC EXAM, FEMUR, 2 VIEWS RADIOLOGIC EXAMINATION, FEMUR; 1 VIEW RADIOLOGIC EXAMINATION, FEMUR; MINIMUM 2 VIEWS RADIOLOGIC EXAM, KNEE; 1/2 VIEWS RADIOLOGIC EXAM, KNEE; 3 VIEWS RADIOLOGIC EXAM, KNEE; COMPLETE, OVER 4 VIEWS RADIOLOGIC EXAM, KNEE; BOTH KNEES, STANDING, ANTER RADIOLOGIC EXAM, KNEE, ARTHROGRAPHY, RADIOLOGICAL RADIOLOGIC EXAM; TIBIA AND FIBULA, TWO VIEWS RADIOLOGIC EXAM; LOWER EXTREMITY, INFANT, OVER 2 V RADIOLOGIC EXAM, ANKLE; 2 VIEWS RADIOLOGIC EXAM, ANKLE; COMPLETE, OVER 3 VIEWS RADIOLOGIC EXAM, ANKLE, ARTHROGRAPHY, RADIOLOGICAL RADIOLOGIC EXAM, FOOT; 2 VIEWS RADIOLOGIC EXAM, FOOT; COMPLETE, OVER 3 VIEWS RADIOLOGIC EXAM; CALCANEUS, OVER 2 VIEWS RADIOLOGIC EXAM; TOE(S), OVER 2 VIEWS CT SCAN, LOWER EXTREMITY; W/O CONTRAST MATL CT SCAN, LOWER EXTREMITY; W/CONTRAST MATL(S) 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y N N Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y N Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 73702 73706 73718 73719 73720 73721 73722 73723 73725 74000 74010 74020 74022 74150 74160 74170 74174 74175 74176 74177 74178 74181 74182 74183 74185 74190 74210 74220 74230 74235 74240 74241 74245 74246 74247 74249 74250 74251 74260 74261 74262 74263 74270 74280 74283 74290 74300 74301 74305 74320 74327 74328 74329 74330 74340 74355 74360 74363 74400 74410 74415 74420 74425 74430 74440 74445 74450 74455 74470 74475 74480 74485 74710 74712 74713 74740 74742 74775 75557 75559 75561 75563 75565 75571 75572 75573 75574 75600 75605 75625 75630 CT SCAN, LOWER EXTREMITY; W/O CONTRAST MATL, THEN CT ANGIOGRAPHY, LOWER EXTREMITY, W/O CONTRAST MATL MRI, LOWER EXTREMITY OTHER THAN JOINT; W/O CONTRAS MRI, LOWER EXTREMITY OTHER THAN JOINT; W/CONTRAST MRI, LOWER EXTREMITY OTHER THAN JOINT; W/O CONTRAS MRI, ANY JOINT, LOWER EXTREMITY; W/O CONTRAST MATL MRI, ANY JOINT, LOWER EXTREMITY; W/CONTRAST MATL(S MRI, ANY JOINT, LOWER EXTREMITY; W/O CONTRAST MATL MRA, LOWER EXTREMITY, W/WO CONTRAST MATL(S) RADIOLOGIC EXAM, ABDOMEN; SINGLE ANTEROPOSTERIOR V RADIOLOGIC EXAM, ABDOMEN; ANTEROPOSTERIOR AND ADDL RADIOLOGIC EXAM, ABDOMEN; COMPLETE, W/DECUBITUS AN RADIOLOGIC EXAMINATION, ABDOMEN; COMPL ACUTE SERIE CT SCAN, ABDOMEN; W/O CONTRAST MATL CT SCAN, ABDOMEN; W/CONTRAST MATL(S) CT SCAN, ABDOMEN; W/O CONTRAST MATL, THEN W/CONTRA CT ANGIO ABD&PLVIS CNTRST MTRL W/WO CNTRST IMGES CT ANGIOGRAPHY, ABDOMEN, W/O CONTRAST MATL(S), FOL CT ABD & PELVIS W/O CONTRAST CT ABD & PELVIS W/CONTRAST CT ABD & PELVIS W/O CONTRST 1+ BODY REGNS MRI, ABDOMEN; W/O CONTRAST MATL(S) MRI, ABDOMEN; W/CONTRAST MATL(S) MRI, ABDOMEN; W/O CONTRAST MATL(S) FOLLOWED BY CON MRA, ABDOMEN, W/WO CONTRAST MATL(S) PERITONEOGRAM, RADIOLOGICAL S AND I RADIOLOGIC EXAM; PHARYNX AND/OR CERVICAL ESOPHAGUS RADIOLOGIC EXAM; ESOPHAGUS SWALLOWING FUNCTION, W/CINERADIOGRAPHY AND/OR VIDE REMOVAL FB, ESOPHAGUS W/BALLOON CATHETER, RADIOLOG RADIOLOGIC EXAM, UPPER GI TRACT; W/WO DELAYED FILM RADIOLOGIC EXAM, UPPER GI TRACT; W/WO DELAYED FILM RADIOLOGIC EXAM, UPPER GI TRACT; W/SMALL INTESTINE RADIOLOGIC EXAM, UPPER GI TRACT W/CONTRAST W/WO GL RADIOLOGIC EXAM, UPPER GI TRACT W/CONTRAST W/WO GL RADIOLOGIC EXAM, UPPER GI TRACT W/CONTRAST W/WO GL RADIOLOGIC EXAM, SMALL BOWEL, W/MULTIPLE SERIAL FI RADIOLOGIC EXAM, SMALL BOWEL, W/MULTIPLE SERIAL FI DUODENOGRAPHY, HYPOTONIC CT COLONOGRPHY DX IMAGE POSTPROCESS NO CONTRAST CT COLONOGRPHY DX IMAGE POSTPROCESS W/CONTRAST CT COLONOGRAPHY SCREENING IMAGE POSTPROCESSING RADIOLOGIC EXAM, COLON; BARIUM ENEMA, W/WO KUB RADIOLOGIC EXAM, COLON; AIR CONTRAST W/SPECIFIC HI THERAPEUTIC ENEMA, REDUCTION, INTUSSUSCEPTION/OBST CHOLECYSTOGRAPHY ORAL CONTRST CHOLANGIOGRAPHY AND/OR PANCREATOGRAPHY; INTRAOPERA CHOLANGIOGRAPHY AND/OR PANCREATOGRAPHY; INTRAOPERA CHOLANGIOGRAPHY AND/OR PANCREATOGRAPHY; THROUGH CA CHOLANGIOGRAPHY, PERCUTANEOUS, TRANSHEPATIC, RADIO POSTOPERATIVE BILE STONE REMOVAL, PERCUTANEOUS, RA ENDOSCOPIC CATHETERIZATION, BILE DUCT, RADIOLOGICA ENDOSCOPIC CATHETERIZATION, PANCREATIC DUCTAL SYST COMBINED ENDOSCOPIC CATHETERIZATION BILE DUCT AND INTRODUCTION LONG GI TUBE W/MULTIPLE FLUOROSCOPIES PERCUTANEOUS PLACEMENT, ENTEROCLYSIS TUBE, RADIOLO INTRALUMINAL DILATION STRICTURES AND/OR OBSTRUCTIO PERCUTANEOUS TRANSHEPATIC DILATION, BILE DUCT STRI UROGRAPHY (PYELOGRAPHY), IV, W/WO KUB, W/WO TOMOGR UROGRAPHY, INFUSION, DRIP TECHNIQUE AND/OR BOLUS T UROGRAPHY, INFUSION, DRIP TECHNIQUE AND/OR BOLUS T UROGRAPHY, RETROGRADE, W/WO KUB UROGRAPHY, ANTEGRADE, RADIOLOGICAL S AND I CYSTOGRAPHY, OVER 3 VIEWS, RADIOLOGICAL S AND I VASOGRAPHY, VESICULOGRAPHY/EPIDIDYMOGRAPHY, RADIOL CORPORA CAVERNOSOGRAPHY, RADIOLOGICAL S AND I URETHROCYSTOGRAPHY, RETROGRADE, RADIOLOGICAL S AND URETHROCYSTOGRAPHY, VOIDING, RADIOLOGICAL S AND I RADIOLOGIC EXAM, RENAL CYST STUDY, TRANSLUMBAR, CO INTRODUCTION CATHETER, RENAL PELVIS, PERCUTANEOUS, INTRODUCTION URETERAL CATHETER/STENT, PERCUTANEOUS DILATION, NEPHROSTOMY/URETERS/URETHRA, RADIOLOGICA PELVIMETRY, W/WO PLACENTAL LOCALIZATION MAGNETIC RESONANCE (EG, PROTON) IMAGING, FETAL, INCLUDING PLACENTAL AND MATERN MAGNETIC RESONANCE (EG, PROTON) IMAGING, FETAL, INCLUDING PLACENTAL AND MATERN HYSTEROSALPINGOGRAPHY, RADIOLOGICAL S AND I TRANSCERVICAL CATHETERIZATION, FALLOPIAN TUBE, RAD PERINEOGRAM CARDIAC MRI MORPHOLOGY & FUNCTION W/O CONTRAST CARDIAC MRI W/O CONTRAST W STRESS IMAGING CARDIAC MRI W/W/O CONTRAST & FURTHER SEQ CARDIAC MRI W/W/O CONTRAST W STRESS CARDIAC MRI FOR VELOCITY FLOW MAPPING CT HEART NO CONTRAST QUANT EVAL CORONRY CALCIUM CT HEART CONTRAST EVAL CARDIAC STRUCTURE&MORPH CT HRT CONTRST CARDIAC STRUCT&MORPH CONG HRT DX CTA HRT CORNRY ART/BYPASS GRFTS CONTRST 3D POST AORTOGRAPHY, THORACIC, W/O SERIALOGRAPHY, RADIOLOG AORTOGRAPHY, THORACIC, SERIALOGRAPHY, RADIOLOGICAL AORTOGRAPHY, ABDOMINAL, SERIALOGRAPHY, RADIOLOGICA AORTOGRAPHY, ABDOMINAL AND BILAT ILIOFEMORAL LOWER 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/1900 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y N N N N Y Y Y N Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y N N N N N N N N All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 75635 75658 75705 75710 75716 75724 75726 75731 75733 75736 75741 75743 75746 75756 75774 75791 75801 75803 75805 75807 75809 75810 75820 75822 75825 75827 75831 75833 75840 75842 75860 75870 75872 75880 75885 75887 75889 75891 75893 75894 75896 75898 75901 75902 75945 75946 75952 75953 75954 75956 75957 75958 75959 75962 75964 75966 75968 75970 75978 75980 75982 75984 75989 76000 76001 76010 76080 76098 76100 76101 76102 76120 76125 76140 76376 76377 76380 76390 76496 76497 76498 76499 76506 76510 76511 76512 76513 76514 76516 76519 76529 CT ANGIO, ABD AORTA AND BILAT ILIOFEM LOWR EXTREM ANGIOGRAPHY, BRACHIAL, RETROGRADE, RADIOLOGICAL S ANGIOGRAPHY, SPINAL, SELECTIVE, RADIOLOGICAL S AND ANGIOGRAPHY, EXTREMITY, UNILAT, RADIOLOGICAL S AND ANGIOGRAPHY, EXTREMITY, BILAT, RADIOLOGICAL S AND ANGIOGRAPHY, RENAL, BILAT, SELECTIVE, RADIOLOGICAL ANGIOGRAPHY, VISCERAL, SELECTIVE/SUPRASELECTIVE, R ANGIOGRAPHY, ADRENAL, UNILAT, SELECTIVE, RADIOLOGI ANGIOGRAPHY, ADRENAL, BILAT, SELECTIVE, RADIOLOGIC ANGIOGRAPHY, PELVIC, SELECTIVE/SUPRASELECTIVE, RAD ANGIOGRAPHY, PULMONARY, UNILAT, SELECTIVE, RADIOLO ANGIOGRAPHY, PULMONARY, BILAT, SELECTIVE, RADIOLOG ANGIOGRAPHY, PULMONARY, NON-SELECTIVE CATHETER/VEN ANGIOGRAPHY, INT MAMMARY, RADIOLOGICAL S AND I ANGIOGRAPHY, SELECTIVE, ADDL VESSEL(S), RADIOLOGIC ANGIOGRPHY AV SHUNT COMPLETE EVAL FLUOR S&I LYMPHANGIOGRAPHY, EXTREMITY ONLY, UNILAT, RADIOLOG LYMPHANGIOGRAPHY, EXTREMITY ONLY, BILAT, RADIOLOGI LYMPHANGIOGRAPHY, PELVIC/ABDOMINAL, UNILAT, RADIOL LYMPHANGIOGRAPHY, PELVIC/ABDOMINAL, BILAT, RADIOLO SHUNTOGRAM, INVESTIGATION PREVIOUS NONVASCULAR SHU SPLENOPORTOGRAPHY, RADIOLOGICAL S AND I VENOGRAPHY, EXTREMITY, UNILAT, RADIOLOGICAL S AND VENOGRAPHY, EXTREMITY, BILAT, RADIOLOGICAL S AND I VENOGRAPHY, CAVAL, INFERIOR, W/SERIALOGRAPHY, RADI VENOGRAPHY, CAVAL, SUPERIOR, W/SERIALOGRAPHY, RADI VENOGRAPHY, RENAL, UNILAT, SELECTIVE, RADIOLOGICAL VENOGRAPHY, RENAL, BILAT, SELECTIVE, RADIOLOGICAL VENOGRAPHY, ADRENAL, UNILAT, SELECTIVE, RADIOLOGIC VENOGRAPHY, ADRENAL, BILAT, SELECTIVE, RADIOLOGICA VENOGRAPHY, SINUS/JUGULAR, CATHETER, RADIOLOGICAL VENOGRAPHY, SUPERIOR SAGITTAL SINUS, RADIOLOGICAL VENOGRAPHY, EPIDURAL, RADIOLOGICAL S AND I VENOGRAPHY, ORBITAL, RADIOLOGICAL S AND I PERCUTANEOUS TRANSHEPATIC PORTOGRAPHY, W/HEMODYNAM PERCUTANEOUS TRANSHEPATIC PORTOGRAPHY, W/O HEMODYN HEPATIC VENOGRAPHY, WEDGE/FREE W/HEMODYNAMIC EVAL HEPATIC VENOGRAPHY, WEDGE/FREE W/O HEMODYNAMIC EVA VENOUS SAMPLE, CATHETER, W/WO ANGIOGRAPHY, RADIOLO TRANSCATHETER THERAPY, EMBOLIZATION, ANY METHOD, R TRANSCATHETER INFUSION OTHER THAN THROMBOLYSIS ANGRPH CATH F-UP STD TCAT OTHER THAN THROMBYLSIS MECHANICAL REMOVE, PERICATHETER OBSTRUCTIVE MATL F MECHANICAL REMOVE, INTRALUMINAL OBSTRUCTIVE MATL F INTRAVASCULAR ULTRASOUND, NON-CORONARY VESSEL, RAD INTRAVASCULAR ULTRASOUND, NON-CORONARY VESSEL, RAD ENDOVASCULAR REPAIR, INFRARENAL ABD AORTIC ANEURYS PLACEMNT, PROX/DISTL EXTEN PROSTHS, ENDVAS REPR, I EVASC RPR ILIAC ART W/ILIO-ILIAC PROSTH RS&I ENDOVASCULAR REPAIR OF DESCENDING THORACIC AORTA, ENDOVASCULAR REPAIR OF DESCENDING THORACIC AORTA, PLACEMENT OF PROXIMAL EXTENSION PROSTHESIS FOR END PLACEMENT OF DISTAL EXTENSION PROTHESIS (DELAYED) TRANSLUMINAL BALLOON ANGIOP PERIPHERAL ART RSI TRLUML BALOON ANGIOP PERIPHER EA ADDL ARTERY RSI TRANSLUMINAL BALLOON ANGIOPLASTY, RENAL/VISCERAL A TRANSLUMINAL BALLOON ANGIOPLASTY, ADDL VISCERAL AR TRANSCATHETER BX, RADIOLOGICAL S AND I TRANSLUMINAL BALLOON ANGIOPLASTY, VENOUS, RADIOLOG PERCUTANEOUS TRANSHEPATIC BILIARY DRAINAGE W/CONTR PERCUTANEOUS BILIARY DRAINAGE, RADIOLOGICAL S AND PERCUTANEOUS DRAINAGE CATHETER CHANGE, W/CONTRAST, RADIOLOGICAL GUIDED, PERCUT DRAINAGE, W/CATHETER P FLUOROSCOPY SPX UP TO 1 HOUR PHYS/QHP TIME FLUOROSCOPY SPX >1 HOUR PHYS/QHP TIME RADIOLOGIC EXAM, NOSE TO RECTUM, FB, SINGLE VIEW, RADIOLOGIC EXAM, ABSCESS/FISTULA/SINUS TRACT, RADI RADIOLOGICAL EXAM, SURGICAL SPECIMEN RADIOLOGIC EXAM, SINGLE PLANE BODY SECTION, OTHER RADIOLOGIC EXAM, COMPLEX MOTION BODY SECTION, NONRADIOLOGIC EXAM, COMPLEX MOTION BODY SECTION, NONCINERADIOGRAPHY/VIDEORADIOLOGY, EXCEPT WHERE SPECI CINERADIOGRAPHY/VIDEORADIOGRAPHY W/ROUTINE EXAM CONSULTATION ON X-RAY EXAM MADE ELSEWHERE, WRITTEN 3D RENDERING W/INTERP & POSTPROCESS SUPERVISION 3D RENDERING W/INTERP&POSTPROC DIFF WORK STATION CT SCAN, LIMITED/LOCALIZED FOLLOW-UP STUDY MR SPECTROSCOPY UNLISTED FLUOROSCOPIC PROCEDURE UNLISTED CT PROCEDURE UNLISTED MR PROCEDURE UNLISTED DX RADIOGRAPHIC PROCEDURE ULTRASOUND, HEAD/BRAIN OPHTHALMIC ULTRASOUND, DIAGNOSITC;B-SCAN AND QUANI OPHTHALMIC ULTRASOUND, ECHOGRAPHY, DX; A-SCAN ONLY OPHTHALMIC ULTRASOUND, ECHOGRAPHY, DX; CONTACT B-S OPHTHALMIC ULTRASOUND, ECHOGRAPHY, DX; ANTERIOR SE OPHTHALMIC ULTRASOUND, ECHOGRAPHY, DX; CORNEAL PAC OPHTHALMIC BIOMETRY, ULTRASOUND ECHOGRAPHY, A-SCAN OPHTHALMIC BIOMETRY, ULTRASOUND ECHOGRAPHY, A-SCAN OPHTHALMIC ULTRASONIC FB LOCALIZATION 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/1900 1/1/1900 1/1/1900 1/1/1900 1/1/1900 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N Y N Y Y N N N N N N N N N N All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 76536 76604 76641 76642 76700 76705 76770 76775 76776 76800 76801 76802 76805 76810 76811 76812 76813 76814 76815 76816 76817 76818 76819 76820 76821 76825 76826 76827 76828 76830 76831 76856 76857 76870 76872 76873 76881 76882 76885 76886 76930 76932 76936 76937 76940 76941 76942 76945 76946 76948 76965 76970 76975 76977 76998 76999 77001 77002 77003 77011 77012 77013 77014 77021 77022 77051 77052 77053 77054 77055 77056 77057 77058 77059 77061 77062 77063 77071 77072 77073 77074 77075 77076 77077 77078 77080 77081 77084 77085 77086 77261 ULTRASOUND, HEAD/NECK TISSUES, B-SCAN/REAL TIME W/ ECHOGRAPHY, CHEST, B-SCAN (INCLUDES MEDIASTINUM) A US BREAST UNI REAL TIME WITH IMAGE COMPLETE US BREAST UNI REAL TIME WITH IMAGE LIMITED ULTRASOUND, ABDOMINAL, B-SCAN AND/OR REAL TIME W/I ULTRASOUND, ABDOMINAL, B-SCAN AND/OR REAL TIME W/I ULTRASOUND, RETROPERITONEUM, B-SCAN/REAL TIME; COM ULTRASOUND, RETROPERITONEUM, B-SCAN/REAL TIME; LIM Us exam k transpl w/doppler ECHOGRAPHY, SPINAL CANAL AND CONTENTS US, PREG UTER, REAL TIME W/IMAGE DOCUMENT, FETAL A US, PREG UTER, REAL TIME W/IMAGE DOCUMENT, FETAL A US, PREG UTER, RLTIME W/IMAGE DOCUMENT, FETAL AND US, PREG UTER, REAL TIME W/IMAGE DOCUMENT EA ADDL US, PREG UTER, REAL TIME W/IMAGE DOC, FETL/MATRNL, US, PREG UTER, REAL TIME W/IMAGE DOC, FETAL/MATERN Ob us nuchal meas, 1 gest Ob us nuchal meas, add-on US, PREGNANT UTERUS, REAL TIME W/IMAGE DOCUMENT, L US, PREGNANT UTERUS, REAL TIME W/IMAGE DOCUMENT, F US, PREGNANT UTERUS, REAL TIME W/IMAGE DOCUMENT, T FETAL BIOPHYSICAL PROFILE; W/NON-STRESS TESTING FETAL BIOPHYSICAL PROFILE; W/O NON-STRESS TESTING DOPPLER VELOCIMETRY, FETAL; UMBILICAL ARTERY DOPPLER VELOCIMETRY, FETAL; MIDDLE CEREBRAL ARTERY ECHOCARDIOGRAPHY, FETAL, CARDIOVASCULAR SYSTEM, RE ECHOCARDIOGRAPHY, FETAL, CARDIOVASCULAR SYSTEM, RE ECHOCARDIOGRAPHY, FETAL, DOPPLER, CARDIOVASCULAR S ECHOCARDIOGRAPHY, FETAL, DOPPLER, CARDIOVASCULAR S ECHOGRAPHY, TRANSVAGINAL SALINE INFUSION SONOHYSTEROGRAPHY, W/COLOR FLOW DO ECHOGRAPHY, PELVIC (NONOBSTETRIC), B-SCAN AND/OR R ECHOGRAPHY, PELVIS, B-SCAN/REAL TIME; LIMITED/FOLL ECHOGRAPHY, SCROTUM AND CONTENTS US, TRANSRECTAL ECHOGRAPHY, TRANSRECTAL; PROSTATE VOLUME STUDY, BR US EXTREMITY NON-VASC REAL-TIME IMG COMPL US EXTREMITY NON-VASC REAL-TIME IMG LMTD US INFT HIPS R-T IMG DYNAMIC REQ PHYS/QHP MANJ US INFT HIPS R-T IMG LMTD STATIC PHYS/QHP MANJ US GUIDANCE, PERICARDIOCENTESIS, IMAGING S AND I US GUIDANCE, ENDOMYOCARDIAL BX, IMAGING S AND I US GUIDANCE, COMPRESSION REPAIR, PSEUDO-ANEURYSM/A US GUIDE, VASC ACCESS, REQ EVAL, POTENTIAL SITES, US GUIDANCE, AND MONITORING, VISCERAL TISSUE ABLAT US GUIDANCE, INTRAUTERINE FETAL TRANSFUSION/CORDOC US GUIDANCE, NEEDLE PLACEMENT, RADIOLOGICAL S AND US GUIDANCE, CHORIONIC VILLUS SAMPLING, IMAGING S US GUIDANCE, AMNIOCENTESIS, IMAGING S AND I US GUIDANCE, ASPIRATION, OVA, IMAGING S AND I US GUIDANCE, INTERSTITIAL RADIOELEMENT APPLICATION US STUDY FOLLOW-UP (SPECIFY) GI ENDOSCOPIC US, S AND I US BONE DENSITY MEASUREMENT AND INTERPRETATION, PE Us guide, intraop UNLISTED ULTRASOUND PROCEDURE Fluoroguide for vein device Needle localization by xray FLUORO NEEDLE/CATH SPINE/PARASPINAL DX/THER Ct scan for localization Ct scan for needle biopsy Ct guide for tissue ablation Ct scan for therapy guide Mr guidance for needle place Mri for tissue ablation COMPUTER-AIDED DETECTION DX MAMMOGRAPHY COMPUTER-AIDED DETECTION SCREENING MAMMOGRAPHY X-ray of mammary duct X-ray of mammary ducts Mammogram, one breast Mammogram, both breasts Mammogram, screening Mri, one breast Mri, both breasts DIGITAL BREAST TOMOSYNTHESIS UNILATERAL DIGITAL BREAST TOMOSYNTHESIS BILATERAL SCREENING DIGITAL BREAST TOMOSYNTHESIS BI MANUAL APPL STRESS PFRMD PHYS/QHP JOINT FILMS X-rays for bone age X-rays, bone length studies X-rays, bone survey, limited X-rays, bone survey complete X-rays, bone survey, infant Joint survey, single view Ct bone density, axial Dxa bone density, axial Dxa bone density/peripheral Magnetic image, bone marrow DXA BONE DENSITY STUDY AXIAL SKELETON VERTEBRAL FRACTURE ASSESSMENT VIA DXA THERAPEUTIC RADIOLOGY TREATMENT PLANNING; SIMPLE 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2015 1/1/2015 1/1/2015 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2015 1/1/2015 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y N N N N N N N N N N N N N N N N N N N Y N N Y N N N All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 77262 77263 77280 77285 77290 77293 77295 77299 77300 77301 77306 77307 77316 77317 77318 77321 77331 77332 77333 77334 77336 77338 77370 77371 77372 77373 77385 77386 77387 77399 77401 77402 77407 77412 77417 77422 77423 77424 77425 77427 77431 77432 77435 77469 77470 77499 77520 77522 77523 77525 77600 77605 77610 77615 77620 77750 77761 77762 77763 77767 77768 77770 77771 77772 77776 77778 77785 77786 77787 77789 77790 77799 78012 78013 78014 78015 78016 78018 78020 78070 78071 78072 78075 78099 78102 78103 78104 78110 78111 78120 78121 THERAPEUTIC RADIOLOGY TREATMENT PLANNING; INTERMED THERAPEUTIC RADIOLOGY TREATMENT PLANNING; COMPLEX THERAPEUTIC RADIOLOGY SIMULATION-AIDED FIELD SETTI THERAPEUTIC RADIOLOGY SIMULATION-AIDED FIELD SETTI THERAPEUTIC RADIOLOGY SIMULATION-AIDED FIELD SETTI RESPIRATORY MOTION MANAGEMENT SIMULATION RADIOTHERAPY PLAN DOSE-VOLUME HISTOGRAMS UNLISTED PROC, THERAPEUTIC RADIOLOGY CLINICAL TREA RADIATION THERAPY, DOSIMETRY PLAN INTENSITY MODULATED RADIOTHERAPY PLAN W/DOSE VOLUM TELETHX ISODOSE PLN SMPL W/DOSIMETRY CALCULATION TELETHX ISODOSE PLN CPLX W/BASIC DOSIMETRY BRACHYTX ISODOSE PLN SMPL W/DOSIMETRY CAL BRACHYTX ISODOSE PLN INTERMED W/DOSIMETRY CAL BRACHYTX ISODOSE PLN CPLX W/DOSIMETRY CAL SPECIAL TELETHERAPY PORT PLAN, PARTICLES, HEMIBODY RADIATION THERAPY, SPECIAL DOSIMETRY TREATMENT DEVICES, DESIGN AND CONSTRUCTION; SIMPLE TREATMENT DEVICES, DESIGN AND CONSTRUCTION; INTERM TREATMENT DEVICES, DESIGN AND CONSTRUCTION; COMPLE CONTINUING MEDICAL PHYSICS CONSULTATION, PER WEEK MLC IMRT DESIGN & CONSTRUCTION PER IMRT PLAN SPECIAL MEDICAL RADIATION PHYSICS CONSULTATION Srs, multisource Srs, linear based Sbrt delivery INTENSITY MODULATED RADIATION TX DLVR SIMPLE INTENSITY MODULATED RADIATION TX DLVR COMPLEX GUIDANCE FOR LOC OF TARGET VOL RADIAJ TX DLVR UNLISTED PROC, RADIATION/PHYSICS/DOSIMETRY AND TRE RADIATION TX DELIVERY SUPERFICIAL&/ORTHO VOLTA RADIATION TREATMENT DELIVERY 1 MEV+ SIMPLE RADIATION TREATMENT DELIVERY 1 MEV+ INTERMEDIATE RADIATION TREATMENT DELIVERY 1 MEV+ COMPLEX THERAPEUTIC RADIOLOGY PORT FILM(S) HIGH ENERGY NEUTRON RAD TX DELIVERY; SINGLE TREATM HIGH ENERGY NEUTRON RAD TX DELIVERY; 1 OR MORE ISO INTRAOP RADIAJ TX DELIVER XRAY SINGLE TX SESSION INTRAOP RADIAJ TX DELIVER ELECTRONS SNGL TX SESS RADIATION TREATMENT MANAGEMENT, 5 TREATMENTS RADIATION THERAPY MANAGEMENT, COMPLETE, 1 TO 2 FRA RADIATION TREATMENT MANAGEMENT, STEREOTACTIC, CERE Sbrt management INTRAOPERATIVE RADIATION TREATMENT MANAGEMENT SPECIAL TREATMENT PROC UNLISTED PROC, THERAPEUTIC RADIOLOGY TREATMENT MAN PROTON TREATMENT DELIVERY; SIMPLE W/O COMPENSATION PROTON TREATMENT DELIVERY; SIMPLE W/COMPENSATION PROTON TREATMENT DELIVERY; INTERMEDIATE PROTON TREATMENT DELIVERY; COMPLEX HYPERTHERMIA, EXTERNALLY GENERATED; SUPERFICIAL HYPERTHERMIA, EXTERNALLY GENERATED; DEEP HYPERTHERMIA GENERATED, INTERSTITIAL PROBE(S); UP HYPERTHERMIA GENERATED, INTERSTITIAL PROBE(S); MOR HYPERTHERMIA GENERATED, INTRACAVITARY PROBE(S) INFUSION/INSTILLATION, RADIOELEMENT SOLUTION INTRACAVITARY RADIATION SOURCE APPLICATION; SIMPLE INTRACAVITARY RADIATION SOURCE APPLICATION; INTERM INTRACAVITARY RADIATION SOURCE APPLICATION; COMPLE REMOTE AFTERLOADING HIGH DOSE RATE RADIONUCLIDE SKIN SURFACE BRACHYTHERAPY, IN REMOTE AFTERLOADING HIGH DOSE RATE RADIONUCLIDE SKIN SURFACE BRACHYTHERAPY, IN REMOTE AFTERLOADING HIGH DOSE RATE RADIONUCLIDE INTERSTITIAL OR INTRACAVITARY B REMOTE AFTERLOADING HIGH DOSE RATE RADIONUCLIDE INTERSTITIAL OR INTRACAVITARY B REMOTE AFTERLOADING HIGH DOSE RATE RADIONUCLIDE INTERSTITIAL OR INTRACAVITARY B INTERSTITIAL RADIATION SOURCE APPLICATION; SIMPLE INTERSTITIAL RADIOELEMENT APPLICATION; COMPLEX REMOTE AFTLD RADIONUCLIDE BRACHYTX 1 CHANNEL REMOTE AFTLD RADIONUCLIDE BRACHYTX 2-12 CHANNEL REMOTE AFTLD RADIONUCLIDE BRACHYTX > 12 CHANNEL SURFACE APPLICATION, RADIATION SOURCE SUPERVISION, HANDLING, LOADING, RADIATION SOURCE UNLISTED PROC, CLINICAL BRACHYTHERAPY THYROID UPTAKE SINGLE/MULTIPLE QUANT MEASUREMENT THYROID IMAGING WITH VASCULAR FLOW THYROID UPTAKE W/BLOOD FLOW SNGLE/MULT QUAN MEAS THYROID CARCINOMA METASTASES IMAGING; LIMITED AREA THYROID CARCINOMA METASTASES IMAGING; W/ADDL STUDI THYROID CARCINOMA METASTASES IMAGING; WHOLE BODY THYROID CARCINOMA METASTASES UPTAKE PARATHYROID PLANAR IMAGING PARATHYROID PLANAR IMAGING W/WO SUBTRACTION PARATHYROID IMAGING W/TOMOGRAPHIC SPECT & CT ADRENAL IMAGING, CORTEX AND/OR MEDULLA UNLISTED ENDOCRINE PROC, DX NUCLEAR MEDICINE BONE MARROW IMAGING; LIMITED AREA BONE MARROW IMAGING; MULTIPLE AREAS BONE MARROW IMAGING; WHOLE BODY PLASMA VOLUME, RADIOPHARMACEUTICAL VOLUME-DILUTION PLASMA VOLUME, RADIOPHARMACEUTICAL VOLUME-DILUTION RED CELL VOLUME DETERMINATION (SEP PROC); SINGLE S RED CELL VOLUME DETERMINATION (SEP PROC); MULTIPLE 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 78122 78130 78135 78140 78185 78190 78191 78195 78199 78201 78202 78205 78206 78215 78216 78226 78227 78230 78231 78232 78258 78261 78262 78264 78265 78266 78267 78268 78270 78271 78272 78278 78282 78290 78291 78299 78300 78305 78306 78315 78320 78350 78351 78399 78414 78428 78445 78451 78452 78453 78454 78456 78457 78458 78459 78466 78468 78469 78472 78473 78481 78483 78491 78492 78494 78496 78499 78579 78580 78582 78597 78598 78599 78600 78601 78605 78606 78607 78608 78609 78610 78630 78635 78645 78647 78650 78660 78699 78700 78701 78707 WHOLE BLOOD VOLUME DETERMINATION, RADIOPHARMACEUTI RED CELL SURVIVAL STUDY RED CELL SURVIVAL STUDY; DIFFERENTIAL ORGAN/TISSUE LABELED RED CELL SEQUESTRATION, DIFFERENTIAL ORGAN SPLEEN IMAGING ONLY, W/WO VASCULAR FLOW KINETICS, STUDY, PLATELET SURVIVAL, W/WO DIFFERENT PLATELET SURVIVAL STUDY LYMPHATICS AND LYMPH GLANDS IMAGING UNLISTED HEMATOPOIETIC/RETICULOENDOTHELIAL/LYMPHAT LIVER IMAGING; STATIC ONLY LIVER IMAGING; W/VASCULAR FLOW LIVER IMAGING (SPECT) LIVER IMAGING (SPECT); W/VASCULAR FLOW LIVER AND SPLEEN IMAGING; STATIC ONLY LIVER AND SPLEEN IMAGING; W/VASCULAR FLOW HEPATOBILIARY SYST IMAGING INCLUDING GALLBLADDER HEPATOBIL SYST IMAG INC GB W/PHARMA INTERVENJ SALIVARY GLAND IMAGING SALIVARY GLAND IMAGING; W/SERIAL IMAGES SALIVARY GLAND FUNCTION STUDY ESOPHAGEAL MOTILITY GASTRIC MUCOSA IMAGING GASTROESOPHAGEAL REFLUX STUDY GASTRIC EMPTYING STUDY GASTRIC EMPTYING IMAGING STUDY (EG, SOLID, LIQUID, OR BOTH); WITH SMALL BOWEL TRA GASTRIC EMPTYING IMAGING STUDY (EG, SOLID, LIQUID, OR BOTH); WITH SMALL BOWEL AND BREATH TEST, C-14 UREA; ACQUISITION AND ANALYSIS BREATH TEST, C-14 UREA; ANALYSIS VITAMIN B-12 ABSORPTION STUDY; W/O INTRINSIC FACTO VITAMIN B-12 ABSORPTION STUDY; W/INTRINSIC FACTOR VITAMIN B-12 ABSORPTION STUDIES COMBINED, W/WO INT ACUTE GI BLOOD LOSS IMAGING GI PROTEIN LOSS BOWEL IMAGING PERITONEAL-VENOUS SHUNT PATENCY TEST UNLISTED GI PROC, DX NUCLEAR MEDICINE BONE AND/OR JOINT IMAGING; LIMITED AREA BONE AND/OR JOINT IMAGING; MULTIPLE AREAS BONE AND/OR JOINT IMAGING; WHOLE BODY BONE AND/OR JOINT IMAGING; THREE PHASE STUDY BONE AND/OR JOINT IMAGING; TOMOGRAPHIC (SPECT) BONE DENSITY (BONE MINERAL CONTENT) STUDY, 1OR MOR BONE DENSITY STUDY, 1 OR MORE SITES; DUAL PHOTON UNLISTED MUSCULOSKELETAL PROC, DX NUCLEAR MEDICINE DETERMINATION, CENTRAL C-V HEMODYNAMICS, NON-IMAGI CARDIAC SHUNT DETECTION NON-CARDIAC VASCULAR FLOW IMAGING MYOCARDIAL SPECT SINGLE STUDY AT REST OR STRESS MYOCARDIAL SPECT MULTIPLE STUDIES MYOCARDIAL PERFUSION PLANAR 1 STUDY REST/STRESS MYOCARDIAL PERFUSION PLANAR MULTIPLE STUDIES IMAGING, PEPTIDE, ACUTE VENOUS THROMBOSIS VENOUS THROMBOSIS, IMAGING, VENOGRAM; UNILAT VENOUS THROMBOSIS, IMAGING, VENOGRAM; BILAT MYOCARDIAL IMAGING, POSITRON EMISSION TOMOGRAPHY ( MYOCARDIAL IMAGING, INFARCT AVID, PLANAR; QUALITAT MYOCARDIAL IMAGING, INFARCT AVID, PLANAR; W/EJECTI MYOCARDIAL IMAGING, INFARCT AVID, PLANAR; TOMOGRAP CARDIAC BLOOD POOL IMAGING, GATED EQUILIBRIUM; PLA CARDIAC BLOOD POOL IMAGING, GATED EQUILIBRIUM; PLA CARDIAC BLOOD POOL IMAGING, PLANAR, 1ST PASS; SING CARDIAC BLOOD POOL IMAGING, PLANAR, 1ST PASS; MULT MYOCARDIAL PET; SINGLE STUDY, REST/STRESS MYOCARDIAL PET; MULTIPLE STUDIES, REST AND/OR STRE CARDIAC BLOOD POOL IMAGING, GATED EQUILIBRIUM, RES CARDIAC BLOOD POOL IMAGING, GATED EQUILIBRIUM, SIN UNLISTED CARDIOVASCULAR PROC, DX NUCLEAR MEDICINE PULMONARY VENTILATION IMAGING PULMONARY PERFUSION IMAGING PARTICULATE PULMONARY VENTILATION & PERFUSION IMAGING QUANT DIFFERENTIAL PULM PERFUSION W/WO IMAGING QUANT DIFF PULM PRFUSION & VENTLAJ W/WO IMAGING UNLISTED RESPIRATORY PROC, DX NUCLEAR MEDICINE BRAIN IMAGING, LIMITED PROC; STATIC BRAIN IMAGING, LIMITED PROC; W/VASCULAR FLOW BRAIN IMAGING, COMPLETE STUDY; STATIC BRAIN IMAGING, COMPLETE STUDY; W/VASCULAR FLOW BRAIN IMAGING, COMPLETE STUDY; TOMOGRAPHIC (SPECT) BRAIN IMAGING, POSITRON EMISSION TOMOGRAPHY (PET); BRAIN IMAGING, POSITRON EMISSION TOMOGRAPHY (PET); BRAIN IMAGING, VASCULAR FLOW ONLY CEREBROSPINAL FLUID FLOW, IMAGING (NOT W/INTRODUCT CEREBROSPINAL FLUID FLOW, IMAGING (NOT W/INTRODUCT CEREBROSPINAL FLUID FLOW, IMAGING (NOT W/INTRODUCT CEREBROSPINAL FLUID FLOW, IMAGING (NOT W/INTRODUCT CSF LEAKAGE DETECTION AND LOCALIZATION RADIOPHARMACEUTICAL DACRYOCYSTOGRAPHY UNLISTED NERVOUS SYSTEM PROC, DX NUCLEAR MEDICINE KIDNEY IMAGING; STATIC ONLY KIDNEY IMAGING; W/VASCULAR FLOW KIDNEY IMAGING W/BLOOD FLOW AND FUNCTION; SINGLE W 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 78708 78709 78710 78725 78730 78740 78761 78799 78800 78801 78802 78803 78804 78805 78806 78807 78808 78811 78812 78813 78814 78815 78816 78999 79005 79101 79200 79300 79403 79440 79445 79999 80047 80048 80050 80051 80053 80055 80061 80069 80074 80076 80081 80150 80155 80156 80157 80158 80159 80162 80163 80164 80165 80168 80169 80170 80171 80173 80175 80176 80177 80178 80180 80183 80184 80185 80186 80188 80190 80192 80194 80195 80197 80198 80199 80200 80201 80202 80203 80299 80300 80301 80302 80303 80304 80320 80321 80322 80323 80324 80325 KIDNEY IMAGING W/BLOOD FLOW AND FUNCTION; SINGLE W KIDNEY IMAGING W/BLOOD FLOW AND FUNCTION; MULTIPLE KIDNEY IMAGING, TOMOGRAPHIC (SPECT) KIDNEY FUNCTION STUDY, NON-IMAGING RADIOISOTOPIC S URINARY BLADDER RESIDUAL STUDY URETERAL REFLUX STUDY (RADIOPHARMACEUTICAL VOIDING TESTICULAR IMAGING; W/VASCULAR FLOW UNLISTED GENITOURINARY PROC, DX NUCLEAR MEDICINE RADIOPHARMACEUTICAL LOCALIZATION, TUMOR, AGENT(S) RADIOPHARMACEUTICAL LOCALIZATION, TUMOR; MULTIPLE RADIOPHARMACEUTICAL LOCALIZATION, TUMOR; WHOLE BOD RADIOPHARMACEUTICAL LOCALIZATION, TUMOR; TOMOGRAPH RADIOPHARMACEUTICAL LOCALIZATION, TUMOR; WHOLE BOD RADIOPHARMACEUTICAL LOCALIZATION, INFLAMMATORY PRO RADIOPHARMACEUTICAL LOCALIZATION, ABSCESS; WHOLE B RADIOPHARMACEUTICAL LOCALIZATION, ABSCESS; TOMOGRA NJX RP LOCLZJ NON-IMG PROBE STUDY INTRAVENOUS TUMOR IMAGING, POSITRON EMISSION TOMOGRAPHY (PET), TUMOR IMAGING, POSITRON EMISSION TOMOGRAPHY (PET), TUMOR IMAGING, POSITRON EMISSION TOMOGRAPHY (PET), TUMOR IMAGING, POSITRON EMISSION TOMOGRAPHY (PET) TUMOR IMAGING, POSITRON EMISSION TOMOGRAPHY (PET) TUMOR IMAGING, POSITRON EMISSION TOMOGRAPHY (PET) UNLISTED MISCELLANEOUS PROC, DX NUCLEAR MEDICINE RADIOPHARMACEUTICAL THERAPY, BY ORAL ADMINISTRATIO RADIOPHARMACEUTICAL THERAPY, BY IV ADMINISTRATION INTRACAVITARY RADIOACTIVE COLLOID THERAPY INTERSTITIAL RADIOACTIVE COLLOID THERAPY RADIOPHARMACEUTICAL THERAPY, RADIOLABELED MONOCLON INTRA-ARTICULAR RADIOPHARMACEUTICAL THERAPY RADIOPHARMACEUTICAL THERAPY, BY INTRA-ARTERIAL PAR UNLISTED RADIOPHARMACEUTICAL THERAPEUTIC PROC BASIC METABOLIC PANEL CALCIUM IONIZED BASIC METABOLIC PANEL GENERAL HEALTH PANEL ELECTROLYTE PANEL COMPREHENSIVE METABOLIC PANEL OBSTETRIC PANEL LIPID PANEL RENAL FUNCTION PANEL ACUTE HEPATITIS PANEL HEPATIC FUNCTION PANEL OBSTETRIC PANEL (INCLUDES HIV TESTING) ASSAY OF AMIKACIN DRUG SCREEN QUANTITATIVE CAFFEINE ASSAY OF CARBAMAZEPINE; TOTAL ASSAY OF CARBAMAZEPINE; FREE ASSAY OF CYCLOSPORINE DRUG SCREEN QUANTITATIVE CLOZAPINE DRUG SCREEN QUANTITATIVE DIGOXIN TOTAL DRUG SCREEN QUANTITATIVE DIGOXIN FREE DRUG ASSAY VALPROIC DIPROPYLACETIC ACID TOTAL DRUG SCREEN QUANT DIPROPYLACETIC ACID FREE ASSAY OF ETHOSUXIMIDE DRUG SCREEN QUANTITATIVE EVEROLIMUS ASSAY OF GENTAMICIN DRUG SCREEN QUANTITATIVE GABAPENTIN ASSAY OF HALOPERIDOL DRUG SCREEN QUANTITATIVE LAMOTRIGINE ASSAY OF LIDOCAINE DRUG SCREEN QUANTITATIVE LEVETIRACETAM ASSAY OF LITHIUM DRUG SCREEN QUANTITATIVE MYCOPHENOLATE DRUG SCREEN QUANTITATIVE OXCARBAZEPINE ASSAY OF PHENOBARBITAL ASSAY OF PHENYTOIN; TOTAL ASSAY OF PHENYTOIN; FREE ASSAY OF PRIMIDONE ASSAY OF PROCAINAMIDE ASSAY OF PROCAINAMIDE; W/METABOLITES ASSAY OF QUINIDINE SIROLIMUS ASSAY OF TACROLIMUS ASSAY OF THEOPHYLLINE DRUG SCREEN QUANTITATIVE TIAGABINE ASSAY OF TOBRAMYCIN ASSAY OF TOPIRAMATE ASSAY OF VANCOMYCIN DRUG SCREEN QUANTITATIVE ZONISAMIDE QUANTITATION DRUG NOT ELSEWHERE SPECIFIED DRUG SCREEN LIST A ANY NMBR NON TLC DEVICES DRUG SCREEN LIST A SINGLE DRUG CLASS METHOD DRUG SCREEN PRESUMPTIVE 1 CLASS METHOD LIST B DRUG SCREEN PRSMPTV 1/MULT CLASS METHOD TLC DRUG SCREEN PRSMPTV 1/MULT CLASS METHOD DRUG SCREEN QUANTITATIVE ALCOHOLS DRUG SCREEN QUANT ALCOHOLS BIOMARKERS 1 OR 2 DRUG SCREEN QUANT ALCOHOLS BIOMARKERS 3 OR MORE ALKALOIDS NOT OTHERWISE SPECIFIED DRUG SCREEN QUANT AMPHETAMINES 1 OR 2 DRUG SCREEN QUANT AMPHETAMINES 3 OR 4 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2015 1/1/2004 1/1/2015 1/1/2004 1/1/2014 1/1/2004 1/1/2014 1/1/2004 1/1/2014 1/1/2004 1/1/2014 1/1/2004 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 80326 80327 80328 80329 80330 80331 80332 80333 80334 80335 80336 80337 80338 80339 80340 80341 80342 80343 80344 80345 80346 80347 80348 80349 80350 80351 80352 80353 80354 80355 80356 80357 80358 80359 80360 80361 80362 80363 80364 80365 80366 80367 80368 80369 80370 80371 80372 80373 80374 80375 80376 80377 80400 80402 80406 80408 80410 80412 80414 80415 80416 80417 80418 80420 80422 80424 80426 80428 80430 80432 80434 80435 80436 80438 80439 80500 80502 81000 81001 81002 81003 81005 81007 81015 81020 81025 81050 81099 81161 81162 81170 DRUG SCREEN QUANT AMPHETAMINES 5 OR MORE DRUG SCREEN QUANT ANABOLIC STEROID 1 OR 2 DRUG SCREEN QUANT ANABOLIC STEROID 3 OR MORE DRUG SCREEN ANALGESICS NON-OPIOID 1 OR 2 DRUG SCREEN ANALGESICS NON-OPIOID 3-5 DRUG SCREEN ANALGESICS NON-OPIOID 6 OR MORE ANTIDEPRESSANTS SEROTONERGIC CLASS 1 OR 2 ANTIDEPRESSANTS SEROTONERGIC CLASS 3-5 ANTIDEPRESSANTS SEROTONERGIC CLASS 6 OR MORE ANTIDEPRESSANTS TRICYCLIC OTHER CYCLICALS 1 OR 2 ANTIDEPRESSANTS TRICYCLIC OTHER CYCLICALS 3-5 ANTIDEPRESSANTS TRICYCLIC OTHER CYCLICALS 6/MORE ANTIDEPRESSANTS NOT OTHERWISE SPECIFIED ANTIEPILEPTICS NOT OTHERWISE SPECIFIED 1-3 ANTIEPILEPTICS NOT OTHERWISE SPECIFIED 4-6 ANTIEPILEPTICS NOT OTHERWISE SPECIFIED 7/MORE ANTIPSYCHOTICS NOT OTHERWISE SPECIFIED 1-3 ANTIPSYCHOTICS NOT OTHERWISE SPECIFIED 4-6 ANTIPSYCHOTICS NOT OTHERWISE SPECIFIED 7/MORE DRUG SCREENING BARBITURATES DRUG SCREENING BENZODIAZEPINES 1-12 DRUG SCREENING BENZODIAZEPINES 13 OR MORE DRUG SCREENING BUPRENORPHINE DRUG SCREENING CANNABINOIDS NATURAL DRUG SCREENING CANNABINOIDS SYNTHETIC 1-3 DRUG SCREENING CANNABINOIDS SYNTHETIC 4-6 DRUG SCREENING CANNABINOIDS SYNTHETIC 7/MORE DRUG SCREENING COCAINE DRUG SCREENING FENTANYL DRUG SCREENING GABAPENTIN NON-BLOOD DRUG SCREENING HEROIN METABOLITE DRUG SCREENING KETAMINE AND NORKETAMINE DRUG SCREENING METHADONE DRUG SCREENING METHYLENEDIOXYAMPHETAMINES DRUG SCREENING METHYLPHENIDATE DRUG SCREENING OPIATES 1 OR MORE DRUG SCREENING OPIOIDS AND OPIATE ANALOGS 1 OR 2 DRUG SCREENING OPIOIDS AND OPIATE ANALOGS 3 OR 4 DRUG SCREENING OPIOIDS & OPIATE ANALOGS 5/MORE DRUG SCREENING OXYCODONE DRUG SCREENING PREGABALIN DRUG SCREENING PROPOXYPHENE DRUG SCREENING SEDATIVE HYPNOTICS DRUG SCREENING SKELETAL MUSCLE RELAXANTS 1 OR 2 DRUG SCREENING SKEL MUSCLE RELAXANTS 3 OR MORE DRUG SCREENING STIMULANTS SYNTHETIC DRUG SCREENING TAPENTADOL DRUG SCREENING TRAMADOL DRUG SCREEN STEREOISOMER ANALYSIS 1 DRUG CLASS DRUG/SUBSTANCE DEFINITIVE QUAL/QUANT NOS 1-3 DRUG/SUBSTANCE DEFINITIVE QUAL/QUANT NOS 4-6 DRUG/SUBSTANCE DEFINITIVE QUAL/QUANT NOS 7/MORE ACTH STIMULATION PANEL; ADRENAL INSUFFICIENCY ACTH STIMULATION PANEL; 21 HYDROXYLASE DEFICIENCY ACTH STIMULATION PANEL; 3 BETA-HYDROXYDEHYDROGENAS ALDOSTERONE SUPPRESSION EVAL PANEL CALCITONIN STIMULATION PANEL CORTICOTROPIC RELEASING HORMONE (CRH) STIMULATION CHORIONIC GONADOTROPIN STIMULATION PANEL; TESTOSTE CHORIONIC GONADOTROPIN STIMULATION PANEL; ESTRADIO RENAL VEIN RENIN STIMULATION PANEL PERIPHERAL VEIN RENIN STIMULATION PANEL COMBINED RAPID ANTERIOR PITUITARY EVAL PANEL DEXAMETHASONE SUPPRESSION PANEL, 48 HR GLUCAGON TOLERANCE PANEL; INSULINOMA GLUCAGON TOLERANCE PANEL; PHEOCHROMOCYTOMA GONADOTROPIN RELEASING HORMONE STIMULATION PANEL GROWTH HORMONE STIMULATION PANEL GROWTH HORMONE SUPPRESSION PANEL (GLUCOSE ADMINIST INSULIN-INDUCED C-PEPTIDE SUPPRESSION PANEL INSULIN TOLERANCE PANEL; ACTH INSUFFICIENCY INSULIN TOLERANCE PANEL; GROWTH HORMONE DEFICIENCY METYRAPONE PANEL TRH STIMULATION PANEL; 1 HR TRH STIMULATION PANEL; 2 HR CLINICAL PATHOLOGY CONSULTATION; LIMITED, W/O REVI CLINICAL PATHOLOGY CONSULTATION; COMPREHENSIVE, W/ URINALYSIS, DIP STICK/TABLET REAGENT; NON-AUTOMATE URINALYSIS, DIP STICK/TABLET REAGENT; AUTOMATED W/ URINALYSIS, DIP STICK/TABLET REAGENT; NON-AUTOMATE URINALYSIS, DIP STICK/TABLET REAGENT; AUTOMATED, W URINALYSIS; QUALITATIVE/SEMIQUANTITATIVE, EXCEPT I URINALYSIS; BACTERIURIA SCREEN, EXCEPT BY CULTURE/ URINALYSIS; MICROSCOPIC ONLY URINALYSIS; TWO/THREE GLASS TEST URINE PREGNANCY TEST, VISUAL COLOR COMPARISON METH VOLUME MEASUREMENT, TIMED COLLECTION, EACH UNLISTED URINALYSIS PROC DMD DUPLICATION/DELETION ANALYSIS BRCA1, BRCA2 (BREAST CANCER 1 AND 2) (EG, HEREDITARY BREAST AND OVARIAN CANCER) G ABL1 (ABL PROTO-ONCOGENE 1, NON-RECEPTOR TYROSINE KINASE) (EG, ACQUIRED IMATINIB 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2016 1/1/2016 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 81200 81201 81202 81203 81205 81206 81207 81208 81209 81210 81211 81212 81213 81214 81215 81216 81217 81218 81219 81220 81221 81222 81223 81224 81225 81226 81227 81228 81229 81235 81240 81241 81242 81243 81244 81245 81246 81250 81251 81252 81253 81254 81255 81256 81257 81260 81261 81262 81263 81264 81265 81266 81267 81268 81270 81272 81273 81275 81276 81280 81281 81282 81287 81288 81290 81291 81292 81293 81294 81295 81296 81297 81298 81299 81300 81301 81302 81303 81304 81310 81311 81313 81314 81315 81316 81317 81318 81319 81321 81322 81323 ASPA GENE ANALYSIS COMMON VARIANTS APC GENE ANALYSIS FULL GENE SEQUENCE APC GENE ANALYSIS KNOWN FAMILIAL VARIANTS APC GENE ANALYSIS DUPLICATION/DELETION VARIANTS BCKDHB GENE ANALYSIS COMMON VARIANTS BCR/ABL1 MAJOR BREAKPNT QUALITATIVE/QUANTITATIVE BCR/ABL1 MINOR BREAKPNT QUALITATIVE/QUANTITATIVE BCR/ABL1 OTHER BREAKPNT QUALITATIVE/QUANTITATIVE BLM GENE ANALYSIS 2281DEL6INS7 VARIANT BRAF GENE ANALYSIS V600E VARIANT BRCA1&BRCA2 FULL SEQ ANALYS/COMM DUP/DEL BRCA1 BRCA1&BRCA2 ANAL 185DELAG5385INSC/6174DELT BRCA1&BRCA2 ANAL UNCOMMON DUP/DEL VARIANTS BRCA1 FULL SEQ ANAL&COMMON DUP/DEL VARIANTS BRCA1 GENE ANALYSIS KNOWN FAMILIAL VARIANT BRCA2 GENE ANALYSIS FULL SEQUENCE ANALYSIS BRCA2 GENE ANALYSIS KNOWN FAMILIAL VARIANT CEBPA (CCAAT/ENHANCER BINDING PROTEIN [C/EBP], ALPHA) (EG, ACUTE MYELOID LEUKEMIA CALR (CALRETICULIN) (EG, MYELOPROLIFERATIVE DISORDERS), GENE ANALYSIS, COMMON VAR CFTR GENE ANALYSIS COMMON VARIANTS CFTR GENE ANALYSIS KNOWN FAMILIAL VARIANTS CFTR GENE ANALYSIS DUPLICATION/DELETION VARIANTS CFTR GENE ANALYSIS FULL GENE SEQUENCE CFTR GENE ANALYSIS INTRON 8 POLY-T ANALYSIS CYP2C19 GENE ANALYSIS COMMON VARIANTS CYP2D6 GENE ANALYSIS COMMON VARIANTS CYP2C9 GENE ANALYSIS COMMON VARIANTS CYTOGENOM CONST MICROARRAY COPY NUMBER VARIANTS CYTOGENOM CONST MICROARRAY COPY NUMBER&SNP VAR EGFR GENE ANALYSIS COMMON VARIANTS F2 GENE ANALYSIS 20210G >A VARIANT F5 COAGULATION FACTOR V ANAL LEIDEN VARIANT FANCC GENE ANALYSIS COMMON VARIANT FMR1 ANALYSIS EVAL TO DETECT ABNORMAL ALLELES FMR1 GENE ANALYSIS CHARACTERIZATION OF ALLELES FLT3 GENE ANALYSIS INTERNAL TANDEM DUP VARIANTS FLT3 GENE ANLYS TYROSINE KINASE DOMAIN VARIANTS G6PC GENE ANALYSIS COMMON VARIANTS GBA GLUCOSIDASE/BETA/ACID ANAL COMM VARIANTS GJB2 GENE ANALYSIS FULL GENE SEQUENCE GJB2 GENE ANALYSIS KNOWN FAMILIAL VARIANTS GJB6 GENE ANALYSIS COMMON VARIANTS HEXA GENE ANALYSIS COMMON VARIANTS HFE HEMOCHROMATOSIS GENE ANAL COMMON VARIANTS HBA1/HBA2 ANALYSIS FOR COMMON DELETIONS/VARIANT IKBKAP GENE ANALYSIS COMMON VARIANTS IGH@ REARRANGE ABNORMAL CLONAL POP AMPLIFIED IGH@ REARRANGE ABNORMAL CLONAL POP DIRECT PROBE IGH@ VARIABLE REGION SOMATIC MUTATION ANALYSIS IGK@ GENE REARRANGE DETECT ABNORMAL CLONAL POP COMPARATIVE ANAL STR MARKERS PATIENT&COMP SPEC COMPARATIVE ANAL STR MARKERS EA ADDL SPECIMEN CHIMERISM W/COMP TO BASELINE W/O CELL SELECTION CHIMERISM W/COMP TO BASELINE W/CELL SELECTION EA JAK2 GENE ANALYSIS P.VAL617PHE VARIANT KIT (V-KIT HARDY-ZUCKERMAN 4 FELINE SARCOMA VIRAL ONCOGENE HOMOLOG) (EG, GASTRO KIT (V-KIT HARDY-ZUCKERMAN 4 FELINE SARCOMA VIRAL ONCOGENE HOMOLOG) (EG, MASTO KRAS GENE ANALYSIS VARIANTS IN CODONS 12 AND 13 KRAS (KIRSTEN RAT SARCOMA VIRAL ONCOGENE HOMOLOG) (EG, CARCINOMA) GENE ANALYS LONG QT SYNDROME FULL SEQUENCE ANALYSIS LONG QT SYNDROME ANAL KNOWN FAMILIAL SEQUENCE LONG QT SYNDROME GENE ANAL DUP/DEL VARIANTS MGMT METHYLATION ANALYSIS MLH1 GENE ANALYSIS PROMOTER METHYLATION ANALYSIS MCOLN1 MUCOLIPIN1 GENE ANALYSIS COMMON VARIANTS MTHFR GENE ANALYSIS COMMON VARIANTS MLH1 GENE ANALYSIS FULL SEQUENCE ANALYSIS MLH1 GENE ANALYSIS KNOWN FAMILIAL VARIANTS MLH1 GENE ANALYSIS DUPLICATION/DELETION VARIANTS MSH2 GENE ANALYSIS FULL SEQUENCE ANALYSIS MSH2 GENE ANALYSIS KNOWN FAMILIAL VARIANTS MSH2 GENE ANALYSIS DUPLICATION/DELETION VARIANTS MSH6 GENE ANALYSIS FULL SEQUENCE ANALYSIS MSH6 GENE ANALYSIS KNOWN FAMILIAL VARIANTS MSH6 GENE ANALYSIS DUPLICATION/DELETION VARIA MICROSATELLITE INSTAB ANAL MISMATCH REPAIR DEF MECP2 GENE ANALYSIS FULL SEQUENCE MECP2 GENE ANALYSIS KNOWN FAMILIAL VARIANT MECP2 GENE ANALYSIS DUPLICATION/DELETION VARIANT NPM1 NUCLEOPHOSMIN GENE ANAL EXON 12 VARIANTS NRAS (NEUROBLASTOMA RAS VIRAL [V-RAS] ONCOGENE HOMOLOG) (EG, COLORECTAL CARCI PCA3/KLK3 PROSTATE SPECIFIC ANTIGEN RATIO PDGFRA (PLATELET-DERIVED GROWTH FACTOR RECEPTOR, ALPHA POLYPEPTIDE) (EG, GASTRO PML/RARALPHA COMMON BREAKPOINTS QUAL/QUANT PML/RARALPHA SINGLE BREAKPOINT QUAL/QUAN PMS2 GENE ANALYSIS FULL SEQUENCE PMS2 GENE ANALYSIS KNOWN FAMILIAL VARIANTS PMS2 GENE ANALYSIS DUPLICATION/DELETION VARIANTS PTEN GENE ANALYSIS FULL SEQUENCE ANALYSIS PTEN GENE ANALYSIS KNOWN FAMILIAL VARIANT PTEN GENE ANALYSIS DUPLICATION/DELETION VARIANT 1/1/2012 1/1/2013 1/1/2013 1/1/2013 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2016 1/1/2016 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2013 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2015 1/1/2012 1/1/2012 1/1/2013 1/1/2013 1/1/2013 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2016 1/1/2016 1/1/2012 1/1/2016 1/1/2012 1/1/2012 1/1/2012 1/1/2014 1/1/2015 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2016 1/1/2015 1/1/2016 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2013 1/1/2013 1/1/2013 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N Y N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N Y N Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 81324 81325 81326 81330 81331 81332 81340 81341 81342 81350 81355 81370 81371 81372 81373 81374 81375 81376 81377 81378 81379 81380 81381 81382 81383 81400 81401 81402 81403 81404 81405 81406 81407 81408 81410 81411 81412 81415 81416 81417 81420 81425 81426 81427 81430 81431 81432 81433 81434 81435 81436 81437 81438 81440 81442 81445 81450 81455 81460 81465 81470 81471 81479 81490 81493 81500 81503 81504 81506 81507 81508 81509 81510 81511 81512 81519 81525 81528 81535 81536 81538 81540 81545 81595 81599 82009 82010 82013 82016 82017 82024 PMP22 GENE ANAL DUPLICATION/DELETION ANALYSIS PMP22 GENE ANALYSIS FULL SEQUENCE ANALYSIS PMP22 GENE ANALYSIS KNOWN FAMILIAL VARIANT SMPD1 GENE ANALYSIS COMMON VARIANTS SNRPN/UBE3A METHYLATION ANALYSIS SERPINA1 GENE ANALYSIS COMMON VARIANTS TRB@ REARRANGEMENT ANAL AMPLIFICATION METHOD TRB@ REARRANGEMENT ANAL DIRECT PROBE METHODOLOGY TRG@ GENE REARRANGEMENT ANALYSIS UGT1A1 GENE ANALYSIS COMMON VARIANTS VKORC1 GENE ANALYSIS COMMON VARIANTS HLA CLASS I&II LOW HLA-A -B -C -DRB1/3/4/5&-DQB1 HLA I&LI LOW RESOLUTION HLA-A -B&-DRB1 HLA CLASS I TYPING LOW RESOLUTION COMPLETE HLA CLASS I TYPING LOW RESOLUTION ONE LOCUS EACH HLA I LOW RESOLUTION ONE ANTIGEN EQUIVALENT EACH HLA II LOW RESOLUTION HLA-DRB1/3/4/5 AND -DQB1 HLA CLASS II TYPING LOW RESOLUTION ONE LOCUS EA HLA II LOW RESOLUTION ONE ANTIGEN EQUIVALENT EA HLA I&II HIGH RESOLUTION HLA-A -B -C AND -DRB1 HLA CLASS I TYPING HIGH RESOLUTION COMPLETE HLA CLASS I TYPING HIGH RESOLUTION ONE LOCUS EA HLA I TYPING HIGH RESOLUTION 1 ALLELE/ALLELE GRP HLA CLASS II TYPING HIGH RESOLUTION ONE LOCUS EA HLA II HIGH RESOLUTION 1 ALLELE/ALLELE GROUP MOLECULAR PATHOLOGY PROCEDURE LEVEL 1 MOLECULAR PATHOLOGY PROCEDURE LEVEL 2 MOLECULAR PATHOLOGY PROCEDURE LEVEL 3 MOLECULAR PATHOLOGY PROCEDURE LEVEL 4 MOLECULAR PATHOLOGY PROCEDURE LEVEL 5 MOLECULAR PATHOLOGY PROCEDURE LEVEL 6 MOLECULAR PATHOLOGY PROCEDURE LEVEL 7 MOLECULAR PATHOLOGY PROCEDURE LEVEL 8 MOLECULAR PATHOLOGY PROCEDURE LEVEL 9 AORTIC DYSFUNCTION/DILATION GENOMIC SEQ ANALYSIS AORTIC DYSFUNCTION/DILATION DUP/DEL ANALYSIS ASHKENAZI JEWISH ASSOCIATED DISORDERS, GENOMIC SEQUENCE ANALYSIS PANEL, MUST IN EXOME SEQUENCE ANALYSIS EXOME SEQUENCE ANALYSIS EACH COMPARATOR EXOME EXOME RE-EVAL OF PREVIOUSLY OBTAINED EXOME SEQ FETAL CHROMOSOMAL ANEUPLOIDY GENOMIC SEQ ANALYS GENOME SEQUENCE ANALYSIS GENOME SEQUENCE ANALYSIS EACH COMPARATOR GENOME GENOME RE-EVALUATION OF PREC OBTAINED GENOME SEQ HEARING LOSS GENOMIC SEQUENCE ANALYSIS 60 GENES HEARING LOSS DUP/DEL ANALYSIS HEREDITARY BREAST CANCER-RELATED DISORDERS; GENOMIC SEQUENCE ANALYSIS PANEL, M HEREDITARY BREAST CANCER-RELATED DISORDERS (EG, HEREDITARY BREAST CANCER, HERED HEREDITARY RETINAL DISORDERS (EG, RETINITIS PIGMENTOSA, LEBER CONGENITAL AMAUROS HEREDITARY COLON CA GENOMIC SEQ ANALYS 7 GENES HEREDITARY COLON CA SYND DUP/DEL ANALYS 8 GENES HEREDITARY NEUROENDOCRINE TUMOR DISORDERS (EG, MEDULLARY THYROID CARCINOMA, HEREDITARY NEUROENDOCRINE TUMOR DISORDERS (EG, MEDULLARY THYROID CARCINOMA, NUCLEAR MITOCHONDRIAL 100 GENE GENOMIC SEQ NOONAN SPECTRUM DISORDERS, GENOMIC SEQUENCE ANALYSIS PANEL, MUST INCLUDE SEQ TARGETED GENOMIC SEQ ANALYS DNA ANALYS 5-50 GENE GENOMIC SEQ ANALYS DNA&RNA ANALYS 5-50 GENE GENOMIC SEQ ANALYS DNA&RNA ANALYS 51/MORE GENES WHOLE MITOCHONDRIAL GENOME WHOLE MITOCHONDRIAL GENOME ANALYSIS PANEL X-LINKED INTELLECTUAL DBLT GENOMIC SEQ ANALYS X-LINKED INTELLECTUAL DBLT DUP/DEL GENE ANALYS UNLISTED MOLELCULAR PATHOLOGY PROCEDURE AUTOIMMUNE (RHEUMATOID ARTHRITIS), ANALYSIS OF 12 BIOMARKERS USING IMMUNOASS CORONARY ARTERY DISEASE, MRNA, GENE EXPRESSION PROFILING BY REAL-TIME RT-PCR OF 2 ONCO (OVARIAN) BIOCHEMICAL ASSAY TWO PROTEINS ONCO (OVARIAN) BIOCHEMICAL ASSAY FIVE PROTEINS ONCOLOGY TISSUE OF ORIGIN SIMILAR SCOR ALGORITHM ENDOCRINOLOGY BIOCHEMICAL ASSAY SEVEN ANAL FETAL ANEUPLOIDY 21 18 13 SEQ ANALY TRISOM RISK FETAL CONGENITAL ABNOR ASSAY TWO PROTEINS FETAL CONGENITAL ABNOR ASSAY 3 PROTEINS FETAL CONGENITAL ABNOR ASSAY THREE ANAL FETAL CONGENITAL ABNOR ASSAY FOUR ANAL FETAL CONGENITAL ABNOR ASSAY FIVE ANAL ONCOLOGY BREAST MRNA GENE EXPRESSION 21 GENES ONCOLOGY (COLON), MRNA, GENE EXPRESSION PROFILING BY REAL-TIME RT-PCR OF 12 GENE ONCOLOGY (COLORECTAL) SCREENING, QUANTITATIVE REAL-TIME TARGET AND SIGNAL AMPL ONCOLOGY (GYNECOLOGIC), LIVE TUMOR CELL CULTURE AND CHEMOTHERAPEUTIC RESPONS ONCOLOGY (GYNECOLOGIC), LIVE TUMOR CELL CULTURE AND CHEMOTHERAPEUTIC RESPONS ONCOLOGY (LUNG), MASS SPECTROMETRIC 8-PROTEIN SIGNATURE, INCLUDING AMYLOID A, U ONCOLOGY (TUMOR OF UNKNOWN ORIGIN), MRNA, GENE EXPRESSION PROFILING BY REAL-T ONCOLOGY (THYROID), GENE EXPRESSION ANALYSIS OF 142 GENES, UTILIZING FINE NEEDLE AS CARDIOLOGY (HEART TRANSPLANT), MRNA, GENE EXPRESSION PROFILING BY REAL-TIME QUA UNLISTED MULTIANALYTE ASSAY ALGORITHMIC ANALYSIS KETONE BODIES SERUM QUALITATIVE KETONE BODIES SERUM QUANTITATIVE ACETYLCHOLINESTERASE ACYLCARNITINES; QUALITATIVE, EACH SPECIMEN ACYLCARNITINES; QUANTITATIVE, EACH SPECIMEN ASSAY OF ADRENOCORTICOTROPIC HORMONE (ACTH) 1/1/2013 1/1/2013 1/1/2013 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2015 1/1/2015 1/1/2016 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2016 1/1/2016 1/1/2016 1/1/2015 1/1/2015 1/1/2016 1/1/2016 1/1/2015 1/1/2016 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2013 1/1/2016 1/1/2016 1/1/2013 1/1/2013 1/1/2014 1/1/2013 1/1/2014 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2015 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N Y Y Y Y Y Y Y Y Y N N N Y Y N N Y N Y Y Y Y Y Y Y Y N N Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 82030 82040 82042 82043 82044 82045 82075 82085 82088 82103 82104 82105 82106 82107 82108 82120 82127 82128 82131 82135 82136 82139 82140 82143 82150 82154 82157 82160 82163 82164 82172 82175 82180 82190 82232 82239 82240 82247 82248 82252 82261 82270 82271 82272 82274 82286 82300 82306 82308 82310 82330 82331 82340 82355 82360 82365 82370 82373 82374 82375 82376 82378 82379 82380 82382 82383 82384 82387 82390 82397 82415 82435 82436 82438 82441 82465 82480 82482 82485 82486 82487 82488 82489 82491 82492 82495 82507 82523 82525 82528 82530 ADENOSINE, 5-MONOPHOSPHATE, CYCLIC (CYCLIC AMP) ALBUMIN; SERUM ALBUMIN; URINE OR OTHER SOURCE, QUANTITATIVE, EACH ALBUMIN; URINE, MICROALBUMIN, QUANTITATIVE ALBUMIN; URINE, MICROALBUMIN, SEMIQUANTITATIVE ALBUMIN; URINE, MICROALBUMIN, ISCHEMIA MODIFIED ASSAY OF ALCOHOL BREATH ALDOLASE ALDOSTERONE ALPHA-1-ANTITRYPSIN; TOTAL ALPHA-1-ANTITRYPSIN; PHENOTYPE ALPHA-FETOPROTEIN; SERUM ALPHA-FETOPROTEIN; AMNIOTIC FLUID Alpha-fetoprotein l3 ALUMINUM AMINES, VAGINAL FLUID, QUALITATIVE AMINO ACIDS; SINGLE, QUALITATIVE, EACH SPECIMEN AMINO ACIDS; MULTIPLE, QUALITATIVE, EACH SPECIMEN AMINO ACIDS; SINGLE, QUANTITATIVE, EACH SPECIMEN AMINOLEVULINIC ACID, DELTA (ALA) AMINO ACIDS, 2-5 AMINO ACIDS, QUANTITATIVE, EACH S AMINO ACIDS, 6 OR MORE AMINO ACIDS, QUANTITATIVE, AMMONIA AMNIOTIC FLUID SCAN (SPECTROPHOTOMETRIC) AMYLASE ANDROSTANEDIOL GLUCURONIDE ANDROSTENEDIONE ANDROSTERONE ANGIOTENSIN II ANGIOTENSIN I - CONVERTING ENZYME (ACE) APOLIPOPROTEIN, EACH ARSENIC ASCORBIC ACID (VITAMIN C), BLOOD ATOMIC ABSORPTION SPECTROSCOPY, EACH ANALYTE BETA-2 MICROGLOBULIN BILE ACIDS; TOTAL BILE ACIDS; CHOLYLGLYCINE BILIRUBIN; TOTAL BILIRUBIN; DIRECT BILIRUBIN; FECES, QUALITATIVE BIOTINIDASE, EACH SPECIMEN BLOOD, OCCULT, BY PEROXIDASE ACTIVITY, QUALITATIVE BLOOD, OCCULT; OTHER SOURCES Blood, occult, by peroxidase activity (eg. guaiac) BLOOD, OCCULT, FECAL HEMOGLOBIN DETERMIN, IMMUNOAS BRADYKININ CADMIUM 25 HYDROXY INCLUDES FRACTIONS IF PERFORMED CALCITONIN CALCIUM; TOTAL CALCIUM; IONIZED CALCIUM; AFTER CALCIUM INFUSION TEST CALCIUM; URINE QUANTITATIVE, TIMED SPECIMEN CALCULUS; QUALITATIVE ANALYSIS CALCULUS; QUANTITATIVE ANALYSIS, CHEMICAL CALCULUS; INFRARED SPECTROSCOPY CALCULUS; X-RAY DIFFRACTION CARBOHYDRATE DEFICIENT TRANSFERRIN CARBON DIOXIDE (BICARBONATE) CARBON MONOXIDE, (CARBOXYHEMOGLOBIN); QUANTITATIVE CARBON MONOXIDE, (CARBOXYHEMOGLOBIN); QUALITATIVE CARCINOEMBRYONIC ANTIGEN (CEA) CARNITINE (TOTAL AND FREE), QUANTITATIVE, EACH SPE CAROTENE CATECHOLAMINES; TOTAL URINE CATECHOLAMINES; BLOOD CATECHOLAMINES; FRACTIONATED CATHEPSIN-D CERULOPLASMIN CHEMILUMINESCENT ASSAY CHLORAMPHENICOL CHLORIDE; BLOOD CHLORIDE; URINE CHLORIDE; OTHER SOURCE CHLORINATED HYDROCARBONS, SCREEN CHOLESTEROL, SERUM/WHOLE BLOOD, TOTAL CHOLINESTERASE; SERUM CHOLINESTERASE; RBC CHONDROITIN B SULFATE, QUANTITATIVE CHROMATOGRAPHY, QUALITATIVE; COLUMN, ANALYTE NOT E CHROMATOGRAPHY, QUALITATIVE; PAPER, 1-DIMENSIONAL, CHROMATOGRAPHY, QUALITATIVE; PAPER, 2-DIMENSIONAL, CHROMATOGRAPHY, QUALITATIVE; THIN LAYER, ANALYTE N CHROMATOGRAPHY, QUANTITATIVE, COLUMN; SINGLE ANALY CHROMATOGRAPHY, QUANTITATIVE, COLUMN; MULTIPLE ANA CHROMIUM CITRATE COLLAGEN CROSS LINKS, ANY METHOD COPPER CORTICOSTERONE CORTISOL; FREE 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 82533 82540 82541 82542 82543 82544 82550 82552 82553 82554 82565 82570 82575 82585 82595 82600 82607 82608 82610 82615 82626 82627 82633 82634 82638 82652 82656 82657 82658 82664 82668 82670 82671 82672 82677 82679 82693 82696 82705 82710 82715 82725 82726 82728 82731 82735 82746 82747 82757 82759 82760 82775 82776 82777 82784 82785 82787 82800 82803 82805 82810 82820 82830 82930 82938 82941 82943 82945 82946 82947 82948 82950 82951 82952 82955 82960 82962 82963 82965 82977 82978 82979 82985 83001 83002 83003 83006 83009 83010 83012 83013 CORTISOL; TOTAL CREATINE COL-CHR/MS QUAL 1 STATIONARY&MOBILE PHASE NES COL-CHR/MS QUAN 1 STATIONARY&MOBILE PHASE NES COL-CHR/MS STABLE ISOTOPE DIL 1 ANALYTE NES COL-CHR/MS STABLE ISOTOPE DIL MLT ANALYTES NES CREATINE KINASE (CK), (CPK); TOTAL CREATINE KINASE (CK), (CPK); ISOENZYMES CREATINE KINASE (CK), (CPK); MB FRACTION ONLY CREATINE KINASE (CK), (CPK); ISOFORMS CREATININE; BLOOD CREATININE; OTHER SOURCE CREATININE; CLEARANCE CRYOFIBRINOGEN CRYOGLOBULIN, QUALITATIVE OR SEMI-QUANTITATIVE CYANIDE CYANOCOBALAMIN (VITAMIN B-12) CYANOCOBALAMIN (VITAMIN B-12); UNSATURATED BINDING CYSTATIN C CYSTINE AND HOMOCYSTINE, URINE, QUALITATIVE DEHYDROEPIANDROSTERONE (DHEA) DEHYDROEPIANDROSTERONE-SULFATE (DHEA-S) DESOXYCORTICOSTERONE, 11DEOXYCORTISOL, 11DIBUCAINE NUMBER DIHYDROXYVITAMIN D, 1,25ELASTASE, PANCREATIC (El-1) FECAL, QUALITATIVE ENZYME ACTIVITY, CELLS/TISSUE NOT ELSEWHERE SPECIF ENZYME ACTIVITY, CELLS/TISSUE NOT ELSEWHERE SPECIF ELECTROPHORETIC TECHNIQUE, NOT ELSEWHERE SPECIFIED ERYTHROPOIETIN ESTRADIOL ESTROGENS; FRACTIONATED ESTROGENS; TOTAL ESTRIOL ESTRONE ETHYLENE GLYCOL ETIOCHOLANOLONE FAT/LIPIDS, FECES; QUALITATIVE FAT/LIPIDS, FECES; QUANTITATIVE FAT DIFFERENTIAL, FECES, QUANTITATIVE FATTY ACIDS, NONESTERIFIED VERY LONG CHAIN FATTY ACIDS FERRITIN FETAL FIBRONECTIN, CERVICOVAGINAL SECRETIONS, SEMI FLUORIDE FOLIC ACID; SERUM FOLIC ACID; RBC FRUCTOSE, SEMEN GALACTOKINASE, RBC GALACTOSE GALACTOSE-1-PHOSPHATE URIDYL TRANSFERASE; QUANTITA GALACTOSE-1-PHOSPHATE URIDYL TRANSFERASE; SCREEN GALECTIN 3 GAMMAGLOBULIN; IGA, IGD, IGG, IGM, EACH GAMMAGLOBULIN; IGE GAMMAGLOBULIN; IMMUNOGLOBULIN SUBCLASSES, (IGG1/2/ GASES, BLOOD, PH ONLY GASES, BLOOD, PH, PCO2, PO2, CO2, HCO3, (W/CALCULA GASES, BLOOD, PH, PCO2, PO2, CO2, HCO3; W/O2 SAT, GASES, BLOOD, O2 SAT ONLY, DIRECT MEASURE, NOT PUL HEMOGLOBIN-OXYGEN AFFINITY (PO2, 50 PCT HEMOGLOBIN GASTRIC ACID ANALYIS W/PH EA SPECIMEN Gastric acid analysis, includes pH if performed, each specimen GASTRIN AFTER SECRETIN STIMULATION GASTRIN GLUCAGON GLUCOSE, BODY FLUID, OTHER THAN BLOOD GLUCAGON TOLERANCE TEST GLUCOSE; QUANTITATIVE, BLOOD (EXCEPT REAGENT STRIP GLUCOSE; BLOOD, REAGENT STRIP GLUCOSE; POST GLUCOSE DOSE (INCLUDES GLUCOSE) GLUCOSE; TOLERANCE TEST (GTT), 3 SPECIMENS (INCLUD GLUCOSE TOLERANCE EA ADDL BEYOND 3 SPECIMENS GLUCOSE-6-PHOSPHATE DEHYDROGENASE (G6PD); QUANTITA GLUCOSE-6-PHOSPHATE DEHYDROGENASE (G6PD); SCREEN GLUCOSE, BLOOD, GLUCOSE MONITORING DEVICE(S) CLEAR GLUCOSIDASE, BETA GLUTAMATE DEHYDROGENASE GLUTAMYLTRANSFERASE, GAMMA (GGT) GLUTATHIONE GLUTATHIONE REDUCTASE, RBC GLYCATED PROTEIN GONADOTROPIN; FOLLICLE STIMULATING HORMONE (FSH) GONADOTROPIN; LUTEINIZING HORMONE (LH) GROWTH HORMONE, HUMAN (HGH) (SOMATOTROPIN) GROWTH STIMULATION EXPRESSED GENE 2 HELICOBACTER PYLORI, BLLOD TEST ANALYSIS FOR UREAS HAPTOGLOBIN; QUANTITATIVE HAPTOGLOBIN; PHENOTYPES H. PYLORI; ANALYSIS FOR UREASE ACTIVITY, NON-RADIO 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2005 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 83014 83015 83018 83020 83021 83026 83030 83033 83036 83037 83045 83050 83051 83060 83065 83068 83069 83070 83080 83088 83090 83150 83491 83497 83498 83499 83500 83505 83516 83518 83519 83520 83525 83527 83528 83540 83550 83570 83582 83586 83593 83605 83615 83625 83630 83631 83632 83633 83655 83661 83662 83663 83664 83670 83690 83695 83698 83700 83701 83704 83718 83719 83721 83727 83735 83775 83785 83788 83789 83825 83835 83857 83861 83864 83872 83873 83874 83876 83880 83883 83885 83915 83916 83918 83919 83921 83930 83935 83937 83945 83950 BREATH TEST, H. PYLORI, MASS SPECTOMETRY, DRUG ADM HEAVY METAL (ARSENIC, BARIUM, BERYLLIUM, BISMUTH, HEAVY METAL (ARSENIC, BARIUM, BERYLLIUM, BISMUTH, HEMOGLOBIN FRACTIONATION AND QUANTITATION; ELECTRO HEMOGLOBIN FRACTIONATION AND QUANTITATION; CHROMAT HEMOGLOBIN; COPPER SULFATE METHOD, NON-AUTOMATED HEMOGLOBIN; F (FETAL), CHEMICAL HEMOGLOBIN; F (FETAL), QUALITATIVE HEMOGLOBIN; GLYCATED HEMOGLOBIN; GLYCOSYLATED (A1C) BY DEVICE HEMOGLOBIN; METHEMOGLOBIN, QUALITATIVE HEMOGLOBIN; METHEMOGLOBIN, QUANTITATIVE HEMOGLOBIN; PLASMA HEMOGLOBIN; SULFHEMOGLOBIN, QUANTITATIVE HEMOGLOBIN; THERMOLABILE HEMOGLOBIN; UNSTABLE, SCREEN HEMOGLOBIN; URINE ASSAY OF HEMOSIDERIN QUALITATIVE B-HEXOSAMINIDASE, EACH ASSAY HISTAMINE HOMOCYSTINE HOMOVANILLIC ACID (HVA) HYDROXYCORTICOSTEROIDS, 17- (17-OHCS) HYDROXYINDOLACETIC ACID, 5-(HIAA) HYDROXYPROGESTERONE, 17-D HYDROXYPROGESTERONE, 20HYDROXYPROLINE; FREE HYDROXYPROLINE; TOTAL IMMUNOASSAY ANALYTE QUAL/SEMIQUAL MULTIPLE STEP IMMUNOASSAY ANALYTE QUAL/SEMIQUAL SINGLE STEP IMMUNOASSAY, ANALYTE, QUANTITATIVE; RADIOPHARMACEU IMMUNOASSAY, ANALYTE, QUANTITATIVE; NOT OTHERWISE INSULIN; TOTAL INSULIN; FREE INTRINSIC FACTOR IRON IRON BINDING CAPACITY ISOCITRIC DEHYDROGENASE (IDH) KETOGENIC STEROIDS, FRACTIONATION KETOSTEROIDS, 17- (17-KS); TOTAL KETOSTEROIDS, 17- (17-KS); FRACTIONATION LACTATE (LACTIC ACID) LACTATE DEHYDROGENASE (LD), (LDH) LACTATE DEHYDROGENASE (LD), (LDH); ISOENZYMES, SEP LACTOFERRIN, FECAL, QUALITATIVE LACTOFERRIN, FECAL, QUANITATIVE LACTOGEN, HUMAN PLACENTAL (HPL) HUMAN CHORIONIC SO LACTOSE URINE QUALITATIVE LEAD FETAL LUNG MATURITY ASSESSMENT; LECITHIN SPHINGOMY FETAL LUNG MATURITY ASSESSMENT; FOAM STABILITY TES FETAL LUNG MATURITY ASSESSMENT; FLUORESCENCE POLAR FETAL LUNG MATURITY ASSESSMENT; LAMELLAR BODY DENS LEUCINE AMINOPEPTIDASE (LAP) LIPASE LIPOPROTEIN (A) Assay lipoprotein pla2 LIPOPROTEIN, BLOOD, ELCTROPHORETIC SEPARATION & QU LIPOPROTEIN, BLOOD, HIGH RESOLUTION FRACTIONATION LIPOPROTEIN, BLOOD, QUANTITATION OF LIPOPROTEIN PA LIPOPROTEIN, DIRECT MEASUREMENT; HIGH DENSITY CHOL LIPOPROTEIN, DIRECT MEASUREMENT; DIRECT MEASUREMEN LIPOPROTEIN, DIRECT MEASUREMENT; DIRECT MEASUREMEN LUTEINIZING RELEASING FACTOR (LRH) MAGNESIUM MALATE DEHYDROGENASE MANGANESE MASS/TANDEM SPECTROMETRY, ANALYTE NOT ELSEWHERE CL MASS/TANDEM SPECTROMETRY, ANALYTE NOT ELSEWHERE CL MERCURY, QUANTITATIVE METANEPHRINES METHEMALBUMIN MICROFLUID ANALYSIS TEAR OSMOLARITY MUCOPOLYSACCHARIDES ACID QUANTITATIVE MUCIN, SYNOVIAL FLUID (ROPES TEST) MYELIN BASIC PROTEIN, CSF MYOGLOBIN MYELOPEROXIDASE MPO NATRIURETIC PEPTIDE NEPHELOMETRY, EACH ANALYTE NOT ELSEWHERE SPECIFIED NICKEL NUCLEOTIDASE 5OLIGOCLONAL IMMUNOGLOBULIN (OLIGOCLONAL BANDS) ORGANIC ACIDS; TOTAL, QUANTITATIVE, EACH SPECIMEN ORGANIC ACIDS; QUALITATIVE, EACH SPECIMEN ORGANIC ACID, SINGLE, QUANTITATIVE OSMOLALITY; BLOOD OSMOLALITY; URINE OSTEOCALCIN (BONE G1A PROTEIN) OXALATE ONCOPROTEIN, HER-2/NEU 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2007 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 83951 83970 83986 83987 83992 83993 84030 84035 84060 84061 84066 84075 84078 84080 84081 84085 84087 84100 84105 84106 84110 84112 84119 84120 84126 84132 84133 84134 84135 84138 84140 84143 84144 84145 84146 84150 84152 84153 84154 84155 84156 84157 84160 84163 84165 84166 84181 84182 84202 84203 84206 84207 84210 84220 84228 84233 84234 84235 84238 84244 84252 84255 84260 84270 84275 84285 84295 84300 84302 84305 84307 84311 84315 84375 84376 84377 84378 84379 84392 84402 84403 84425 84430 84431 84432 84436 84437 84439 84442 84443 84445 ONCOPROTEIN DES-GAMMA-CARBOXY-PROTHROMBIN DCP PARATHORMONE (PARATHYROID HORMONE) PH, BODY FLUID, EXCEPT BLOOD PH EXHALED BREATH CONDENSATE PHENCYCLIDINE (PCP) CALPROTECTIN FECAL PHENYLALANINE (PKU), BLOOD PHENYLKETONES, QUALITATIVE PHOSPHATASE, ACID; TOTAL PHOSPHATASE, ACID; FORENSIC EXAM PHOSPHATASE, ACID; PROSTATIC PHOSPHATASE, ALKALINE PHOSPHATASE, ALKALINE; HEAT STABLE (TOTAL NOT INCL PHOSPHATASE, ALKALINE; ISOENZYMES PHOSPHATIDYLGLYCEROL PHOSPHOGLUCONATE, 6-, DEHYDROGENASE, RBC PHOSPHOHEXOSE ISOMERASE PHOSPHORUS INORGANIC (PHOSPHATE) PHOSPHORUS INORGANIC (PHOSPHATE); URINE PORPHOBILINOGEN, URINE; QUALITATIVE PORPHOBILINOGEN, URINE; QUANTITATIVE EVAL C/V AMNIOTIC FLUID PROTEIN QUAL EA SPECIMEN PORPHYRINS, URINE; QUALITATIVE PORPHYRINS, URINE; QUANTITATION AND FRACTIONATION PORPHYRINS FECES QUANTITATIVE POTASSIUM; SERUM POTASSIUM; URINE PREALBUMIN PREGNANEDIOL PREGNANETRIOL PREGNENOLONE 17-HYDROXYPREGNENOLONE PROGESTERONE PROCALCITONIN (PCT) PROLACTIN PROSTAGLANDIN, EACH PROSTATE SPECIFIC ANTIGEN (PSA); COMPLEXED (DIRECT PROSTATE SPECIFIC ANTIGEN (PSA); TOTAL PROSTATE SPECIFIC ANTIGEN (PSA); FREE PROTEIN, TOTAL, EXCEPT REFRACTOMETRY; SERUM PROTEIN, TOTAL, EXCEPT REFRACTOMETRY; URINE PROTEIN, TOTAL, EXCEPT REFRACTOMETRY; OTHER PROTEIN, TOTAL, EXCEPT REFRACTOMETRY; ANY SOURCE PREGNANCY-ASSOCIATED PLASMA PROTEIN PROTEIN, ELECTROPHORETIC FRACTIONATION AND QUANTIT PROTEIN, ELECTROPHORETIC FRACTIONATION AND QUANTIT PROTEIN; WESTERN BLOT, W/INTERPRETATION AND REPORT PROTEIN; WESTERN BLOT, W/INTERPRETATION AND REPORT PROTOPORPHYRIN, RBC; QUANTITATIVE PROTOPORPHYRIN, RBC; SCREEN PROINSULIN PYRIDOXAL PHOSPHATE (VITAMIN B-6) PYRUVATE PYRUVATE KINASE QUININE RECEPTOR ASSAY; ESTROGEN RECEPTOR ASSAY; PROGESTERONE RECEPTOR ASSAY; ENDOCRINE, OTHER THAN ESTROGEN/PRO RECEPTOR ASSAY; NON-ENDOCRINE RENIN RIBOFLAVIN (VITAMIN B-2) SELENIUM SEROTONIN SEX HORMONE BINDING GLOBULIN (SHBG) SIALIC ACID SILICA SODIUM; SERUM SODIUM; URINE SODIUM; OTHER SOURCE SOMATOMEDIN SOMATOSTATIN SPECTROPHOTOMETRY, ANALYTE NOT ELSEWHERE SPECIFIED SPECIFIC GRAVITY (EXCEPT URINE) SUGARS, CHROMATOGRAPHIC, TLC/PAPER CHROMATOGRAPHY SUGARS (MONO, DI, AND OLIGOSACCHARIDES); SINGLE QU SUGARS (MONO, DI, AND OLIGOSACCHARIDES); MULTIPLE SUGARS (MONO, DI, AND OLIGOSACCHARIDES); SINGLE QU SUGARS (MONO, DI, AND OLIGOSACCHARIDES); MULTIPLE SULFATE, URINE TESTOSTERONE; FREE TESTOSTERONE; TOTAL THIAMINE (VITAMIN B-1) THIOCYANATE THROMBOXANE METABOLITE W/WO THROMBOXANE URINE THYROGLOBULIN THYROXINE; TOTAL THYROXINE; REQUIRING ELUTION THYROXINE; FREE THYROXINE BINDING GLOBULIN (TBG) THYROID STIMULATING HORMONE (TSH) THYROID STIMULATING IMMUNOGLOBULINS (TSI) 1/1/2009 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 84446 84449 84450 84460 84466 84478 84479 84480 84481 84482 84484 84485 84488 84490 84510 84512 84520 84525 84540 84545 84550 84560 84577 84578 84580 84583 84585 84586 84588 84590 84591 84597 84600 84620 84630 84681 84702 84703 84704 84830 84999 85002 85004 85007 85008 85009 85013 85014 85018 85025 85027 85032 85041 85044 85045 85046 85048 85049 85055 85060 85097 85130 85170 85175 85210 85220 85230 85240 85244 85245 85246 85247 85250 85260 85270 85280 85290 85291 85292 85293 85300 85301 85302 85303 85305 85306 85307 85335 85337 85345 85347 TOCOPHEROL ALPHA (VITAMIN E) TRANSCORTIN (CORTISOL BINDING GLOBULIN) TRANSFERASE; ASPARTATE AMINO (AST) (SGOT) TRANSFERASE; ALANINE AMINO (ALT) (SGPT) TRANSFERRIN TRIGLYCERIDES THYROID HORMONE (T3/T4) UPTAKE/THYROID HORMONE BIN TRIIODOTHYRONINE T3; TOTAL (TT-3) TRIIODOTHYRONINE T3; FREE TRIIODOTHYRONINE T3; REVERSE TROPONIN, QUANTITATIVE TRYPSIN; DUODENAL FLUID TRYPSIN; FECES, QUALITATIVE TRYPSIN; FECES, QUANTITATIVE, 24-HOUR COLLECTION TYROSINE TROPONIN, QUALITATIVE UREA NITROGEN; QUANTITATIVE UREA NITROGEN; SEMIQUANTITATIVE UREA NITROGEN, URINE UREA NITROGEN, CLEARANCE URIC ACID; BLOOD URIC ACID; OTHER SOURCE UROBILINOGEN, FECES, QUANTITATIVE UROBILINOGEN, URINE; QUALITATIVE UROBILINOGEN, URINE; QUANTITATIVE, TIMED SPECIMEN UROBILINOGEN, URINE; SEMIQUANTITATIVE VANILLYLMANDELIC ACID (VMA), URINE VASOACTIVE INTESTINAL PEPTIDE (VIP) VASOPRESSIN (ANTIDIURETIC HORMONE, ADH) VITAMIN A VITAMIN, NOT OTHERWISE SPECIFIED VITAMIN K ASSAY OF VOLATILES XYLOSE ABSORPTION TEST, BLOOD AND/OR URINE ZINC C-PEPTIDE GONADOTROPIN, CHORIONIC (HCG); QUANTITATIVE GONADOTROPIN, CHORIONIC (HCG); QUALITATIVE GONADOTROPIN CHORIONIC HCG FREE BETA CHAIN OVULATION TESTS, VISUAL COLOR COMPARISON METHODS, UNLISTED CHEMISTRY PROC BLEEDING TIME BLOOD COUNT; AUTOMATED DIFFERENTIAL WBC COUNT BLOOD COUNT; BLOOD SMEAR, MICROSCOP EXAM W/MANUAL BLOOD COUNT; BLOOD SMEAR, MICROSCOP EXAM W/O MANUA BLOOD COUNT; MANUAL DIFFERENTIAL WBC COUNT, BUFFY BLOOD COUNT; SPUN MICROHEMATOCRIT BLOOD COUNT; HEMATOCRIT BLOOD COUNT; HEMOGLOBIN BLOOD COUNT; COMPLETE CBC, AUTOMATED (HGB, HCT, RB BLOOD COUNT; COMPLETE CBC, AUTOMATED (HGB, HCT, RB BLOOD COUNT; MANUAL CELL (ERYTHROCYTE, LEUKOCYTE, BLOOD COUNT; RED BLOOD CELL (RBC), AUTOMATED BLOOD COUNT; RETICULOCYTE, MANUAL BLOOD COUNT; RETICULOCYTE, AUTOMATED BLOOD COUNT; RETICULOCYTES, HEMOGLOBIN CONCENTRATI BLOOD COUNT; LEUKOCYTE (WBC), AUTOMATED BLOOD COUNT; PLATELET, AUTOMATED RETICULATED PLATELET ASSAY BLOOD SMEAR, PERIPHERAL, INTERPRETATION, PHYSICIAN BONE MARROW, SMEAR INTERPRETATION CHROMOGENIC SUBSTRATE ASSAY CLOT RETRACTION CLOT LYSIS TIME, WHOLE BLOOD DILUTION CLOTTING; FACTOR II, PROTHROMBIN, SPECIFIC CLOTTING; FACTOR V (ACG/PROACCELERIN), LABILE FACT CLOTTING; FACTOR VII (PROCONVERTIN, STABLE FACTOR) CLOTTING; FACTOR VIII (AHG), ONE STAGE CLOTTING; FACTOR VIII RELATED ANTIGEN CLOTTING; FACTOR VIII, VW FACTOR, RISTOCETIN COFAC CLOTTING; FACTOR VIII, VW FACTOR ANTIGEN CLOTTING; FACTOR VIII, VW FACTOR, MULTIMETRIC ANAL CLOTTING; FACTOR IX (PTC/CHRISTMAS) CLOTTING; FACTOR X (STUART-PROWER) CLOTTING; FACTOR XI (PTA) CLOTTING; FACTOR XII (HAGEMAN) CLOTTING; FACTOR XIII (FIBRIN STABILIZING) CLOTTING; FACTOR XIII (FIBRIN STABILIZING), SCREEN CLOTTING; PREKALLIKREIN ASSAY (FLETCHER FACTOR ASS CLOTTING; HIGH MOLECULAR WT KININOGEN ASSAY (FITZG CLOTTING INHIBITORS/ANTICOAGULANTS; ANTITHROMBIN I CLOTTING INHIBITORS/ANTICOAGULANTS; ANTITHROMBIN I CLOTTING INHIBITORS/ANTICOAGULANTS; PROTEIN C, ANT CLOTTING INHIBITORS/ANTICOAGULANTS; PROTEIN C, ACT CLOTTING INHIBITORS/ANTICOAGULANTS; PROTEIN S, TOT CLOTTING INHIBITORS/ANTICOAGULANTS; PROTEIN S, FRE ACTIVATED PROTEIN C (APC) RESISTANCE ASSAY FACTOR INHIBITOR TEST THROMBOMODULIN COAGULATION TIME; LEE AND WHITE COAGULATION TIME; ACTIVATED 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 85348 85360 85362 85366 85370 85378 85379 85380 85384 85385 85390 85396 85397 85400 85410 85415 85420 85421 85441 85445 85460 85461 85475 85520 85525 85530 85536 85540 85547 85549 85555 85557 85576 85597 85598 85610 85611 85612 85613 85635 85651 85652 85660 85670 85675 85705 85730 85732 85810 85999 86000 86001 86003 86005 86021 86022 86023 86038 86039 86060 86063 86077 86078 86079 86140 86141 86146 86147 86148 86152 86153 86155 86156 86157 86160 86161 86162 86171 86185 86200 86215 86225 86226 86235 86243 86255 86256 86277 86280 86294 86300 COAGULATION TIME; OTHER METHODS EUGLOBULIN LYSIS FIBRIN(OGEN) DEGRADATION (SPLIT) PRODUCTS (FDP)(FS FIBRIN(OGEN) DEGRADATION (SPLIT) PRODUCTS (FDP)(FS FIBRIN(OGEN) DEGRADATION (SPLIT) PRODUCTS (FDP)(FS FIBRIN DEGRADATION PRODUCTS, D-DIMER; QUALITATIVE FIBRIN DEGRADATION PRODUCTS, D-DIMER; QUANTITATIVE FIBRIN DEGRADATION PRODUCTS, D-DIMER; ULTRASENSITI FIBRINOGEN; ACTIVITY FIBRINOGEN; ANTIGEN FIBRINOLYSINS/COAGULOPATHY SCREEN, INTERPRETATION COAGULATION/FIBRINOLYSIS, WHOLE BLOOD, W/ USE, ADD COAGJ&FIBRINOLYSIS FUNCTIONAL ACTV NOS EA ANAL FIBRINOLYTIC FACTORS AND INHIBITORS; PLASMIN FIBRINOLYTIC FACTORS AND INHIBITORS; ALPHA-2 ANTIP FIBRINOLYTIC FACTORS AND INHIBITORS; PLASMINOGEN A FIBRINOLYTIC FACTORS AND INHIBITORS; PLASMINOGEN, FIBRINOLYTIC FACTORS AND INHIBITORS; PLASMINOGEN, HEINZ BODIES; DIRECT HEINZ BODIES; INDUCED, ACETYL PHENYLHYDRAZINE HEMOGLOBIN/RBCS, FETAL, FETOMATERNAL HEMORRHAGE; D HEMOGLOBIN/RBCS, FETAL, FETOMATERNAL HEMORRHAGE; R HEMOLYSIN, ACID HEPARIN ASSAY HEPARIN NEUTRALIZATION HEPARIN-PROTAMINE TOLERANCE TEST IRON STAIN PERIPHERAL BLOOD LEUKOCYTE ALKALINE PHOSPHATASE W/COUNT MECHANICAL FRAGILITY, RBC MURAMIDASE OSMOTIC FRAGILITY, RBC; UNINCUBATED OSMOTIC FRAGILITY, RBC; INCUBATED PLATELET, AGGREGATION (IN VITRO), EACH AGENT PHOSPHOLIPID NEUTRALIZATION PLATELET PHOSPHOLIPID NEUTRALIZATION HEXAGONAL PROTHROMBIN TIME PROTHROMBIN TIME; SUBSTITUTION, PLASMA FRACTIONS, RUSSELL VIPER VENOM TIME (INCLUDES VENOM); UNDILUT RUSSELL VIPER VENOM TIME (INCLUDES VENOM); DILUTED REPTILASE TEST SEDIMENTATION RATE, ERYTHROCYTE; NON-AUTOMATED SEDIMENTATION RATE, ERYTHROCYTE; AUTOMATED SICKLING, RBC, REDUCTION THROMBIN TIME; PLASMA THROMBIN TIME; TITER THROMBOPLASTIN INHIBITION; TISSUE THROMBOPLASTIN TIME, PARTIAL (PTT); PLASMA/WHOLE B THROMBOPLASTIN TIME, PARTIAL (PTT); SUBSTITUTION, VISCOSITY UNLISTED HEMATOLOGY AND COAGULATION PROC AGGLUTININS, FEBRILE, EACH ANTIGEN ALLERGEN SPECIFIC IGG QUANTITATIVE/SEMIQUANTITATIV ALLERGEN SPECIFIC IGE; QUANTITATIVE/SEMIQUANTITATI ALLERGEN SPECIFIC IGE; QUALITATIVE, MULTIALLERGEN ANTIBODY IDENTIFICATION; LEUKOCYTE ANTIBODIES ANTIBODY IDENTIFICATION; PLATELET ANTIBODIES ANTIBODY IDENTIFICATION; PLATELET ASSOCIATED IMMUN ANTINUCLEAR ANTIBODIES (ANA) ANTINUCLEAR ANTIBODIES (ANA); TITER ANTISTREPTOLYSIN 0; TITER ANTISTREPTOLYSIN 0; SCREEN BLOOD BANK PHYSICIAN SERVICES; DIFFICULT CROSS MAT BLOOD BANK PHYSICIAN SERVICES; INVESTIGATION, TRAN BLOOD BANK PHYSICIAN SERVICES; AUTHORIZATION, DEVI C-REACTIVE PROTEIN C-REACTIVE PROTEIN; HIGH SENSITIVITY (HSCRP) BETA 2 GLYCOPROTEIN I ANTIBODY, EA CARDIOLIPIN (PHOSPHOLIPID) ANTIBODY, EA IG CLASS ANTI-PHOSPHATIDYLSERINE (PHOSPHOLIPID) ANTIBODY CELL ENUMERATION IMMUNE SELECTJ & ID FLUID SPEC CELL ENUMERATION IMMUNE SELECTJ & ID PHYS INTERP CHEMOTAXIS ASSAY, SPECIFY METHOD COLD AGGLUTININ; SCREEN COLD AGGLUTININ; TITER COMPLEMENT; ANTIGEN, EACH COMPONENT COMPLEMENT; FUNCTIONAL ACTIVITY, EACH COMPONENT COMPLEMENT; TOTAL HEMOLYTIC (CH50) COMPLEMENT FIXATION TESTS, EACH ANTIGEN COUNTERIMMUNOELECTROPHORESIS, EACH ANTIGEN CYCLIC CITRULLINATED PEPTIDE (CCP), ANTIBODY DEOXYRIBONUCLEASE, ANTIBODY DEOXYRIBONUCLEIC ACID (DNA) ANTIBODY; NATIVE/DOUBL DEOXYRIBONUCLEIC ACID (DNA) ANTIBODY; SINGLE STRAN EXTRACTABLE NUCLEAR ANTIGEN, ANTIBODY TO, ANY METH FC RECEPTOR FLUORESCENT NONINFECTIOUS AGENT ANTIBODY; SCREEN, FLUORESCENT NONINFECTIOUS AGENT ANTIBODY; TITER, E GROWTH HORMONE, HUMAN (HGH), ANTIBODY HEMAGGLUTINATION INHIBITION TEST (HAI) IMMUNOASSAY, TUMOR ANTIGEN, QUALITATIVE/SEMIQUANTI IMMUNOASSAY, TUMOR ANTIGEN, QUANTITATIVE; CA 15-3 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 86301 86304 86305 86308 86309 86310 86316 86317 86318 86320 86325 86327 86329 86331 86332 86334 86335 86336 86337 86340 86341 86343 86344 86352 86353 86355 86356 86357 86359 86360 86361 86367 86376 86378 86382 86384 86386 86403 86406 86430 86431 86480 86481 86485 86486 86490 86510 86580 86590 86592 86593 86602 86603 86606 86609 86611 86612 86615 86617 86618 86619 86622 86625 86628 86631 86632 86635 86638 86641 86644 86645 86648 86651 86652 86653 86654 86658 86663 86664 86665 86666 86668 86671 86674 86677 86682 86684 86687 86688 86689 86692 IMMUNOASSAY, TUMOR ANTIGEN, QUANTITATIVE; CA 19-9 IMMUNOASSAY, TUMOR ANTIGEN, QUANTITATIVE; CA 125 HUMAN EPIDIDYMIS PROTEIN 4 (HE4) HETEROPHILE ANTIBODIES; SCREENING HETEROPHILE ANTIBODIES; TITER HETEROPHILE ANTIBODIES; TITERS AFTER ABSORPTION W/ IMMUNOASSAY, TUMOR ANTIGEN; OTHER ANTIGEN, QUANTIT IMMUNOASSAY, INFECTIOUS AGENT ANTIBODY, QUANTITATI IMMUNOASSAY, INFECTIOUS AGENT ANTIBODY, QUALITATIV IMMUNOELECTROPHORESIS; SERUM IMMUNOELECTROPHORESIS; OTHER FLUIDS W/CONCENTRATIO IMMUNOELECTROPHORESIS; CROSSED (2-DIMENSIONAL ASSA IMMUNODIFFUSION; NOT ELSEWHERE SPECIFIED IMMUNODIFFUSION; GEL DIFFUSION, QUALITATIVE (OUCHT IMMUNE COMPLEX ASSAY IMMUNOFIXATION ELECTROPHORESIS IMMUNOFIXATION ELECTROPHORESIS, OTHER FLUIDS WITH INHIBIN A INSULIN ANTIBODIES INTRINSIC FACTOR ANTIBODIES ISLET CELL ANTIBODY LEUKOCYTE HISTAMINE RELEASE TEST (LHR) LEUKOCYTE PHAGOCYTOSIS CELLULAR FUNCTION ASSAY STIMUL&DETECT BIOMARKER LYMPHOCYTE TRANSFORMATION, MITOGEN (PHYTOMITOGEN)/ B CELLS; TOTAL COUNT MONONUCLEAR CELL ANTIGEN QUANTITATIVE NOS EA NATURAL KILLER (NK) CELLS, TOTAL COUNT T CELLS; TOTAL COUNT T CELLS; ABSOLUTE CD4 AND CD8 COUNT, W/RATIO T CELLS; ABSOLUTE CD4 COUNT STEM CELLS (IE CD34); TOTAL COUNT MICROSOMAL ANTIBODIES, EACH MIGRATION INHIBITORY FACTOR TEST (MIF) NEUTRALIZATION TEST, VIRAL NITROBLUE TETRAZOLIUM DYE TEST (NTD) NUCLEAR MATRIX PROTEIN 22 NMP22 QUALITATIVE PARTICLE AGGLUTINATION; SCREEN, EACH ANTIBODY PARTICLE AGGLUTINATION; TITER, EACH ANTIBODY RHEUMATOID FACTOR; QUALITATIVE RHEUMATOID FACTOR; QUANTITATIVE TB TEST, CELL MEDIATED IMMUNITY MEASUREMENT OF GAM TB ANTIGEN RESPONSE GAMMA INTERFERON T-CELL SUSP SKIN TEST; CANDIDA SKIN TEST UNLISTED ANTIGEN EACH SKIN TEST; COCCIDIOIDOMYCOSIS SKIN TEST; HISTOPLASMOSIS SKIN TEST; TUBERCULOSIS, INTRADERMAL STREPTOKINASE, ANTIBODY SYPHILIS TEST NON TREPONEMAL ANTIBODY QUAL SYPHILIS TEST; QUANTITATIVE ANTIBODY; ACTINOMYCES ANTIBODY; ADENOVIRUS ANTIBODY; ASPERGILLUS ANTIBODY; BACTERIUM, NOT ELSEWHERE SPECIFIED ANTIBODY; BARTONELLA ANTIBODY; BLASTOMYCES ANTIBODY; BORDETELLA ANTIBODY; BORRELIA BURGDORFERI (LYME DISEASE) CONF ANTIBODY; BORRELIA BURGDORFERI (LYME DISEASE) ANTIBODY; BORRELIA (RELAPSING FEVER) ANTIBODY; BRUCELLA ANTIBODY; CAMPYLOBACTER ANTIBODY; CANDIDA ANTIBODY; CHLAMYDIA ANTIBODY; CHLAMYDIA, IGM ANTIBODY; COCCIDIOIDES ANTIBODY; COXIELLA BRUNETII (Q FEVER) ANTIBODY; CRYPTOCOCCUS ANTIBODY; CYTOMEGALOVIRUS (CMV) ANTIBODY; CYTOMEGALOVIRUS (CMV), IGM ANTIBODY; DIPHTHERIA ANTIBODY; ENCEPHALITIS, CALIFORNIA (LA CROSSE) ANTIBODY; ENCEPHALITIS, EASTERN EQUINE ANTIBODY; ENCEPHALITIS, ST. LOUIS ANTIBODY; ENCEPHALITIS, WESTERN EQUINE ANTIBODY; ENTEROVIRUS ANTIBODY; EPSTEIN-BARR (EB) VIRUS, EARLY ANTIGEN ( ANTIBODY; EPSTEIN-BARR (EB) VIRUS, NUCLEAR ANTIGEN ANTIBODY; EPSTEIN-BARR (EB) VIRUS, VIRAL CAPSID (V ANTIBODY; EHRLICHIA ANTIBODY; FRANCISELLA TULARENSIS ANTIBODY; FUNGUS, NOT ELSEWHERE SPECIFIED ANTIBODY; GIARDIA LAMBLIA ANTIBODY; HELICOBACTER PYLORI ANTIBODY; HELMINTH, NOT ELSEWHERE SPECIFIED ANTIBODY; HEMOPHILUS INFLUENZA ANTIBODY; HTLV-I ANTIBODY; HTLV-II ANTIBODY; HTLV/HIV ANTIBODY, CONFIRMATORY TEST ANTIBODY; HEPATITIS, DELTA AGENT 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2006 1/1/2008 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2011 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 86694 86695 86696 86698 86701 86702 86703 86704 86705 86706 86707 86708 86709 86710 86711 86713 86717 86720 86723 86727 86729 86732 86735 86738 86741 86744 86747 86750 86753 86756 86757 86759 86762 86765 86768 86771 86774 86777 86778 86780 86784 86787 86788 86789 86790 86793 86800 86803 86804 86805 86806 86807 86808 86812 86813 86816 86817 86821 86822 86825 86826 86828 86829 86830 86831 86832 86833 86834 86835 86849 86850 86860 86870 86880 86885 86886 86890 86891 86900 86901 86902 86904 86905 86906 86910 86911 86920 86921 86922 86923 86927 ANTIBODY; HERPES SIMPLEX, NON-SPECIFIC TYPE TEST ANTIBODY; HERPES SIMPLEX, TYPE I ANTIBODY; HERPES SIMPLEX, TYPE 2 ANTIBODY; HISTOPLASMA ANTIBODY; HIV-1 ANTIBODY; HIV-2 ANTIBODY HIV-1&HIV-2 SINGLE RESULT HEPATITIS B CORE ANTIBODY (HBCAB); TOTAL HEPATITIS B CORE ANTIBODY (HBCAB); IGM ANTIBODY HEPATITIS B SURFACE ANTIBODY (HBSAB) HEPATITIS BE ANTIBODY (HBEAB) HEPATITIS A ANTIBODY (HAAB); TOTAL HEPATITIS A ANTIBODY (HAAB); IGM ANTIBODY ANTIBODY; INFLUENZA VIRUS ANTIBODY JOHN CUNNINGHAM VIRUS ANTIBODY; LEGIONELLA ANTIBODY; LEISHMANIA ANTIBODY; LEPTOSPIRA ANTIBODY; LISTERIA MONOCYTOGENES ANTIBODY; LYMPHOCYTIC CHORIOMENINGITIS ANTIBODY; LYMPHOGRANULOMA VENEREUM ANTIBODY; MUCORMYCOSIS ANTIBODY; MUMPS ANTIBODY; MYCOPLASMA ANTIBODY; NEISSERIA MENINGITIDIS ANTIBODY; NOCARDIA ANTIBODY; PARVOVIRUS ANTIBODY; PLASMODIUM (MALARIA) ANTIBODY; PROTOZOA, NOT ELSEWHERE SPECIFIED ANTIBODY; RESPIRATORY SYNCYTIAL VIRUS ANTIBODY; RICKETTSIA ANTIBODY; ROTAVIRUS ANTIBODY; RUBELLA ANTIBODY; RUBEOLA ANTIBODY; SALMONELLA ANTIBODY; SHIGELLA ANTIBODY; TETANUS ANTIBODY; TOXOPLASMA ANTIBODY; TOXOPLASMA, IGM ANTIBODY TREPONEMA PALLIDUM ANTIBODY; TRICHINELLA ANTIBODY; VARICELLA-ZOSTER West nile virus ab, igm West nile virus antibody ANTIBODY; VIRUS, NOT ELSEWHERE SPECIFIED ANTIBODY; YERSINIA THYROGLOBULIN ANTIBODY HEPATITIS C ANTIBODY HEPATITIS C ANTIBODY; CONFIRMATORY TEST LYMPHOCYTOTOXICITY ASSAY, VISUAL CROSSMATCH; W/TIT LYMPHOCYTOTOXICITY ASSAY, VISUAL CROSSMATCH; W/O T SERUM SCREENING, CYTOTOXIC PERCENT REACTIVE ANTIBO SERUM SCREENING, CYTOTOXIC PERCENT REACTIVE ANTIBO HLA TYPING; A, B, OR C, SINGLE ANTIGEN HLA TYPING; A, B, OR C, MULTIPLE ANTIGENS HLA TYPING; DR/DQ, SINGLE ANTIGEN HLA TYPING; DR/DQ, MULTIPLE ANTIGENS HLA TYPING; LYMPHOCYTE CULTURE, MIXED (MLC) HLA TYPING; LYMPHOCYTE CULTURE, PRIMED (PLC) HLA CROSSMATCH NONCYTOTOXIC 1ST SERUM/DILUTION HLA CROSSMATCH NONCYTOTOXIC EA+ SERUM/DILUTION ANTIBODY HLA CLASS I & CLASS II ANTIGENS QUAL ANTIBODY HLA CLASS I OR CLASS II ANTIGENS QUAL ANTIBODY HLA CLASS I PHENOTYPE PANEL QUALITATIVE ANTIBODY HLA CLASS II PHENOTYPE PANEL QUAL ANTIBODY HLA CLASS I HIGH DEFINITION PANEL QUAL ANTIBODY HLA CLASS II HIGH DEFINITION PANEL QUAL ANTIBODY HLA CLASS I SEMIQUANTITATIVE PANEL ANTIBODY HLA CLASS II SEMIQUANTITATIVE PANEL UNLISTED IMMUNOLOGY PROC ANTIBODY SCREEN, RBC, EACH SERUM TECHNIQUE ANTIBODY ELUTION (RBC), EACH ELUTION ANTIBODY IDENTIFICATION, RBC ANTIBODIES, EACH PANE ANTIHUMAN GLOBULIN TEST (COOMBS TEST); DIRECT, EAC ANTIHUMAN GLOBULIN TEST (COOMBS TEST); INDIRECT, Q ANTIHUMAN GLOBULIN TEST (COOMBS TEST); INDIRECT, T AUTOLOGOUS BLOOD/COMPONENT, COLLECT/PROCESS/STORAG AUTOLOGOUS BLOOD/COMPONENT, COLLECT/PROCESS/STORAG BLOOD TYPING SEROLOGIC ABO BLOOD TYPING SEROLOGIC RH (D) BLOOD TYPE ANTIGEN DONOR REAGENT SERUM EACH BLOOD TYPING ANTIGEN SCREEN PATIENT SERUM/UNIT BLOOD TYPING RBC ANTIGENS OTH/THN ABO/RH D EACH BLOOD TYPING SEROLOGIC RH PHENOTYPING COMPLETE BLOOD TYPING, PATERNITY TESTING, PER INDIVIDUAL; A BLOOD TYPING, PATERNITY TESTING, PER INDIVIDUAL; E COMPATIBILITY TEST EACH UNIT; IMMEDIATE SPIN TECHN COMPATIBILITY TEST EACH UNIT; INCUBATION TECHNIQUE COMPATIBILITY TEST EACH UNIT; ANTIGLOBULIN TECHNIQ COMPATIBILITY TEST EACH UNIT; ELECTRONIC FRESH FROZEN PLASMA, THAWING, EACH UNIT 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2010 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 86930 86931 86932 86940 86941 86945 86950 86960 86965 86970 86971 86972 86975 86976 86977 86978 86985 86999 87003 87015 87040 87045 87046 87070 87071 87073 87075 87076 87077 87081 87084 87086 87088 87101 87102 87103 87106 87107 87109 87110 87116 87118 87140 87143 87147 87149 87150 87152 87153 87158 87164 87166 87168 87169 87172 87176 87177 87181 87184 87185 87186 87187 87188 87190 87197 87205 87206 87207 87209 87210 87220 87230 87250 87252 87253 87254 87255 87260 87265 87267 87269 87270 87271 87272 87273 87274 87275 87276 87277 87278 87279 FROZEN BLOOD, EACH UNIT; FREEZING (W/PREPARATION) FROZEN BLOOD, EACH UNIT; THAWING FROZEN BLOOD, EACH UNIT; FREEZING (W/PREPARATION)A HEMOLYSINS AND AGGLUTININS; AUTO, SCREEN, EACH HEMOLYSINS AND AGGLUTININS; INCUBATED IRRADIATION, BLOOD PRODUCT, EACH UNIT LEUKOCYTE TRANSFUSION VOLUME REDUCTION OF BLOOD OR BLOOD PRODUCT, EACH U POOLING, PLATELETS/OTHER BLOOD PRODUCTS PRETREATMENT RBCS, ANTIBODY DETECTION/ID/COMPATIBI PRETREATMENT RBCS, ANTIBODY DETECTION/ID/COMPATIBI PRETREATMENT RBCS, ANTIBODY DETECTION/ID/COMPATIBI PRETREATMENT, SERUM, USE IN RBC ANTIBODY IDENTIFIC PRETREATMENT, SERUM, USE IN RBC ANTIBODY IDENTIFIC PRETREATMENT, SERUM, USE IN RBC ANTIBODY IDENTIFIC PRETREATMENT SERUM, USE IN RBC ANTIBODY IDENTIFICA SPLITTING, BLOOD/BLOOD PRODUCTS, EACH UNIT UNLISTED TRANSFUSION MEDICINE PROC ANIMAL INOCULATION SMALL ANIMAL W/OBS&DSJ CONCENTRATION (ANY TYPE), FOR INFECTIOUS AGENTS CULTURE, BACTERIAL; BLOOD, AEROBIC, W/ ISOLATN/PRE CULTURE, BACTERIAL; STOOL, AEROBIC, W/ ISOLATN/PRE CULTURE, BACTERIAL; STOOL, AEROBIC, ADDL PATHOGENS CULTURE, BACTERIAL; ANY OTHER SOURCE EXCEPT URINE/ CULTURE, BACTERIAL; QUANTIT, AEROBIC W/ISOLATN AND CULTURE, BACTERIAL; QUANTIT, ANAEROBIC W/ISOLATN A CULTURE, BACTERIAL; ANY SOURCE, EXCEPT BLOOD, ANAE CULTURE, BACTERIAL; ANAEROBIC ISOLATE, ADDL METHOD CULTURE, BACTERIAL; AEROBIC ISOLATE, ADDL METHODS CULTURE, PRESUMPTIVE, PATHOGENIC ORGANISMS, SCREEN CULTURE, PRESUMPTIVE, PATHOGENIC ORGANISMS, SCREEN CULTURE, BACTERIAL; QUANTITATIVE COLONY COUNT, URI CULTURE, BACTERIAL; W/ISOLATION AND PRESUMPTIVE ID CULTURE, FUNGI (MOLD/YEAST) ISOLATION, W/PRESUMPTI CULTURE, FUNGI (MOLD/YEAST) ISOLATION, W/PRESUMPTI CULTURE, FUNGI (MOLD/YEAST) ISOLATION, W/PRESUMPTI CULTURE, FUNGI, DEFINITIVE ID, EACH ORGANISM; YEAS CULTURE, FUNGI, DEFINITIVE ID, EACH ORGANISM; MOLD CULTURE, MYCOPLASMA, ANY SOURCE CULTURE, CHLAMYDIA, ANY SOURCE CULTURE, TUBERCLE/ACID-FAST BACILLI ANY SOURCE, W/ CULTURE, MYCOBACTERIAL, DEFINITIVE ID, EA ISOLATE CULTURE, TYPING; IMMUNOFLUORESCENT METHOD, EA ANTI CULTURE, TYPING; GAS LIQUID CHROMATOGRAPHY (GLC)/H CULTURE, TYPING; IMMUNOLOGIC METHOD, OTHER THAN IM CULTURE, TYPING; ID BY NUCLEIC ACID PROBE CULTYP NUC ACID AMP PRB CULT/ISOLATE EA ORGNISM CULTURE, TYPING; ID BY PULSE FIELD GEL TYPING CULTYP NUCLEIC ACID SEQUENCING METH EA ISOLATE CULTURE, TYPING; OTHER METHODS DARK FIELD EXAM, ANY SOURCE; W/SPECIMEN COLLECTION DARK FIELD EXAM, ANY SOURCE; W/O COLLECTION MACROSCOPIC EXAM; ARTHROPOD MACROSCOPIC EXAM; PARASITE PINWORM EXAM HOMOGENIZATION, TISSUE, FOR CULTURE OVA AND PARASITES, DIRECT SMEARS, CONCENTRATION AN SUSCEPTIBILITY STUDIES, ANTIMICROBIAL AGENT; AGAR SUSCEPTIBILITY STUDIES, ANTIMICROBIAL AGENT; DISK SUSCEPTIBILITY STUDIES, ANTIMICROBIAL AGENT; ENZYM SUSCEPT STUDIES, ANTIMICROB AGNT; MICRODILUT/AGAR SUSCEPTIBILITY STUDIES, ANTIMICROB AGENT; MICRODIL SUSCEPTIBILITY STUDIES, ANTIMICROB AGENT; MACROBRO SUSCEPTIBILITY STUDIES, ANTIMICROB AGENT; MYCOBACT SERUM BACTERICIDAL TITER (SCHLICTER TEST) SMEAR, PRIMARY SOURCE W/INTERPRETATION; GRAM OR GI SMEAR, PRIME SRCE, W/INTERPR; FLUORESC AND/OR ACID SMEAR, PRIMARY SOURCE W/INTERPRETATION; SPECIAL ST SMEAR; COMPLEX SPECIAL STAIN FOR OVA AND PARASITES SMEAR, PRIMARY SOURCE W/INTERPRETATION; WET MOUNT, TISSUE EXAM BY KOH SLIDE OF SAMPLES FROM SKIN/HAIR TOXIN/ANTITOXIN ASSAY, TISSUE CULTURE VIRUS ISOLATION; INOCULATION EGGS/SMALL ANIMAL, OB VIRUS ISOLATION; TISSUE CULTURE INOCULATION, OBSER VIRUS ISOLATION; TISSUE CULTURE, ADDL STUDIES/DEFI VIRUS ISOLATION; CENTRIFUGE ENHANCED (SHELL VIAL) VIRUS ISOLATION; ID, NON-IMMUNOLOGIC METHOD, OTHER INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION, IMMUNOFLUORESC INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORO INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORO INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 87280 87281 87283 87285 87290 87299 87300 87301 87305 87320 87324 87327 87328 87329 87332 87335 87336 87337 87338 87339 87340 87341 87350 87380 87385 87389 87390 87391 87400 87420 87425 87427 87430 87449 87450 87451 87470 87471 87472 87475 87476 87477 87480 87481 87482 87485 87486 87487 87490 87491 87492 87493 87495 87496 87497 87498 87500 87501 87502 87503 87505 87506 87507 87510 87511 87512 87515 87516 87517 87520 87521 87522 87525 87526 87527 87528 87529 87530 87531 87532 87533 87534 87535 87536 87537 87538 87539 87540 87541 87542 87550 INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOFLUORE IMMUNOFLUORESCENT TECHNIQUE, POLYVALENT FOR MULTIP ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA Aspergillus ag, eia ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA) QUALITATIVE/SEMIQUANTITAT ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA) QUALITATIVE/SEMIQUANTITAT ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA) QUALITAT/SEMIQUANTITAT, M ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA IAAD EIA HIV-1 AG W/HIV-1&HIV-2 ANTBDY SINGLE ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA), QUALITATIVE/SEMIQUANTITA ENZYME IMMUNOASSAY (EIA) QUALITATIVE/SEMIQUANTITAT ENZYME IMMUNOASSAY (EIA) QUALITATIVE/SEMIQUANTITAT ENZYME IMMUNOASSAY QUALIT/SEMIQUANTIT; MULTI STEP INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); BARTONEL INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); BARTONEL INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); BARTONEL INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); BORRELIA INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); BORRELIA INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); BORRELIA INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CANDIDA, INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CANDIDA, INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CANDIDA, INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CHLAMYDI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CHLAMYDI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CHLAMYDI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CHLAMYDI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CHLAMYDI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CHLAMYDI INF AGENT DET NUC ACID CLOSTRIDIUM AMP PROBE INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CYTOMEGA INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CYTOMEGA INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); CYTOMEGA IADNA ENTEROVIRUS AMPLIF PROBE & REVRSE TRNSCRIP INFECTIOUS AGENT DNA/RNA VANCOMYCIN RESISTANCE INFECTIOUS AGENT DNA/RNA INFLUENZA EA TYPE INFECTIOUS AGENT DNA/RNA INFLUENZA 1ST 2 TYPES NFCT AGENT DNA/RNA INFLUENZA 1+ TYPES EA ADDL NFCT AGENT DNA/RNA GASTROINTESTINAL PATHOGEN IADNA-DNA/RNA GI PTHGN MULTIPLEX PROBE TQ 6-11 IADNA-DNA/RNA GI PTHGN MULTIPLEX PROBE TQ 12-25 INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); GARDNERE INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); GARDNERE INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); GARDNERE INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HEPATITI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HEPATITI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HEPATITI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HEPATITI IADNA HEPATITIS C AMPLIFIED PROBE&REVRSE TRANSCR IADNA HEPATITIS C QUANT & REVERSE TRANSCRIPTION INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HEPATITI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HEPATITI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HEPATITI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HERPES S INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HERPES S INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HERPES S INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HERPES V INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HERPES V INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HERPES V INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HIV-1, D IADNA HIV-1 AMPLIFIED PROBE & REVERSE TRANSCRPJ IADNA HIV-1 QUANT & REVERSE TRANSCRIPTION INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); HIV-2, D IADNA HIV-2 AMPLIFIED PROBE & REVERSE TRANSCRIPJ IADNA HIV-2 QUANT & REVERSE TRANSCRIPTION INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); LEGIONEL INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); LEGIONEL INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); LEGIONEL INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOBACT 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2008 1/1/2011 1/1/2011 1/1/2011 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 87551 87552 87555 87556 87557 87560 87561 87562 87580 87581 87582 87590 87591 87592 87623 87624 87625 87631 87632 87633 87640 87641 87650 87651 87652 87653 87660 87661 87797 87798 87799 87800 87801 87802 87803 87804 87806 87807 87808 87809 87810 87850 87880 87899 87900 87901 87902 87903 87904 87905 87906 87910 87912 87999 88000 88005 88007 88012 88014 88016 88020 88025 88027 88028 88029 88036 88037 88040 88045 88099 88104 88106 88108 88112 88120 88121 88125 88130 88140 88141 88142 88143 88147 88148 88150 88152 88153 88154 88155 88160 88161 INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOBACT INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOBACT INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOBACT INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOBACT INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOBACT INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOBACT INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOBACT INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOBACT INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOPLAS INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOPLAS INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); MYCOPLAS INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); NEISSERI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); NEISSERI INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); NEISSERI IADNA HUMAN PAPILLOMAVIRUS LOW-RISK TYPES IADNA HUMAN PAPILLOMAVIRUS HIGH-RISK TYPES IADNA HUMAN PAPILLOMAVIRUS TYPES 16 & 18 ONLY IADNA RESPIRATRY PROBE & REV TRNSCR 3-5 TARGETS IADNA RESPIRATRY PROBE & REV TRNSCR 6-11 TARGETS IADNA RESPIRATRY PROBE & REV TRNSCR 12-25 TARGET Staph a, dna, amp probe Mr-staph, dna, amp probe INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); STREPTOC INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); STREPTOC INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); STREPTOC Strep b, dna, amp probe INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA); TRICHOMO IADNA TRICHOMONAS VAGINALIS AMPLIFIED PROBE TECH INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA), NOS; DIR INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA), NOS; AMP INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA), NOS; QUA INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA), MULTIPLE INFECTIOUS AGENT, NUCLEIC ACID (DNA/RNA), MULTIPLE INFECTIOUS AGENT, IMMUNOASSAY, DIRECT OBSERVATION; INFECTIOUS AGENT, IMMUNOASSAY, DIRECT OBSERVATION; INFECTIOUS AGENT, IMMUNOASSAY, DIRECT OBSERVATION; IAADIADOO HIV1 ANTIGEN W/HIV1 & HIV2 ANTIBODIES INFECTIOUS AGENT, IMMUNOASSAY, DIRECT OBSERVATION; Trichomonas assay w/optic INFECTIOUS AGENT IMMUNOASSAY OPTICAL ADENOVIRUS INFECTIOUS AGENT, IMMUNOASSAY, DIRECT OBSERVATION; INFECTIOUS AGENT, IMMUNOASSAY, DIRECT OBSERVATION; INFECTIOUS AGENT, IMMUNOASSAY, DIRECT OBSERVATION; INFECTIOUS AGENT, IMMUNOASSAY, DIRECT OBSERVATION; INFECTIOUS AGENT DRUG SUSCEPTIBILITY PHENO TYPE PR NFCT AGT GEXYP HIV 1 REV TRANSCRIP&PROTEAS REGNS INFECTIOUS AGENT GENOTYPE ANALYSIS, NUCLEIC ACID ( INFECT AGNT PHENOTYP ANALYS (DNA/RNA) W/DRUG RESIS INFECT AGNT PHENOTYP ANALYS (DNA/RNA) W/DRUG RESIS INFECTIOUS AGENT ENZYMATIC ACTV OTH/THN VIRUS NFCT GEXYP DNA/RNA HIV 1 OTHER REGION NFCT AGT GENOTYPE NUCLEIC ACID CYTOMEGALOVIRUS NFCT AGENT GENOTYPE HEPATITIS B VIRUS UNLISTED MICROBIOLOGY PROC NECROPSY (AUTOPSY), GROSS EXAM ONLY; W/O CNS NECROPSY (AUTOPSY), GROSS EXAM ONLY; W/BRAIN NECROPSY (AUTOPSY), GROSS EXAM ONLY; W/BRAIN AND S NECROPSY (AUTOPSY), GROSS EXAM ONLY; INFANT W/BRAI NECROPSY (AUTOPSY), GROSS EXAM ONLY; STILLBORN/NEW NECROPSY (AUTOPSY), GROSS EXAM ONLY; MACERATED STI NECROPSY (AUTOPSY), GROSS AND MICROSCOPIC; W/O CNS NECROPSY (AUTOPSY), GROSS AND MICROSCOPIC; W/BRAIN NECROPSY (AUTOPSY), GROSS AND MICROSCOPIC; W/BRAIN NECROPSY (AUTOPSY), GROSS AND MICROSCOPIC; INFANT NECROPSY (AUTOPSY), GROSS AND MICROSCOPIC; STILLBO NECROPSY (AUTOPSY), LIMITED, GROSS AND/OR MICROSCO NECROPSY (AUTOPSY), LIMITED, GROSS AND/OR MICROSCO NECROPSY (AUTOPSY); FORENSIC EXAM NECROPSY (AUTOPSY); CORONERS CALL UNLISTED NECROPSY (AUTOPSY) PROC CYTOPATHOLOGY EXCEPT CERVICAL/VAGINAL; SMEARS W/IN CYTOPATHOLOGY EXCEPT CERVICAL/VAGINAL; FILTER W/IN CYTOPATHOLOGY, CONCENTRATION TECHNIQUE, SMEARS AND CYTOPATHOLOGY, SELECTIVE CELLULAR ENHANCEMENT, W/ CYTP INSITU HYBRID URINE SPEC 3-5 PROBES EA MNL CYTP INSITU HYBRID URNE SPEC 3-5 PROBES CPTR EA CYTOPATHOLOGY, FORENSIC SEX CHROMATIN IDENTIFICATION; BARR BODIES SEX CHROMATIN IDENTIFICATION; PERIPHERAL BLOOD SME CYTOPATHOLOGY, CERVICAL/VAGINAL; REQUIRING INTERPR CYTOPATHOLOGY, CERVICAL/VAGINAL, PRESERVATIVE FLUI CYTOPATHOLOGY, CERVICAL/VAGINAL, PRESERVATIVE FLUI CYTOPATHOLOGY, CERVICAL/VAGINAL; AUTOMATED SCREEN CYTOPATHOLOGY, CERVICAL/VAGINAL; AUTOMATED SCREEN, CYTOPATHOLOGY, SLIDES, CERVICAL/VAGINAL; MANUAL SC CYTOPATHOLOGY, SLIDES, CERVICAL/VAGINAL; MANUAL SC CYTOPATHOLOGY, SLIDES, CERVICAL/VAGINAL; MANUAL SC CYTOPATHOLOGY, SLIDES, CERVICAL/VAGINAL; MANUAL SC CYTOPATHOLOGY, SLIDES, CERVICAL/VAGINAL, HORMONAL CYTOPATHOLOGY, SMEARS, OTHER SOURCE; SCREENING AND CYTOPATHOLOGY, SMEARS, OTHER SOURCE; PREPARATION, 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2013 1/1/2013 1/1/2013 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2005 1/1/2007 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2011 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 88162 88164 88165 88166 88167 88172 88173 88174 88175 88177 88182 88184 88185 88187 88188 88189 88199 88230 88233 88235 88237 88239 88240 88241 88245 88248 88249 88261 88262 88263 88264 88267 88269 88271 88272 88273 88274 88275 88280 88283 88285 88289 88291 88299 88300 88302 88304 88305 88307 88309 88311 88312 88313 88314 88319 88321 88323 88325 88329 88331 88332 88333 88334 88341 88342 88344 88346 88347 88348 88350 88355 88356 88358 88360 88361 88362 88363 88364 88365 88366 88367 88368 88369 88371 88372 88373 88374 88375 88377 88380 88381 CYTOPATHOLOGY, SMEARS, OTHER SOURCE; EXTENDED STUD CYTOPATHOLOGY, SLIDES, CERVICAL/VAGINAL, BETHESDA; CYTOPATHOLOGY, SLIDES, CERVICAL/VAGINAL, BETHESDA; CYTOPATHOLOGY, SLIDES, CERVICAL/VAGINAL, BETHESDA; CYTOPATHOLOGY, SLIDES, CERVICAL/VAGINAL, BETHESDA; CYTP FINE NDL ASPIRATE IMMT CYTOHIST STD DX 1ST CYTOPATHOLOGY, EVAL FINE NEEDLE ASPIRATE; INTERPRE CYTOPATHOLOGY, CERVICAL/VAGINAL, AUTO THIN LAYER P CYTOPATHOLOGY, CERVICAL/VAGINAL, AUTO THIN LAYER P CYTP C/V AUTO THIN LYR PREPJ ADEQUACY EA EVAL FLOW CYTOMETRY; CELL CYCLE/DNA ANALYSIS FLOW CYTOMETRY, CELL SURFACE, FIRST MARKER FLOW CYTOMETRY, CELL SURFACE, ADDITIONAL MARKER FLOW CYTOMETRY, INTERPRETATION, 2 TO 8 MARKERS FLOW CYTOMETRY, INTERPRETATION, 9 TO 15 MARKERS FLOW CYTOMETRY, INTERPRETATION, 16 OR MORE MARKERS UNLISTED CYTOPATHOLOGY PROC TISSUE CULTURE, NON-NEOPLASTIC DISORDERS; LYMPHOCY TISSUE CULTURE, NON-NEOPLASTIC DISORDERS; SKIN/OTH TISSUE CULTURE, NON-NEOPLASTIC DISORDERS; AMNIOTIC TISSUE CULTURE, NEOPLASTIC DISORDERS; BONE MARROW, TISSUE CULTURE, NEOPLASTIC DISORDERS; SOLID TUMOR CRYOPRESERVATION, FREEZING AND STORAGE, CELLS, EAC THAWING AND EXPANSION, FROZEN CELLS, EACH ALIQUOT CHROMOSOME ANALYSIS, BREAKAGE SYNDROMES; BASELINE CHROMOSOME ANALYSIS, BREAKAGE SYNDROMES; BASELINE CHROMOSOME ANALYSIS, BREAKAGE SYNDROMES; 100 CELLS CHROMOSOME ANALYSIS; COUNT 5 CELLS, 1 KARYOTYPE, W CHROMOSOME ANALYSIS; COUNT 15-20 CELLS, 2 KARYOTYP CHROMOSOME ANALYSIS; COUNT 45 CELLS, MOSAICISM, 2 CHROMOSOME ANALYSIS; ANALYZE 20-25 CELLS CHROMOSOME ANALYSIS AMNIOTIC FLUID/CHORIONIC VILLU CHROMOSOME ANALYSIS IN SITU, AMNIOTIC FLUID CELLS, MOLECULAR CYTOGENETICS; DNA PROBE, EACH MOLECULAR CYTOGENETICS; CHROMOSOMAL IN SITU HYBRID MOLECULAR CYTOGENETICS; CHROMOSOMAL IN SITU HYBRID MOLECULAR CYTOGENETICS; INTERPHASE IN SITU HYBRIDI MOLECULAR CYTOGENETICS; INTERPHASE IN SITU HYBRIDI CHROMOSOME ANALYSIS; ADDL KARYOTYPES, EACH STUDY CHROMOSOME ANALYSIS; ADDL SPECIALIZED BANDING TECH CHROMOSOME ANALYSIS; ADDL CELLS COUNTED, EACH STUD CHROMOSOME ANALYSIS; ADDL HIGH RESOLUTION STUDY CYTOGENETICS AND MOLECULAR CYTOGENETICS, INTERPRET UNLISTED CYTOGENETIC STUDY LEVEL I - SURGICAL PATHOLOGY, GROSS EXAM ONLY LEVEL II - SURGICAL PATHOLOGY, GROSS AND MICROSCOP LEVEL III - SURGICAL PATHOLOGY, GROSS AND MICROSCO LEVEL IV - SURGICAL PATHOLOGY, GROSS AND MICROSCOP LEVEL V - SURGICAL PATHOLOGY, GROSS AND MICROSCOPI LEVEL VI - SURGICAL PATHOLOGY, GROSS AND MICROSCOP DECALCIFICATION PROC SPECIAL STAINS; GROUP I, MICROORGANISMS, EACH SPCL STN 2 I&R EXCPT MICROORG/ENZYME/IMCYT&IMHIS SPECIAL STAINS; HISTOCHEMICAL STAINING W/FROZEN SE SPECIAL STAIN I&R GROUP III ENZYME CONSITUENTS CONSULTATION AND REPORT, REFERRED SLIDES PREPARED CONSULTATION AND REPORT, REFERRED MATL REQUIRING P CONSULTATION AND REPORT, REFERRED MATL, COMPREHENS PATHOLOGY CONSULTATION DURING SURGERY PATHOLOGY CONSULTATION DURING SURGERY; FIRST TISSU PATH CONSLTJ SURG EA BLK FROZEN SCTJ PATHOLOGY CONSULT DURING SURG; CYTOLOGICAL EXAMINA PATH CONSLTJ SURG CYTOL XM EA ADDL IMHISTOCHEM/CYTCHM EA ADDL ANTIBODY SLIDE IMHISTOCHEM/CYTCHM INIT ANTIBODY STAIN PROCEDURE IMHISTOCHEM/CYTCHM EA MULTIPLEX ANTIBODY SLIDE IMMUNOFLUORESCENT STUDY, EACH ANTIBODY; DIRECT MET IMMUNOFLUORESCENT STUDY, EACH ANTIBODY; INDIRECT M ELECTRON MICROSCOPY DIAGNOSTIC IMMUNOFLUORESCENCE, PER SPECIMEN; EACH ADDITIONAL SINGLE ANTIBODY STAIN PROCED MORPHOMETRIC ANALYSIS; SKELETAL MUSCLE MORPHOMETRIC ANALYSIS; NERVE MORPHOMETRIC ANALYSIS; TUMOR (DNA PLOIDY) M/PHMTRC ALYS TUMOR IMHCHEM EA ANTIBODY MANUAL M/PHMTRC ALYS TUMOR IMHCHEM EA ANTBDY CMPTR ASST NERVE TEASING PREPARATIONS EXAM & SELECT ARCHIVE TISSUE MOLECULAR ANALYSIS IN SITU HYBRIDIZATION EA ADDL PROBE STAIN IN SITU HYBRIDIZATION 1ST PROBE STAIN IN SITU HYBRIDIZATION EA MULTIPLEX PROBE STAIN M/PHMTRC ALYS ISH CPTR-ASST TECH 1ST PROBE STAIN M/PHMTRC ALYS IN SITU HYBRIDIZATION EA PROBE MNL M/PHMTRC ALYS ISH QUANT/SEMIQ MNL PER SPEC EACH PROTEIN ANALYSIS, TISSUE, WESTERN BLOT, W/INTERPRE PROTEIN ANALYSIS, TISSUE, WESTERN BLOT, W/INTERPRE M/PHMTRC ALYS ISH QUANT/SEMIQ CPTR PER SPEC EACH M/PHMTRC ALYS ISH QUANT/SEMIQ CPTR EACH MULTIPRB OPTICAL ENDOMICROSCOPIC IMAGE INTERP & REPORT M/PHMTRC ALYS ISH QUANT/SEMIQ MNL EACH MULTIPRB MICRODISSECTION (MECHANICAL, LASER CAPTURE) MICRODISSECTION PREP IDENTIFIED TARGET MANUAL 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2005 1/1/2005 1/1/2005 1/1/2005 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2003 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2015 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2011 1/1/2015 1/1/2004 1/1/2015 1/1/2005 1/1/2005 1/1/2015 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2013 1/1/2015 1/1/2004 1/1/2008 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 88387 88388 88399 88720 88738 88740 88741 88749 89049 89050 89051 89055 89060 89125 89160 89190 89220 89230 89240 89250 89251 89253 89254 89255 89257 89258 89259 89260 89261 89264 89268 89272 89280 89281 89290 89291 89300 89310 89320 89321 89322 89325 89329 89330 89331 89335 89337 89342 89343 89344 89346 89352 89353 89354 89356 89398 90281 90283 90284 90287 90288 90291 90296 90371 90375 90376 90378 90384 90385 90386 90389 90393 90396 90399 90460 90461 90471 90472 90473 90474 90476 90477 90581 90585 90586 90620 90621 90625 90630 90632 90633 MACRO EXAM DISSECT&PREP TISS NONMICRO STD EA MACR EXM DISS&PRP NONMICR IMPRNT/CONSLT/FRZ SEC UNLISTED SURGICAL PATHOLOGY PROC BILIRUBIN TOTAL TRANSCUTANEOUS HGB QUANTITATIVE TRANSCUTANEOUS HEMOGLOBIN QUAN TC PER DAY CARBOXYHEMOGLOBIN HEMOGLOBIN QUANTITATIVE TC PER DAY METHEMOGLOBIN UNLISTED IN VIVO LAB SERVICE CAFFEINE HALOTHAN CONTRACTURE TEST (CHCT) FOR MALI CELL COUNT, MISCELLANEOUS BODY FLUIDS, EXCEPT BLOO CELL COUNT, MISCELLANEOUS BODY FLUIDS, EXCEPT BLOO LEUKOCYTE ASSESSMENT, FECAL, QUALITATIVE/SEMIQUANT CRYSTAL ID, LIGHT MICROSCOPY, BODY FLUID, EXCEPT U FAT STAIN, FECES, URINE/RESPIRATORY SECRETIONS MEAT FIBERS, FECES NASAL SMEAR, EOSINOPHILS SPUTUM, OBTAINING SPECIMEN, AEROSOL INDUCED TECHNI SWEAT COLLECTION BY IONTOPHORESIS UNLISTED MISCELLANEOUS PATHOLOGY TEST CULTURE, OOCYTE(S)/EMBRYO(S), LESS THAN 4 DAYS; CULTURE, OOCYTE(S)/EMBRYO(S), LESS THAN 4 DAYS; W/ ASSISTED EMBRYO HATCHING, MICROTECHNIQUES (ANY MET OOCYTE IDENTIFICATION, FOLLICULAR FLUID PREPARATION, EMBRYO, TRANSFER (ANY METHOD) SPERM IDENTIFICATION, ASPIRATION (OTHER THAN SEMIN CRYOPRESERVATION; EMBRYO(S) CRYOPRESERVATION; SPERM SPERM ISOLATION; SIMPLE PREP, FOR INSEMINATION/DIA SPERM ISOLATION; COMPLEX PREP, FOR INSEMINATION/DI SPERM IDENTIFICATION, TESTIS TISSUE, FRESH/CRYOPRE INSEMINATION, OOCYTES EXTENDED CULTURE, OOCYTE(S)/EMBRYO(S), 4-7 DAYS ASSISTED OOCYTE FERTILIZATION, MICROTECHNIQUE; LES ASSISTED OOCYTE FERTILIZATION, MICROTECHNIQUE; GRE BX, OOCYTE POLAR BODY/EMBRYO BLASTOMERE, MICROTECH BX, OOCYTE POLAR BODY/EMBRYO BLASTOMERE, MICROTECH SEMEN ANALYSIS; PRESENCE AND/OR MOTILITY, SPERM W/ SEMEN ANALYSIS; MOTILITY AND COUNT W/O HUHNER TEST SEMEN ANALYSIS; COMPLETE (VOLUME, COUNT, MOTILITY, SEMEN ANALYSIS, PRESENCE AND/OR MOTILITY OF SPERM SEMEN ANALYSIS STRICT MORPHOLOGIC CRITERIA SPERM ANTIBODIES SPERM EVALUATION; HAMSTER PENETRATION TEST SPERM EVALUATION; CERVICAL MUCUS PENETRATION TEST, SPERM EVALUATION RETROGRADE EJACULATION URINE CRYOPRESERVATION, REPRODUCTIVE TISSUE, TESTICULAR CRYOPRESERVATION MATURE OOCYTE(S) STORAGE, (PER YEAR); EMBRYO(S) STORAGE, (PER YEAR); SPERM/SEMEN STORAGE, (PER YEAR); REPRODUCTIVE TISSUE, TESTICUL STORAGE, (PER YEAR); OOCYTE THAWING, CRYOPRESERVED; EMBRYO(S) THAWING, CRYOPRESERVED; SPERM/SEMEN, EACH ALIQUOT THAWING, CRYOPRESERVED; REPRODUCTIVE TISSUE, TESTI THAWING, CRYOPRESERVED; OOCYTES, EACH ALIQUOT UNLISTED REPRODUCTIVE MEDICINE LAB PROCEDURE IMMUNE GLOBULIN (IG), HUMAN, IM USE IMMUNE GLOBULIN (IGIV), HUMAN, IV USE IMMUNE GLOBULIN HUMAN SUBQ INFUSION 100 MG EA BOTULINUM ANTITOXIN, EQUINE, ANY ROUTE BOTULISM IMMUNE GLOBULIN, HUMAN, IV USE CYTOMEGALOVIRUS IMMUNE GLOBULIN (CMV-IGIV), HUMAN, DIPHTHERIA ANTITOXIN, EQUINE, ANY ROUTE HEPATITIS B IMMUNE GLOBULIN (HBIG), HUMAN, IM USE RABIES IMMUNE GLOBULIN (RIG), HUMAN, IM AND/OR SUB RABIES IMMUNE GLOBULIN, HEAT-TREATED, HUMAN, IM AN RESPIRATORY SYNCYTIAL VIRUS IMMUNE GLOBULIN (RSV-I RHO(D) IMMUNE GLOBULIN (RHIG), HUMAN, FULL-DOSE, I RHO(D) IMMUNE GLOBULIN (RHIG), HUMAN, MINI-DOSE, I RHO(D) IMMUNE GLOBULIN (RHIGIV), HUMAN, IV USE TETANUS IMMUNE GLOBULIN (TIG), HUMAN, IM USE VACCINIA IMMUNE GLOBULIN, HUMAN, IM USE VARICELLA-ZOSTER IMMUNE GLOBULIN, HUMAN, IM USE UNLISTED IMMUNE GLOBULIN IM ADM THRU 18YR ANY RTE 1ST/ONLY COMPT VAC/TOX IM ADM THRU 18YR ANY RTE ADDL VAC/TOX COMPT IMMUNIZATION ADMINISTRATION; 1 SINGLE/COMBINATION IMMUNIZATION ADMINISTRATION; EACH ADDL SINGLE/COMB IMMUNIZATION ADMINISTRATION, INTRANASAL/ORAL; 1 SI IMMUNIZATION ADMINISTRATION, INTRANASAL/ORAL; EA A ADENOVIRUS VACCINE, TYPE 4, LIVE, ORAL USE ADENOVIRUS VACCINE, TYPE 7, LIVE, ORAL USE ANTHRAX VACCINE SUBCUTANEOUS/IM USE BACILLUS CALMETTE-GUERIN VACCINE (BCG), TUBERCULOS BACILLUS CALMETTE-GUERIN VACCINE (BCG), BLADDER CA MENB RECOMBINANT PROT W/OUT MEMBR VESIC VACC IM MENB RECOMBINANT LIPOPROTEIN VACCINE IM CHOLERA VACCINE, LIVE, ADULT DOSAGE, 1 DOSE SCHEDULE, FOR ORAL USE IIV4 INFLUENZA VACCINE 4 VALENT PRSRV FREE ID HEPATITIS A VACCINE, ADULT DOSAGE, IM USE HEPATITIS A VACCINE, PEDIATRIC/ADOLESCENT DOSAGE-2 1/1/2010 1/1/2010 1/1/2004 1/1/2009 1/1/2010 1/1/2009 1/1/2009 1/1/2011 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2015 1/1/2016 1/1/2015 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 90634 90636 90644 90645 90646 90647 90648 90649 90650 90651 90653 90654 90655 90656 90657 90658 90660 90661 90662 90664 90666 90667 90668 90669 90670 90672 90673 90675 90676 90680 90681 90685 90686 90687 90688 90690 90691 90692 90693 90696 90697 90698 90700 90702 90703 90704 90705 90706 90707 90708 90710 90712 90713 90714 90715 90716 90717 90719 90720 90721 90723 90725 90727 90732 90733 90734 90735 90736 90738 90739 90740 90743 90744 90746 90747 90748 90749 90785 90791 90792 90832 90833 90834 90836 90837 90838 90839 90840 90845 90846 90847 HEPATITIS A VACCINE, PEDIATRIC/ADOLESCENT DOSAGE-3 HEPATITIS A AND HEPATITIS B VACCINE (HEPA-HEPB), A MENINGOCOCCAL & HIB-MENCY VACCINE 4 DOSE IM HEMOPHILUS INFLUENZA B VACCINE (HIB), HBOC CONJUGA HEMOPHILUS INFLUENZA B VACCINE (HIB), PRP-D CONJUG HEMOPHILUS INFLUENZA B VACCINE, PRP-OMP, 3 DOSE SC HEMOPHILUS INFLUENZA B VACCINE (HIB),PRP-T CONJUGA HUMAN PAPILLOMA VIRUS (HPV) VACCINE, FOR IM USE HPV TYP 16 18 BIVALENT 3 DOSE SCHED IM HPV HUMAN PAPILLOMA VIRUS VACC 9 VAL 3 DOSE IM INFLUENZA VACCINE INACT SUBUNIT ADJUVANT IM INFLUENZA VACC IIV3 SPLIT VIRUS PRSRV FREE ID INFLUENZA VACC TRIVALENT PRSRV FREE 6-35 MO IM INFLUENZA VIRUS VACC SPLIT PRSRV FREE 3 YRS/> IM INFLUENZA VIRUS VACCINE SPLIT VIRUS 6-35 MO IM INFLUENZA VIRUS VACCINE SPLIT VIRUS 3/> YRS IM INFLUENZA VIRUS VACCINE LIVE INTRANASAL INFLUENZA VACCINE CELL CULT PRSRV FREE IM INFLUENZA VACCINE SPLT PRSRV FREE INC ANTIGEN IM INFLUENZA VACCINE PANDEMIC LIVE INTRANASAL USE INFLUENZA VACCINE PANDEMIC PRSV FREE IM USE INFLUENZA VACCINE PANDEMIC ADJUVANT IM USE INFLUENZA VACCINE PANDEMIC IM USE PNEUMOCOCCAL CONJUGATE VACCINE, POLYVALENT, CHILDR PNEUMOCOCCAL VACCINE 13 VALENT IM INFLUENZA VIRUS VAC QUADRIVALENT LIVE INTRANASAL INFLUENZA VIRUS VACCINE TRIVALEN RIV3 PRSR FR IM RABIES VACCINE, IM USE RABIES VACCINE, INTRADERMAL USE ROTAVIRUS VACCINE, TETRAVALENT, LIVE, ORAL USE ROTAVIRUS VACCINE PENTAV 2 DOSE SCHED LIVE ORAL INFLUENZA VAC QUADRIVALENT PRSRV FREE 6-35 MO IM INFLUENZA VAC 4 VALENT PRSRV FREE 3 YRS PLUS IM INFLUENZA VACCINE QUADRIVALENT 6-35 MO IM INFLUENZA VACCINE QUADRIVALENT 3 YRS PLUS IM TYPHOID VACCINE, LIVE, ORAL TYPHOID VACCINE, VI CAPSULAR POLYSACCHARIDE (VICPS TYPHOID VACCINE, HEAT- AND PHENOL-INACTIVATED (H-P TYPHOID VACCINE, ACETONE-KILLED, DRIED (AKD), SUBDTAP-IPV VACCINE 4-6 YR IM DTAP-IPV-HIB-HEPB VACCINE INTRAMUSCULAR DTAP - HIB - IPV VACCINE, IM USE DIPHTHERIA, TETANUS TOXOIDS, AND ACELLULAR PERTUSS DIPHTHERIA AND TETANUS TOXOIDS (DT) ADSORBED, INDI TETANUS TOXOID ADSORBED, IM USE INDIVIDUAL PSYCHOTHERAPY, OFFICE, 20-30 MIN MEASLES VIRUS VACCINE, LIVE, SUB-Q USE RUBELLA VIRUS VACCINE, LIVE, SUB-Q USE MEASLES, MUMPS AND RUBELLA VIRUS VACCINE (MMR), LI MEASLES AND RUBELLA VIRUS VACCINE, LIVE, SUB-Q USE MEASLES, MUMPS, RUBELLA, AND VARICELLA VACCINE (MM POLIOVIRUS VACCINE, (ANY TYPE(S)) (OPV), LIVE, ORA POLIOVIRUS VACCINE, INACTIVATED, (IPV), SUBQ USE TETANUSAND DIPTHERIA TOXOIDS; ABSORBED; 7 YRS OR O TETANUS, DIPHTHERIA TOXOIDS AND ACELLULAR PERTUSIS VARICELLA VIRUS VACCINE, LIVE, SUBQ USE YELLOW FEVER VACCINE, LIVE, SUBQ USE DIPHTHERIA TOXOID, IM USE DTP/HIB VACCINE, IM DTAP/HIB VACCINE INTRAMUSCULAR DTAP-HEPB-IPV VACCINE INTRAMUSCULAR CHOLERA VACCINE, INJECTABLE USE PLAGUE VACCINE, IM USE PNEUMOCOCCAL POLYSACCHARIDE VACCINE, 23-VALENT, AD MENINGOCOCCAL POLYSACCHARIDE VACCINE (ANY GROUP(S) MENINGOCOCCAL CONJ VACCINE QUADRAVALENT IM JAPANESE ENCEPHALITIS VIRUS VACCINE, SUBQ USE ZOSTER VACCINE, LIVE FOR SUBQ USE JAPANESE ENCEPHALITIS VIRUS VACCINE INACTIVE IM HEPATITIS B VACCINE ADULT 2 DOSE IM HEPATITIS B VACCINE, DIALYSIS/IMMUNOSUPPRESSED PAT HEPATITIS B VACCINE, ADOLESCENT (2-DOSE SCHEDULE), HEPATITIS B VACCINE, PEDIATRIC/ADOLESCENT, (3-DOSE HEPATITIS B VACCINE ADULT 3 DOSE IM HEPATITIS B VACCINE, DIALYSIS/IMMUNOSUPPRESSED PAT HEPATITIS B AND HEMOPHILUS INFLUENZA B VACCINE (HE UNLISTED VACCINE/TOXOID PSYCHOTHERAPY COMPLEX INTERACTIVE PSYCHIATRIC DIAGNOSTIC EVALUATION PSYCHIATRIC DIAGNOSTIC EVAL W/MEDICAL SERVICES PSYCHOTHERAPY PATIENT &/ FAMILY 30 MINUTES PSYCHOTHERAPY PT&/FAMILY W/E&M SRVCS 30 MIN PSYCHOTHERAPY PATIENT &/ FAMILY 45 MINUTES PSYCHOTHERAPY PT&/FAMILY W/E&M SRVCS 45 MIN PSYCHOTHERAPY PATIENT &/ FAMILY 60 MINUTES PSYCHOTHERAPY PT&/FAMILY W/E&M SRVCS 60 MIN PSYCHOTHERAPY FOR CRISIS INITIAL 60 MINUTES PSYCHOTHERAPY FOR CRISIS EACH ADDL 30 MINUTES PSYCHOANALYSIS FAMILY PSYCHOTHERAPY (W/O PATIENT PRESENT) FAMILY PSYCHOTHERAPY (CONJOINT PSYCHOTHERAPY) (W/P 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2008 1/1/2015 1/1/2013 1/1/2011 1/1/2004 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 7/1/2010 7/1/2010 7/1/2010 7/1/2010 1/1/2004 7/1/2009 1/1/2013 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 7/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 7/1/2008 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 90849 90853 90863 90865 90867 90868 90869 90870 90875 90876 90880 90882 90885 90887 90889 90899 90901 90911 90935 90937 90940 90945 90947 90951 90952 90953 90954 90955 90956 90957 90958 90959 90960 90961 90962 90963 90964 90965 90966 90967 90968 90969 90970 90989 90993 90997 90999 91010 91013 91020 91022 91030 91034 91035 91037 91038 91040 91065 91110 91111 91112 91117 91120 91122 91132 91133 91200 91299 92002 92004 92012 92014 92015 92018 92019 92020 92025 92060 92065 92071 92072 92081 92082 92083 92100 92132 92133 92134 92136 92140 92145 MULTIPLE-FAMILY GROUP PSYCHOTHERAPY GROUP PSYCHOTHERAPY (OTHER THAN, MULTIPLE-FAMILY G PHARMACOLOGIC MANAGEMENT W/PSYCHOTHERAPY NARCOSYNTHESIS, PSYCHIATRIC DX AND THERAPEUTIC PUR REPET TMS TX INITIAL W/MAP/MOTR THRESHLD/DEL&MNG THERAP REPETITIVE TMS TX SUBSEQ DELIVERY & MNGT REPET TMS TX SUBSEQ MOTR THRESHLD W/DELIV & MNGT ELECTROCONVULSIVE THERAPY (INCL NECESSARY MONITORI INDIV PSYCHOPHYS BIOFEED TRAIN W/PSYTX 30 MIN INDIV PSYCHOPHYS BIOFEED TRAIN W/PSYTX 45 MIN HYPNOTHERAPY ENVIRONMENTAL INTERVENTION, MEDICAL PURPOSES, PSYC PSYCHIATRIC EVAL, RECORDS, MEDICAL DIAGNOSTIC PURP INTERPRETATION/EXPLANATION RESULTS, PSYCHIATRIC/ME PREP REPORT PT PSYCH STATUS AGENCY/PAYER UNLISTED PSYCHIATRIC SERVICE/PROC BIOFEEDBACK TRAINING, ANY MODALITY BIOFEEDBACK, PERINEAL MUSCLES/ANORECTAL/URETHRAL S HEMODIALYSIS PROCEDURE W/ PHYS/QHP EVALUATION HEMODIALYSIS, REPEATED EVAL, W/WO REVISION DIALYSI HEMODIALYSIS ACCESS FLOW STUDY, BY INDICATOR DILUT DIALYSIS OTHER/THAN HEMODIALYSIS 1 PHYS/QHP EVAL DIALYSIS OTH/THN HEMODIALY REPEAT PHYS/QHP EVALS ESRD RELATED SVC MONTHLY & <2 YR OLD 4/> VISITS ESRD RELATED SVC MONTHLY <2 YR OLD 2/3 VISITS ESRD RELATED SVC MONTHLY <2 YR OLD 1 VISIT ESRD RELATED SVC MONTHLY 2-11 YR OLD 4/> VISITS ESRD RELATED SVC MONTHLY 2-11 YR OLD 2/3 VISITS ESRD RELATED SVC MONTHLY 2-11 YR OLD 1 VISIT ESRD RELATED SVC MONTHLY 12-19 YR OLD 4/>VISITS ESRD RELATED SVC MONTHLY 12-19 YR OLD 2/3 VISITS ESRD RELATED SVC MONTHLY 12-19 YR OLD 1 VISIT ESRD RELATED SVC MONTHLY 20&/> YR OLD 4/> VISITS ESRD RELATED SVC MONTHLY 20/>YR OLD 2/3 VISITS ESRD RELATED SVC MONTHLY 20&/>YR OLD 1 VISIT ESRD SVC HOME DIALYSIS FULL MONTH <2YR OLD ESRD SVC HOME DIALYSIS FULL MONTH 2-11 YR OLD ESRD SVC HOME DIALYSIS FULL MONTH 12-19 YR OLD ESRD SVC HOME DIALYSIS FULL MONTH 20 YR OLD ESRD RELATED SVC <FULL MONTH < 2 YR OLD ESRD RELATED SVC <FULL MONTH 2-11 YR OLD ESRD RELATED SVC <FULL MONTH 12-19 YR OLD ESRD RELATED SVC <FULL MONTH 20&> YR OLD DIALYSIS TRAINING, PATIENT, W/HELPER WHERE APPLICA DIALYSIS TRAINING, PATIENT, W/HELPER WHERE APPLICA HEMOPERFUSION UNLISTED DIALYSIS PROC, INPATIENT/OUTPATIENT ESOPHAGEAL MOTILITY STUDY W/INTERP&RPT ESOPHAGEAL MOTILITY STD W/I&R STIM/PERFUSION GASTRIC MOTILITY (MANOMETRIC) STUDIES DUODENAL MOTILITY STUDY ESOPHAGUS, ACID PERFUSION TEST, ESOPHAGITIS ESOPHAGUS, GASTRO REFLUX TEST, RECORDING, ANALYSIS ESOPHAGUS, GASTRO REFLUX TEST, RECORDING, WITH MUC ESOPHAGEAL FUNCTION TEST,GASTRO REFLUX TEST WITH A ESOPHAGEAL FUNCTION TEST,GASTRO REFLUX TEST WITH A ESOPHAGEAL BALLOON DISTENSION PROVOCATION STUDY BREATH HYDROGEN/METHANE TEST GI IMAG INTRALUMINAL ESOPHAGUS-ILEUM W/I&R GASTROINTESTINAL TRACT IMAGING ESOPHAGUS W/I&R GI TRANSIT & PRES MEAS WIRELESS CAPSULE W/INTERP COLON MOTILITY STDY MIN 6 HR CONT RECORD W/I&R RECTAL SENSATION, TONE, AND COMPLIANCE TEST ANORECTAL MANOMETRY ELECTROGASTROGRAPHY, DIAGNOSTIC, TRANSCUTANEOUS ELECTROGASTROGRAPHY, DIAGNOSTIC, TRANSCUTANEOUS; W LIVER ELASTOGRAPHY W/O IMAG W/I&R UNLISTED DX GASTROENTEROLOGY PROC OPHTHALMOLOGICAL MEDICAL EXAM AND EVAL; INTERMEDIA OPHTHALMOLOGICAL MEDICAL EXAM AND EVAL; COMPREHENS OPHTHALMOLOGICAL MEDICAL EXAM AND EVAL; INTERMEDIA OPHTHALMOLOGICAL MEDICAL EXAM AND EVAL; COMPREHENS DETERMINATION, REFRACTIVE STATE OPHTHALMOLOGICAL EXAM AND EVAL, UNDER GEN ANES, WI OPHTHALMOLOGICAL EXAM & EVAL, UNDER GEN ANES; LTD GONIOSCOPY (SEP PROC) Corneal topography SENSORIMOTOR EXAM W/MULTIPLE MEASUREMENTS, OCULAR ORTHOPTIC AND/OR PLEOPTIC TRAINING, W/CONTINUING M FIT CONTACT LENS TX OCULAR SURFACE DISEASE FITTING CONTACT LENS FOR MNGT OF KERATOCONUS VISUAL FIELD EXAM, UNILAT/BILAT W/INTERPRETATION A VISUAL FIELD EXAM, UNILAT/BILAT W/INTERPRETATION A VISUAL FIELD XM UNI/BI W/INTERP EXTENDED EXAM SERIAL TONOMETRY (SEP PROC) MULTIPLE MEASURE, EXTE CMPTR OPHTHALMIC DX IMG ANT SEGMT W/I&R UNI/BI COMPUTERIZED OPHTHALMIC IMAGING OPTIC NERVE COMPUTERIZED OPHTHALMIC IMAGING RETINA OPHTHALMIC BIOMETRY BY PARTIAL COHERENCE INTERFERO PROVOCATIVE TESTS, GLAUCOMA, W/INTERPRETATION AND CORNEA HYSTERESIS DETERMIN IMPULSE STIMJ UNI/BI 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2011 1/1/2011 1/1/2012 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2006 1/1/2004 1/1/2005 1/1/2005 1/1/2005 1/1/2005 1/1/2005 1/1/2004 1/1/2004 1/1/2007 1/1/2013 1/1/2011 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2015 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N Y Y N N N N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 92225 92226 92227 92228 92230 92235 92240 92250 92260 92265 92270 92275 92283 92284 92285 92286 92287 92310 92311 92312 92313 92314 92315 92316 92317 92325 92326 92340 92341 92342 92352 92353 92354 92355 92358 92370 92371 92499 92502 92504 92507 92508 92511 92512 92516 92520 92521 92522 92523 92524 92526 92531 92532 92533 92534 92537 92538 92540 92541 92542 92543 92544 92545 92546 92547 92548 92550 92551 92552 92553 92555 92556 92557 92558 92559 92560 92561 92562 92563 92564 92565 92567 92568 92570 92571 92572 92575 92576 92577 92579 92582 EXTENDED OPHTHALMOSCOPY W/RETINAL DRAWING, W/INTER EXTENDED OPHTHALMOSCOPY W/RETINAL DRAWING, W/INTER REMOTE IMG DX RETINL DIS W/ALYS & REPORT UNI/BI REMOTE IMG MGT RETINL DIS W/I&R UNI/BI FLUORESCEIN ANGIOSCOPY W/INTERPRETATION AND REPORT FLUORESCEIN ANGIOGRAPHY (W/MULTIFRAME IMAGING) W/I INDOCYANINE-GREEN ANGIOGRAPHY (W/MULTIFRAME IMAGIN FUNDUS PHOTOGRAPHY W/INTERPRETATION AND REPORT OPHTHALMODYNAMOMETRY NEEDLE OCULOELECTROMYOGRAPHY, PLUS 1 EXTRAOCULAR M ELECTRO-OCULOGRAPHY W/INTERPRETATION AND REPORT ELECTRORETINOGRAPHY W/INTERPRETATION AND REPORT COLOR VISION EXAM, EXTENDED DARK ADAPTATION EXAM W/INTERPRETATION AND REPORT EXT OCULAR PHOTOGRAPHY W/INTERPRETATION AND REPORT ANT SGM IMAGING W/MICROSCOPY ENDOTHELIAL ANALY ANT SGM IMAGING W/FLUOROSCEIN ANGIO & I&R PRESCRIPTION/FITTING CONTACT LENS W/MEDICAL SUPERV PRESCRIPTION/FITTING CONTACT LENS W/MEDICAL SUPERV PRESCRIPTION/FITTING CONTACT LENS W/MEDICAL SUPERV PRESCRIPTION/FITTING CONTACT LENS W/MEDICAL SUPERV PRESCRIPTION/FITTING CONTACT LENS W/MEDICAL SUPERV PRESCRIPTION/FITTING CONTACT LENS W/MEDICAL SUPERV PRESCRIPTION/FITTING CONTACT LENS W/MEDICAL SUPERV PRESCRIPTION/FITTING CONTACT LENS W/MEDICAL SUPERV MODIFICATION, CONTACT LENS (SEP PROC), W/MEDICAL S REPLACEMENT, CONTACT LENS FITTING, SPECTACLES, EXCEPT APHAKIA; MONOFOCAL FITTING, SPECTACLES, EXCEPT APHAKIA; BIFOCAL FITTING, SPECTACLES, EXCEPT APHAKIA; MULTIFOCAL, O FITTING, SPECTACLE PROSTHESIS, APHAKIA; MONOFOCAL FITTING, SPECTACLE PROSTHESIS, APHAKIA; MULTIFOCAL FITTING, SPECTACLE MOUNTED LOW VISION AID; SINGLE FITTING, SPECTACLE MOUNTED LOW VISION AID; TELESCO PROSTHESIS SERVICE, APHAKIA, TEMPORARY (DISPOSABLE REPAIR AND REFITTING SPECTACLES; EXCEPT APHAKIA REPAIR AND REFITTING SPECTACLES; SPECTACLE PROSTHE UNLISTED OPHTHALMOLOGICAL SERVICE/PROC OTOLARYNGOLOGIC EXAM UNDER GENERAL ANESTHESIA BINOCULAR MICROSCOPY (SEP DX PROC) SPEECH/HEARING/VOICE/COMMUNICATION THERAPY; INDIVI SPEECH/HEARING/VOICE/COMMUNICATION THERAPY; GROUP, NASOPHARYNGOSCOPY W/ENDOSCOPE (SEP PROC) NASAL FUNCTION STUDIES FACIAL NERVE FUNCTION STUDIES LARYNGEAL FUNCTION STUDIES EVALUATION OF SPEECH FLUENCY (STUTTER CLUTTER) EVALUATION OF SPEECH SOUND PRODUCTION ARTICULATE EVAL SPEECH SOUND PRODUCT LANGUAGE COMPREHENSION BEHAVIORAL & QUALIT ANALYSIS VOICE AND RESONANCE TREATMENT, SWALLOWING DYSFUNCTION AND/OR ORAL FUNC SPONTANEOUS NYSTAGMUS, W/GAZE POSITIONAL NYSTAGMUS CALORIC VESTIBULAR TEST, EACH IRRIGATION OPTOKINETIC NYSTAGMUS TEST CALORIC VESTIBULAR TEST WITH RECORDING, BILATERAL; BITHERMAL (IE, ONE WARM AND O CALORIC VESTIBULAR TEST WITH RECORDING, BILATERAL; MONOTHERMAL (IE, ONE IRRIGATIO VSTBLR FUNCJ NYSTAG FOVL&PERPH STIMJ OSCIL TRKG SPONTANEOUS NYSTAGMUS TEST, W/GAZE AND FIXATION NY POSITIONAL NYSTAGMUS TEST, MINIMUM, 4 POSITIONS, W CALORIC VESTIBULAR TEST, EACH IRRIGATION, W/RECORD OPTOKINETIC NYSTAGMUS TEST, BIDIRECTIONAL, FOVEAL/ OSCILLATING TRACKING TEST, W/RECORDING SINUSOIDAL VERTICAL AXIS ROTATIONAL TESTING USE, VERTICAL ELECTRODES COMPUTERIZED DYNAMIC POSTUROGRAPHY TYMPANOMETRY AND REFLEX THRESHOLD MEASUREMENTS SCREENING TEST, PURE TONE, AIR ONLY PURE TONE AUDIOMETRY (THRESHOLD); AIR ONLY PURE TONE AUDIOMETRY (THRESHOLD); AIR AND BONE SPEECH AUDIOMETRY THRESHOLD SPEECH AUDIOMETRY THRESHOLD; W/SPEECH RECOGNITION COMPREHENSIVE AUDIOMETRY THRESHOLD EVAL AND SPEECH EVOKED OTOACOUSTIC EMISSIONS SCREEN AUTO ANALYS AUDIOMETRIC TESTING, GROUPS BEKESY AUDIOMETRY; SCREENING BEKESY AUDIOMETRY; DX LOUDNESS BALANCE TEST, ALTERNATE BINAURAL/MONAURAL TONE DECAY TEST SHORT INCREMENT SENSITIVITY INDEX (SISI) STENGER TEST, PURE TONE TYMPANOMETRY (IMPEDANCE TESTING) ACOUSTIC REFLEX THRESHOLD ACOUSTIC IMMIT TEST TYMPANOM/ACOUST REFLX/DECAY FILTERED SPEECH TEST STAGGERED SPONDAIC WORD TEST SENSORINEURAL ACUITY LEVEL TEST SYNTHETIC SENTENCE ID TEST STENGER TEST, SPEECH VISUAL REINFORCEMENT AUDIOMETRY (VRA) CONDITIONING PLAY AUDIOMETRY 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 92583 92584 92585 92586 92587 92588 92590 92591 92592 92593 92594 92595 92596 92597 92601 92602 92603 92604 92605 92606 92607 92608 92609 92610 92611 92612 92613 92614 92615 92616 92617 92618 92620 92621 92625 92626 92627 92630 92633 92640 92700 92920 92921 92924 92925 92928 92929 92933 92934 92937 92938 92941 92943 92944 92950 92953 92960 92961 92970 92971 92973 92974 92975 92977 92978 92979 92986 92987 92990 92992 92993 92997 92998 93000 93005 93010 93015 93016 93017 93018 93024 93025 93040 93041 93042 93050 93224 93225 93226 93227 93228 SELECT PICTURE AUDIOMETRY ELECTROCOCHLEOGRAPHY AUDITORY EVOKED POTENTIALS, EVOKED RESPONSE AUDIOM AUDITORY EVOKED POTENTIALS, EVOKED RESPONSE AUDIOM DISTORT PRODUCT EVOKED OTOACOUSTIC EMISNS LIMITD DISTRT PROD EVOKD OTOACOUSTIC EMSNS COMP/DX EVAL HEARING AID EXAM AND SELECTION; MONAURAL HEARING AID EXAM AND SELECTION; BINAURAL HEARING AID CHECK; MONAURAL HEARING AID CHECK; BINAURAL ELECTROACOUSTIC EVALUATION, HEARING AID; MONAURAL ELECTROACOUSTIC EVALUATION, HEARING AID; BINAURAL EAR PROTECTOR ATTENUATION MEASUREMENTS EVAL FOR USE AND/OR FITTING VOICE PROSTHETIC DEVIC DX ANALYSIS COCHLEAR IMPLANT, PATIENT UNDER 7 YRS; DX ANALYSIS COCHLEAR IMPLANT, PATIENT UNDER 7 YRS; DX ANALYSIS COCHLEAR IMPLANT, AGE 7 YEARS OR OLDER DX ANALYSIS COCHLEAR IMPLANT, AGE 7 YEARS OR OLDER; SUBSEQUENT REPROGRAMMING EVAL RX N-SP-GEN AUGMT ALT COMMUN DEV F2F 1ST HR THERAPEUTIC SERVICE(S), USE NON-SPEECH GENERATIING EVAL, PRESCRIPTION, SPEECH-GENERATING AUGMENTATIVE EVAL, PRESCRIP, SPEECH-GENERATING AUGMENTATIVE AND THERAPEUTIC SERVICES, NON-SPEECH GENERATIVE DEVICE EVAL, ORAL AND PHARYNGEAL SWALLOW FUNCTION MOTION FLUOROSCOPIC EVAL, SWALLOW FUNCTION, CINE/V FLEXIBLE FIBEROPTIC ENDOSCOPIC EVAL, SWALLOW, CINE FLX FIBOPT NDSC EVAL SWLNG C/V REC PHYS I&R FLEXIBLE FIBEROPTIC ENDOSCOPIC EVAL, LARYNGEAL SEN FLX FIBOPT NDSC EVAL LARYN SENS PHYS I&R FLEXIBLE FIBEROPTIC ENDOSCOPIC EVAL, SWALLOWING AN FLX FIBOPT NDSC EVAL SWLNG&LARYN SENS PHYS I&R EVAL RX N-SP-GEN AUGMT ALT COMMUN DEV ADD 30 MIN EVALUATION OF CENTRAL AUDITORY FUNCTION, INITIAL 6 EVAL CENTRAL AUDITORY FUNCJ W/REPRT EA 15 MIN ASSESSMENT OF TINNITUS EVAL OF AUDITORY REHABILITATION STATUS; FIRST HOUR EVAL OF AUDITORY REHABILITATION STATUS; EACH ADD'L AUDITORY REHABILITATION; PRE-LINGUAL HEARING LOSS AUDITORY REHABILITATION; POST-LINGUAL HEARING LOSS Aud brainstem implt programg UNLISTED OTORHINOLARYNGOLOGICAL SERVICE/PROCEDURE PRQ TRLUML CORONARY ANGIOPLASTY ONE ART/BRANCH PRQ TRLUML CORONARY ANGIOPLASTY ADDL BRANCH PRQ TRLUML CORONARY ANGIO/ATHERECT ONE ART/BRNCH PRQ TRLUML CORONARY ANGIO/ATHEREC ADDL ART/BRNCH PRQ TRLUML CORONARY STENT W/ANGIO ONE ART/BRNCH PRQ TRLUML CORONARY STENT W/ANGIO ADDL ART/BRNCH PRQ TRLUML CORONRY STENT/ATH/ANGIO ONE ART/BRNCH PRQ TRLUML CORONARY STENT/ATH/ANGIO ADDL BRANCH PRQ TRLUML CORONARY BYP GRFT REVASC ONE VESSEL PRQ TRLUML CORONARY BYP GRFT REVASC ADDL VESSEL PRQ TRLUML CORONRY TOT OCCLUS REVASC MI ONE VSL PRQ TRLUML CORONRY CHRONIC OCCLUS REVASC ONE VSL PRQ TRLUML CORONRY CHRNIC OCCLUS REVASC ADDL VSL CARDIOPULMONARY RESUSCITATION TEMPORARY TRANSCUTANEOUS PACING CARDIOVERSION, ELECTIVE; EXTERNAL CARDIOVERSION, ELECTIVE; INTERNAL (SEP PROC) CARDIOASSIST-METHOD, CIRCULATORY ASSIST; INTERNAL CARDIOASSIST-METHOD, CIRCULATORY ASSIST; EXTERNAL PRQ TRANSLUMINAL CORONARY MECHANICL THROMBECTOMY TRANSCATHETER PLACEMENT, RADIATION DELIVERY DEVICE THROMBOLYSIS, CORONARY; INTRACORONARY INFUSION, W/ THROMBOLYSIS, CORONARY; IV INFUSION INTRAVASCULAR ULTRASOUND, CORONARY VESSEL, DX/THER INTRAVASCULAR ULTRASOUND, CORONARY VESSEL, DX/THER PERCUTANEOUS BALLOON VALVULOPLASTY; AORTIC VALVE PERCUTANEOUS BALLOON VALVULOPLASTY; MITRAL VALVE PERCUTANEOUS BALLOON VALVULOPLASTY; PULMONARY VALV ATRIAL SEPTECTOMY/SEPTOSTOMY; TRANSVENOUS BALLOON, ATRIAL SEPTECTOMY/SEPTOSTOMY; BLADE METHOD W/CARDI PERCUTANEOUS TRANSLUMINAL PULMONARY ARTERY BALLOON PERCUTANEOUS TRANSLUMINAL PULMONARY ANGIOPLASTY; A ELECTROCARDIOGRAM, ROUTINE W/AT LEAST 12 LEADS; W/ ELECTROCARDIOGRAM, ROUTINE W/AT LEAST 12 LEADS; T ELECTROCARDIOGRAM, ROUTINE W/AT LEAST 12 LEADS; IN CV STRS TST XERS&/OR RX CONT ECG W/SI&R CV STRS TST XERS&/OR RX CONT ECG W/O I&R CARDIOVASCULAR STRESS TEST W/ECG MONITOR; TRACING CARDIOVASCULAR STRESS TEST W/ECG MONITOR; INTERPRE ERGONOVINE PROVOCATION TEST MICROVOLT T-WAVE ALTERNANS, ASSESSMENT, VENTRICULA RHYTHM ECG, 1-3 LEADS; W/INTERPRETATION AND REPORT RHYTHM ECG, 1-3 LEADS; TRACING ONLY W/O INTERPRETA RHYTHM ECG, 1-3 LEADS; INTERPRETATION AND REPORT O ARTERIAL PRESSURE WAVEFORM ANALYSIS FOR ASSESSMENT OF CENTRAL ARTERIAL PRESSUR XTRNL ECG & 48 HR RECORD SCAN STOR W/R&I XTRNL ECG UP TO 48 HR RECORDING EXTERNAL ECG SCANNING ANALYSIS REPORT XTRNL ECG CONTINUOUS RHYTHM W/I&R UP TO 48 HRS XTRNL MOBILE CV TELEMETRY W/I&REPORT 30 DAYS 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2005 1/1/2005 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2007 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y N N Y Y Y Y Y Y Y N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 93229 93260 93261 93268 93270 93271 93272 93278 93279 93280 93281 93282 93283 93284 93285 93286 93287 93288 93289 93290 93291 93292 93293 93294 93295 93296 93297 93298 93299 93303 93304 93306 93307 93308 93312 93313 93314 93315 93316 93317 93318 93320 93321 93325 93350 93351 93352 93355 93451 93452 93453 93454 93455 93456 93457 93458 93459 93460 93461 93462 93463 93464 93503 93505 93530 93531 93532 93533 93561 93562 93563 93564 93565 93566 93567 93568 93571 93572 93580 93581 93582 93583 93600 93602 93603 93609 93610 93612 93613 93615 93616 XTRNL MOBILE CV TELEMETRY W/TECHNICAL SUPPORT PRGRMG DEV EVAL IMPLANTABLE SUBQ LEAD DFB SYSTEM INTERROGATION EVAL F2F IMPLANT SUBQ LEAD DEFIB XTRNL PT ACTIV ECG TRANSMIS W/R&I </30 DAYS XTRNL PT ACTIVATED ECG RECORD MONITOR 30 DAYS XTRNL PT ACTIVATED ECG REC DWNLD 30 DAYS XTRNL PT ACTIVTD ECG DWNLD W/R&I </30 DAYS SIGNAL-AVERAGED ELECTROCARDIOGRAPHY (SAECG), W/WO PROGRAM EVAL IMPLANTABLE IN PRSN 1 LD PACEMAKER PROGRAM EVAL IMPLANTABLE IN PERSN DUAL LD PACER PROGRAM EVAL IMPLANTABLE IN PRSN MULTI LD PACER PRGRMNG DEV EVAL IMPLANTABLE IN PERSN 1 LD DFB PRGRMG EVAL IMPLANTABLE IN PRSN DUAL LEAD DFB PRGRMG EVAL IMPLANTABLE IN PERSON MULTI LEAD DFB PROGRAM EVAL IMPLANTABLE DEV IN PRSN ILR SYSTEM PERI-PX EVAL&PROGRAM IN PRSN PACEMAKER SYSTEM PERI-PX DEV EVAL & PROG SING/DUAL/MULTI LEAD DFB INTERROGATION EVAL IN PERSON 1/DUAL/MLT LEAD PM INTERROGATION EVAL F2F 1/DUAL/MLT LEADS CVDFB INTERROGATION EVAL F2F IMPLANTABLE CV MNTR SYS INTERROGATION EVALUATION IN PERSON ILR SYSTEM INTERROGATION EVAL IN PERSON WR DEFIBRILLATOR TRANSTELEPHONIC RHYTHM STRIP PACEMAKER EVAL INTERROGATION EVAL REMOTE </90 D 1/2/MLT LEAD PM INTERROGATION EVAL REMOTE </90 D 1/2/MLT LD DFB INTERROGATION REMOTE </90 D TECHNICIAN REVIEW INTERROGATION EVAL REMOTE </30 D CV MNTR SYS INTERROGATION EVALUATION REMOTE </30 D ILR SYS INTERROGATION EVAL REMOTE </30 D TECH REVIEW TRANSTHORACIC ECHOCARDIOGRAPHY, CONGENITAL CARDIAC TRANSTHORACIC ECHOCARDIOGRAPHY, CONGENITAL CARDIAC ECHO TTHRC R-T 2D -+M-MODE COMPL SPEC&COLOR DOP ECHOCARDIOGRAPHY, TRANSTHORACIC, 2D, W/WO M-MODE; ECHOCARDIOGRAPHY, TRANSTHORACIC, 2D, W/WO M-MODE; ECHOCARDIOGRAPHY, TRANSESOPHAGEAL, 2D; W/PROBE, IM ECHOCARDIOGRAPHY, TRANSESOPHAGEAL, 2D;TRANSESOPHAG ECHOCARDIOGRAPHY, TRANSESOPHAGEAL, 2D; IMAGE ACQUI ECHOCARDIOGRAPHY, TRANSESOPHAGEAL, CONGENITAL ANOM ECHOCARDIOGRAPHY, TRANSESOPHAGEAL, CONGENITAL ANOM ECHOCARDIOGRAPHY, TRANSESOPHAGEAL, CONGENITAL ANOM TEE FOR MONITORING, W/PROBE, REAL TIME 2D ACQUIS A DOPPLER ECHOCARDIOGRAPHY; COMPLETE DOPPLER ECHOCARDIOGRAPHY; FOLLOW-UP/LIMITED DOPPLER COLOR FLOW MAPPING ECHOCARDIOGRAPHY, TRANSTHORACIC, REAL-TIME 2D, CAR ECHO TTHRC R-T 2D W/WO M-MODE REST&STRS CONT ECG USE OF ECHO CONTRAST AGENT DURING STRESS ECHO ECHO TEE GUID TCAT ICAR/VESSEL STRUCTURAL INTVN RIGHT HEART CATH O2 SATURATION & CARDIAC OUTPUT L HRT CATH W/NJX L VENTRICULOGRAPHY IMG S&I R & L HRT CATH W/NJX L VENTRICULOG IMG S&I CATH PLMT & NJX CORONARY ART ANGIO IMG S&I CATH PLMT & NJX CORONARY ART/GRFT ANGIO IMG S&I CATH PLMT R HRT & ARTS W/NJX & ANGIO IMG S&I CATH PLMT R HRT/ARTS/GRFTS W/NJX&ANGIO IMG S&I CATH PLMT L HRT & ARTS W/NJX & ANGIO IMG S&I CATH PLMT L HRT/ARTS/GRFTS WNJX & ANGIO IMG S&I R & L HRT CATH WINJX HRT ART& L VENTR IMG S&I R&L HRT CATH W/INJEC HRT ART/GRFT&L VENT IMG S&I LEFT HEART CATH BY TRANSEPTAL PUNCTURE MEDICATION ADMIN & HEMODYNAMIC MEASURMENT PHYSIOLOGIC EXERCISE STUDY & HEMODYNAMIC MEASURE INSERTION AND PLACEMENT, FLOW DIRECTED CATHETER, M ENDOMYOCARDIAL BX RIGHT HEART CATHETERIZATION, CONGENITAL CARDIAC AN COMBINED RIGHT/RETROGRADE LEFT HEART CATHETERIZATI COMBINED RIGHT/TRANSSEPTAL LEFT HEART CATHETERIZAT COMBINED RIGHT/TRANSSEPTAL LEFT HEART CATHETERIZAT INDIC DIL STD ARTL&/OR VEN CATHJ W/OUTP MEAS INDIC DIL STD ARTL&/OR VEN CATHJ SBSQ OUTP MEAS NJX SEL HRT ART CONGENITAL HRT CATH W/S&I NJX SEL HRT ART/GRFT CONGENITAL HRT CATH W/S&I NJX SEL L VENT/ATRIAL ANGIO HRT CATH W/S&I NJX SEL R VENT/ATRIAL ANGIO HRT CATH W/S&I NJX SUPRAVALV AORTOG HRT CATH W/S&I NJX PULMONARY ANGIO HRT CATH W/S&I INTRAVASCULAR DOPPLER VELOCITY AND/OR PRESSURE COR INTRAVASCULAR DOPPLER VELOCITY AND/OR PRESSURE COR PERC TRANSCATHETER CLOSURE, CONGENITAL INTERATRIAL PERC TRANSCATHETER CLOSURE, CONGENITAL VENTRICULAR PERCUTAN TRANSCATH CLOSURE PAT DUCT ARTERIOSUS PERCUTANEOUS TRANSCATHETER SEPTAL REDUCTION THER BUNDLE OF HIS RECORDING INTRA-ATRIAL RECORDING RIGHT VENTRICULAR RECORDING INTRAVENTRICULAR AND/OR INTRA-ATRIAL MAPPING, TACH INTRA-ATRIAL PACING INTRAVENTRICULAR PACING INTRACARDIAC ELECTROPHYSIOLOGIC 3-DIMENSIONAL MAPP ESOPHAGEAL RECORDING, ATRIAL ELECTROGRAM W/WO VENT ESOPHAGEAL RECORDING, ATRIAL ELECTROGRAM, W/WO VEN 1/1/2009 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2015 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y OUTPT ASC 13X AND 83X & POS 22 OR 24 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 All POS except 21, 23 & 51 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 93618 93619 93620 93621 93622 93623 93624 93631 93640 93641 93642 93644 93650 93653 93654 93655 93656 93657 93660 93662 93668 93701 93702 93724 93740 93745 93750 93770 93784 93786 93788 93790 93797 93798 93799 93880 93882 93886 93888 93890 93892 93893 93895 93922 93923 93924 93925 93926 93930 93931 93965 93970 93971 93975 93976 93978 93979 93980 93981 93982 93990 93998 94002 94003 94004 94005 94010 94011 94012 94013 94014 94015 94016 94060 94070 94150 94200 94250 94375 94400 94450 94452 94453 94610 94620 94621 94640 94642 94644 94645 94660 INDUCTION, ARRHYTHMIA, ELECTRICAL PACING ELECTROPHYS EVAL, W/RIGHT ATRIAL/VENTRICULAR PACE/ ELECTROPHYS EVAL, INSERT CATH, W/ARRHYTHMIA INDUCT ELECTROPHYS EVAL, INSERT CATH, W/ARRHYTHMIA INDUCT ELECTROPHYS EVAL, INSERT CATH, W/ARRYTHMIA INDUCTI PROGRAMMED STIMULATION AND PACING AFTER IV DRUG ELECTROPHYS, FOLLOW-UP STUDY W/PACING AND RECORDIN ELECTROPHYS, INTRA-OPERATIVE EPICARDIAL/ENDOCARDIA ELECTROPHYS EVAL, SINGLE/DUAL PACING CARDIO/DEFIB ELECTROPHYS EVAL, SINGLE/DUAL PACING CARDIO/DEFIB EPHYS EVAL PACG CVDFB PRGRMG/REPRGRMG PARAMETERS EPHYS EVAL SUBQ IMPLANTABLE DEFIBRILLATOR INTRACARDIAC CATHETER ABLATION, ATRIOVENTRICULAR N EPHYS EVAL W/ ABLATION SUPRAVENT ARRHYTHMIA EPHYS EVAL W/ABLATION VENTRICULAR TACHYCARDIA ICAR CATHETER ABLATION ARRHYTHMIA ADD ON EPHYS EVL TRNSPTL TX ATRIAL FIB ISOLAT PULM VEIN ABLATE L/R ATRIAL FIBRIL W/ ISOLATED PULM VEIN CARDIOVASCULAR FUNCTION EVAL W/TILT TABLE/CONTINUO ECHOCARDIOGRAPHY, INTRACARDIAC, DURING DX/THERAPEU PERIPHERAL ARTERIAL DISEASE VASCULAR REHABILITATIO BIOIMPEDANCE CV ANALYSIS BIS EXTRACELLULAR FLUID ALYS LYMPHEDEMA ASSMNT ELECTRONIC ANALYSIS, PACEMAKER, ANTITACHYCARDIA TEMPERATURE GRADIENT STUDIES 1ST SET-UP & PRGRMG PHYS/QHP OF WEARABLE CVDFB INTERROGATION VAD IN PRSON W/PHYS/QHP ANALYSIS DETERMINATION, VENOUS PRESSURE AMBULATORY BP, OVER 24 HR, MONITORING; W/RECORDING AMBULATORY BP, OVER 24 HR, MONITORING; RECORDING O AMBULATORY BP, OVER 24 HR, MONITORING; SCAN ANALYS AMBL BLD PRESS TAPE&/DISK 24/> HR REVIEW OUTPATIENT CARDIAC REHAB W/O CONT ECG MONITOR OUTPATIENT CARDIAC REHAB W/CONT ECG MONITORING UNLISTED CARDIOVASCULAR SERVICE/PROC DUPLEX SCAN, EXTRACRANIAL ARTERIES; COMPLETE BILAT DUPLEX SCAN, EXTRACRANIAL ARTERIES; UNILAT/LIMITED TRANSCRANIAL DOPPLER STUDY, INTRACRANIAL ARTERIES; TRANSCRANIAL DOPPLER STUDY, INTRACRANIAL ARTERIES; TRANSCRANIAL DOPPLER STUDY, VASOREACTIVITY STUDY TRANSCRANIAL DOPPLER STUDY, EMBOLI DETECTION WITHO TRANSCRANIAL DOPPLER STUDY, EMBOLI DETECTION WITH CAROTID INTIMA MEDIA & CAROTID ATHEROMA EVAL BI NON-INVAS PHYSIOLOGIC STD EXTREMITY ART 2 LEVEL NON-INVASIVE PHYSIOLOGIC STUDY EXTREMITY 3 LEVLS N-INVAS PHYSIOLOGIC STD LXTR ART COMPL BI DUPLEX SCAN, LOWER EXTREMITY ARTERIES/ARTERIAL BYP DUPLEX SCAN, LOWER EXTREMITY ARTERIES/ARTERIAL BYP DUPLEX SCAN, UPPER EXTREMITY ARTERIES/ARTERIAL BYP DUPLEX SCAN, UPPER EXTREMITY ARTERIES/ARTERIAL BYP NON-INVASIVE VEIN STUDY, EXTREMITY, COMPLETE BILAT DUPLEX SCAN, VEINS, EXTREMITY; COMPLETE BILAT STUD DUPLEX SCAN, VEINS, EXTREMITY; UNILAT/LIMITED STUD DUPLEX SCAN, ARTERIAL INFLOW, VENOUS OUTFLOW, ABDO DUPLEX SCAN, ARTERIAL INFLOW, VENOUS OUTFLOW, ABDO DUPLEX SCAN, AORTA, INFERIOR VENA CAVA, ILIAC VASC DUPLEX SCAN, AORTA, INFERIOR VENA CAVA, ILIAC VASC DUPLEX SCAN, ARTERIAL INFLOW AND VENOUS OUTFLOW, P DUPLEX SCAN, ARTERIAL INFLOW AND VENOUS OUTFLOW, P NONINV PHYS STDY IMPL WIRELSS PRESS SNSR ANEURYSM DUPLEX SCAN, HEMODIALYSIS ACCESS UNLISTED NONINVASIVE VASCULAR DIAGNOSTIC STUDY Vent mgmt inpat, init day Vent mgmt inpat, subq day Vent mgmt nf per day Home vent mgmt supervision SPIROMETRY W/GRAPHIC RECORD/VITAL CAPACITY/FLOW RA MEAS SPIROMTRC FORCD EXPIRATORY FLO INFANT-2 YR MEAS SPIRO FORCD EXP FLO PRE&POST BRONCH INF-2Y MEAS LUNG VOLUMES INFANT OR CHILD THRU 2 YRS PT-INITIATE SPIROMETRIC RECORDING PHYS/QHP R&I SPIROMETRIC RECORDING, PATIENT INITIATED, 30 DAY P PATIENT-INITIATED SPIROMETRIC PHYS/QHP R&I ONLY SPIROMETRY, EVAL BRONCHOSPASM, BEFORE/AFTER BRONCH SPIROMETRY, EVAL BRONCHOSPASM, PROLONGED POSTEXPOS VITAL CAPACITY, TOTAL (SEP PROC) MAXIMUM BREATHING CAPACITY, MAXIMAL VOLUNTARY VENT EXPIRED GAS COLLECTION, QUANTITATIVE, SINGLE PROC RESPIRATORY FLOW VOLUME LOOP BREATHING RESPONSE TO CO2 (CO2 RESPONSE CURVE) BREATHING RESPONSE TO HYPOXIA (HYPOXIA RESPONSE CU HIGH ALTITUDE SIMULATJ TEST W/PHYS INTERP&REPORT HIGH ALTITUDE SIMULATJ W/PHYS I&R W/O2 TITRATION INTRAPULMONARY SURFACTANT ADMINISTJ PHYS/QHP PULMONARY STRESS TESTING; SIMPLE PULMONARY STRESS TESTING; COMPLEX PRESSURIZED/NONPRESSURIZED INHALATION RX, AIRWAY O AEROSOL INHALATION, PENTAMIDINE, PNEUMOCYSTIS CARI Cbt, 1st hour Cbt, each addl hour CONTINUOUS POSITIVE AIRWAY PRESSURE VENTILATION (C 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2015 1/1/2004 1/1/2004 1/1/2005 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2005 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2012 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2010 1/1/2010 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 94662 94664 94667 94668 94669 94680 94681 94690 94726 94727 94728 94729 94750 94760 94761 94762 94770 94772 94774 94775 94776 94777 94780 94781 94799 95004 95012 95017 95018 95024 95027 95028 95044 95052 95056 95060 95065 95070 95071 95076 95079 95115 95117 95120 95125 95130 95131 95132 95133 95134 95144 95145 95146 95147 95148 95149 95165 95170 95180 95199 95250 95251 95782 95783 95800 95801 95803 95805 95806 95807 95808 95810 95811 95812 95813 95816 95819 95822 95824 95827 95829 95830 95831 95832 95833 95834 95851 95852 95857 95860 95861 CONTINUOUS NEGATIVE PRESSURE VENTILATION (CNP), IN DEMONSTRATE AND/OR EVAL, PT USE, AEROSOL GENERATOR CHEST WALL MANIPULATION, FACILITATE LUNG FUNCTION; CHEST WALL MANIPULATION, FACILITATE LUNG FUNCTION; MECHANICAL CHEST WALL OSCILLATION LUNG FUNCTION OXYGEN UPTAKE, EXPIRED GAS ANALYSIS; REST AND EXER OXYGEN UPTAKE, EXPIRED GAS ANALYSIS; W/CO2 OUTPUT, OXYGEN UPTAKE, EXPIRED GAS ANALYSIS; REST, INDIREC PLETHYSMOGRAPHY LUNG VOLUMES W/WO AIRWAY RESIST GAS DILUT/WASHOUT LUNG VOL W/WO DISTRIB VENT&VOL AIRWAY RESISTANCE BY IMPULSE OSCILLOMETRY C02/MEMBANE DIFFUSE CAPACITY PULMONARY COMPLIANCE STUDY NONINVASIVE EAR/PULSE OXIMETRY, OXYGEN SATURATION; NONINVASIVE EAR/PULSE OXIMETRY, OXYGEN SATURATION; NONINVASIVE EAR/PULSE OXIMETRY, OXYGEN SATURATION; CARBON DIOXIDE, EXPIRED GAS DETERMINATION, INFRARE CIRCADIAN RESPIRATORY PATTERN RECORDING, 12-24 HR PEDIATRIC APNEA MONITOR ATTACHMENT PHYS I&R Ped home apnea rec, hk-up Ped home apnea rec, downld PEDIATRIC APNEA MONITOR PHYS/QHP REVIEW CAR SEAT/BED TESTING W/INTERP & REPORT 60 MIN CAR SEAT/BED TESTNG W/INTERP & REPORT +30MIN UNLISTED PULMONARY SERVICE/PROC PERCUTANEOUS TESTS W/ALLERGENIC EXTRACTS Exhaled nitric oxide meas ALLG TSTG PERQ & IC VENOMS IMMED REACT W/ I&R ALLG TEST PERQ & IC DRUG/BIOL IMMED REACT W/ I&R INTRACUTANEOUS TESTS W/ALLERGENIC EXTRACTS INTRACUTANEOUS TESTS W/ALLERGENIC XTRCS AIRBORNE ALLERGY TESTS, INTRADERMAL, ALLERGENIC EXTRACTS, D PATCH/APPLICATION TEST(S) (SPECIFY NUMBER) PHOTO PATCH TEST(S) (SPECIFY NUMBER) PHOTO TESTS OPHTHALMIC MUCOUS MEMBRANE TESTS DIRECT NASAL MUCOUS MEMBRANE TEST INHALATION BRONCHIAL CHALLENGE TESTS; W/HISTAMINE/ INHALATION BRONCHIAL CHALLENGE TESTS; W/ANTIGENS/G INGESTION CHALLENGE TEST INITIAL 120 MINUTES INGESTION CHALLENGE TEST EACH ADDL 60 MINUTES PROFESSIONAL SVC, ALLERGEN IMMUNOTHERAPY NON-PROVI PROFESSIONAL SVC, ALLERGEN IMMUNOTHERAPY NON-PROVI PROF SVCS ALLG IMMNTX W/PRV ALLGIC XTRC 1 NJX PROF SVCS ALLG IMMNTX W/PRV ALLGIC XTRC 2/> NJX PROF SVCS ALLG IMMNTX W/PRV XTRC 1 STING INSECT PROF SVCS ALLG IMMNTX W/PRV XTRC 2 STING INSECT PROF SVCS ALLG IMMNTX W/PRV XTRC 3 STING INSECT PROF SVCS ALLG IMMNTX W/PRV XTRC 4 STING INSECT PROF SVCS ALLG IMMNTX W/PRV XTRC 5 STING INSECT PROFES SVC, SUPERVIS, PREPARA, PROVISION, ANTIGEN, PROFES SVC, SUPERVIS, PREPARA, PROVISION, ANTIGEN, PROFESSIONAL SVC, SUPERVISION, PROVISION, ANTIGENS PROFESSIONAL SVC, SUPERVISION, PROVISION, ANTIGENS PROFESSIONAL SVC, SUPERVISION, PROVISION, ANTIGENS PROFESSIONAL SVC, SUPERVISION, PROVISION, ANTIGENS PROFES SVC, SUPERVIS, PREPARA, PROVISION, ANTIGENS PROFES SVC, SUPERVIS, PREPARA, PROVISION, ANTIGENS RAPID DESENSITIZATION PROC, EACH HOUR UNLISTED ALLERGY/CLINICAL IMMUNOLOGIC SERVICE/PROC GLUCOSE MONITORING, 72 HRS, CONT REC AND STORAGE, AMBULATORY CONT GLUCOSE MONITORING ; UP TO 72 HOUR POLYSOM <6 YRS SLEEP STAGE 4/> ADDL PARAM ATTND POLYSOM <6 YRS SLEEP W/CPAP/BILVL VENT 4/> PARAM SLP STDY UNATND W/HRT RATE/O2 SAT/RESP/SLP TIME SLP STDY UNATND W/MIN HRT RATE/O2 SAT/RESP ANAL ACTIGRAPHY TESTING RECORDING ANALYSIS I&R MULTIPLE SLEEP LATENCY TEST, MULTIPLE TRAILS SLEEP STUDY, UNATTENDED SLEEP STUDY, ATTENDED POLYSOM ANY AGE SLEEP STAGE 1-3 ADDL PARAM ATTND POLYSOM 6/>YRS SLEEP 4/> ADDL PARAM ATTND POLYSOM 6/>YRS SLEEP W/CPAP 4/> ADDL PARAM ATTND ELECTROENCEPHALOGRAM (EEG) EXTENDED MONITORING; 41 ELECTROENCEPHALOGRAM (EEG) EXTENDED MONITORING; OV ELECTROENCEPHALOGRAM (EEG); W/AWAKE AND DROWSY REC ELECTROENCEPHALOGRAM (EEG); W/AWAKE AND ASLEEP REC ELECTROENCEPHALOGRAM (EEG); COMA/SLEEP RECORD ONLY ELECTROENCEPHALOGRAM (EEG); CEREBRAL DEATH EVALUAT ELECTROENCEPHALOGRAM (EEG); ALL NIGHT RECORD ELECTROCORTICOGRAM AT SURGERY (SEP PROC) INSERTION SPHENOIDAL ELECTRODES EEG PHYS/QHP MUSCLE TESTING, MANUAL (SEP PROC) W/REPORT; EXTREM MUSCLE TESTING, MANUAL (SEP PROC) W/REPORT; HAND, MUSCLE TESTING, MANUAL (SEP PROC) W/REPORT; TOTAL MUSCLE TESTING, MANUAL (SEP PROC) W/REPORT; TOTAL RANGE, MOTION MEASUREMENTS AND REPORT; EACH EXTREM RANGE, MOTION MEASUREMENTS AND REPORT; HAND, W/WO CHOLINESTERASE INHIBITOR CHALLENGE TEST EMG, NEEDLE; 1 EXTREMITY W/WO RELATED PARASPINAL A EMG, NEEDLE; 2 EXTREMITIES W/WO RELATED PARASPINAL 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2007 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2013 1/1/2013 1/1/2011 1/1/2011 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 95863 95864 95865 95866 95867 95868 95869 95870 95872 95873 95874 95875 95885 95886 95887 95905 95907 95908 95909 95910 95911 95912 95913 95921 95922 95923 95924 95925 95926 95927 95928 95929 95930 95933 95937 95938 95939 95940 95941 95943 95950 95951 95953 95954 95955 95956 95957 95958 95961 95962 95965 95966 95967 95970 95971 95972 95973 95974 95975 95978 95979 95980 95981 95982 95990 95991 95992 95999 96000 96001 96002 96003 96004 96020 96040 96101 96102 96103 96105 96110 96111 96116 96118 96119 96120 96125 96127 96150 96151 96152 96153 EMG, NEEDLE; 3 EXTREMITIES W/WO RELATED PARASPINAL EMG, NEEDLE; 4 EXTREMITIES W/WO RELATED PARASPINAL NEEDLE ELECTROMYOGRAPHY; LARYNX NEEDLE ELECTROMYOGRAPHY; HEMIDIAPHRAGM EMG, NEEDLE; CRANIAL NERVE SUPPLIED MUSCLE(S), UNI EMG, NEEDLE; CRANIAL NERVE SUPPLIED MUSCLES, BILAT EMG, NEEDLE; THORACIC PARASPINAL MUSCLES, EXCLUDIN EMG, NEEDLE; 1 EXTREMITY/NON-LIMB (UNILAT/BILAT), EMG, NEEDLE, 1 FIBER ELECTRODE W/QUANTITATIVE MEAS ELECTRICAL STIMULATION FOR GUIDANCE IN CONJUNCTION NEEDLE ELECTROMYOGRAPHY FOR GUIDANCE IN CONJUNCTIO ISCHEMIC LIMB EXERCISE W/SERIAL SPECIMEN(S) ACQUIS NEEDLE EMG EA EXTREMITY W/PARASPINL AREA LIMITED NEEDLE EMG EA EXTREMTY W/PARASPINL AREA COMPLETE NEEDLE EMG NONEXTREMTY MSCLES W/NERVE CONDUCTION MOTOR &/SENS NRV CNDJ PRECONF ELTRD ARRAY LIMB NERVE CONDUCTION STUDIES 1-2 STUDIES NERVE CONDUCTION STUDIES 3-4 STUDIES NERVE CONDUCTION STUDIES 5-6 STUDIES NERVE CONDUCTION STUDIES 7-8 STUDIES NERVE CONDUCTION STUDIES 9-10 STUDIES NERVE CONDUCTION STUDIES 11-12 STUDIES NERVE CONDUCTION STUDIES 13/> STUDIES TESTING, AUTONOMIC NERVOUS SYSTEM; CARDIOVAGAL INN TESTING, AUTONOMIC NERVOUS SYSTEM; SYMPATHETIC ADR TESTING, AUTONOMIC NERVOUS SYSTEM; SUDOMOTOR TSTG ANS FUNCJ PARASYMP&SYMP W/5 MIN PASIVE TILT SHORT-LATENCY SOMATOSENSORY EVOKED POTENTIAL STUDY SHORT-LATENCY SOMATOSENSORY EVOKED POTENTIAL STUDY SHORT-LATENCY SOMATOSENSORY EVOKED POTENTIAL STUDY CENTRAL MOTOR EVOKED POTENTIAL STUDY, UPPER LIMBS CENTRAL MOTOR EVOKED POTENTIAL STUDY, LOWER LIMBS VISUAL EVOKED POTENTIAL (VEP) TESTING CENTRAL NERV ORBICULARIS OCULI REFLEX, BY ELECTRODX TESTING NEUROMUSCULAR JUNCTION TEST, EACH NERVE, ANY ONE M SHORT-LATENCY SOMATOSENS EP STD UPR & LOW LIMBS CTR MOTR EP STD TRANSCRNL MOTR STIM UPR&LOW LIMB IONM 1 ON 1 IN OR W/ATTENDANCE EACH 15 MINUTES IONM REMOTE/NEARBY/>1 PATIENT IN OR PER HOUR PARASYMP & SYMP NRV FUNCJ HRT RATE VARIABILITY MONITORING, ID AND LATERALIZATION, CEREBRAL SEIZUR MONITORING, LOCALIZATION, CEREBRAL SEIZURE FOCUS, LOCALIZE CEREBRAL SEIZURE CPTR PORTABLE EEG RX/PHYSICAL EEG ACTIVAJ PHYS/QHP ATTENDANCE ELECTROENCEPHALOGRAM (EEG) DURING NONINTRACRANIAL MNTR SEIZURE CMPTR 16>CHAN EEG ATND EA 24 HR DIGITAL ANALYSIS, ELECTROENCEPHALOGRAM (EEG) WADA ACTIVATION TEST, HEMISPHERIC FUNCTION, W/EEG FUNCJAL CORT&SUBCORT MAPG PHYS/QHP ATTND INIT HR FUNCJAL CORT&SUBCORT MAPG PHYS/QHP ATTND ADDL HR MAGNETOENCEPHALOGRAPHY (MEG), RECORD AND ANALYSIS; MAGNETOENCEPHALOGRAPHY (MEG), RECORD AND ANALYSIS; MAGNETOENCEPHALOGRAPHY (MEG), RECORD AND ANALYSIS; ELECTRONIC ANALYSIS, IMPLANT NEUUROSTIMULATOR; SIM ELEC ALYS NSTIM PLS GEN SMPL SC/PERPH W/PRGRMG ELEC ALYS NSTIM PLS GEN CPLX SC/PERPH 1ST HR ELEC ALYS NSTIM PLS GEN CPLX SC/PERPH EA 30 MIN ELEC ALYS NSTIM PLS GEN CPLX CRNL NRV 1ST HR ELEC ALYS NSTIM PLS GEN CPLX CRNL NRV EA 30 MIN ELECTRONIC ANALYSIS OF IMPLANTED NEUROSTIMULATOR P ELECTRONIC ANALYSIS OF IMPLANTED NEUROSTIMULATOR P ELEC ALYS NSTIM PLS GEN GASTRIC INTRAOP W/PRGRMG ELEC ALYS NSTIM GEN GASTRIC SBSQ W/O REPRGRMG ELEC ALYS NSTIM PLS GEN GASTRIC SBSQ W/REPRGRMG REFILL&MAINTENANCE PUMP DRUG DLVR SPINAL/BRAIN RFL&MAIN IMPLT PMP/RSVR DLVR SPI/BRN PHY/QHP CANALITH REPOSITIONING PROCEDURE UNLISTED NEUROLOGICAL/NEUROMUSCULAR DX PROC COMPREHENSIVE COMPUTER-BASED MOTION ANALYSIS BY VI COMPREHENSIVE COMPUTER-BASED MOTION ANAL; W/DYNAMI DYNAMIC SURFACE ELECTROMYOGRAPHY, WALKING/FUNCTION DYNAMIC FINE WIRE ELECTROMYOGRAPHY, WALKING/FUNCTI PHYS/QHP R&I CPTR MTN ALYS WALK/FUNCJL ACTV REPR TEST SELECT & ADMN FUNCTL BRAIN MAP PHYS/QHP Genetic counseling, 30 min PSYCHOLOGICAL TESTING; PER HR FOR PSYCHOLOGIST OR PSYCHOLOGICAL TESTING; PER HR FOR TECHNICIAN TIME PSYCHOLOGICAL TESTING; ADMINISTRATED BY COMPUTER ASSESSMENT, APHASIA, INTERPRETATION AND REPORT, PE DEVELOPMENTAL SCREEN W/SCORING & DOC STD INSTRM DEVELOPMENTAL TESTING; EXTENDED, W/INTERPRETATION NEUROBEHAVIORAL STATUS EXAM; PER HOUR OF PHYS OR P NEUROPSYCHOLOGICAL TESTING; PER HOUR OF PHYS OR PS NEUROPSYCHOLOGICAL TESTING; PER HOUR OF TECHNICIAN NEUROPSYCHOLOGICAL TESTING; ADMINISTERED BY A COMP STANDARDIZED COGNITIVE PERFORMANCE TESTING BEHAV ASSMT W/SCORE & DOCD/STAND INSTRUMENT HEALTH AND BEHAVIOR ASSESSMENT, EA 15 MINUTES; INI HEALTH AND BEHAVIOR ASSESSMENT, EA 15 MINUTES; REHEALTH AND BEHAVIOR INTERVENTION, EA 15 MINUTES; I HEALTH AND BEHAVIOR INTERVENTION, EA 15 MINUTES; G 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2012 1/1/2012 1/1/2012 1/1/2010 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2008 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 96154 96155 96360 96361 96365 96366 96367 96368 96369 96370 96371 96372 96373 96374 96375 96376 96379 96401 96402 96405 96406 96409 96411 96413 96415 96416 96417 96420 96422 96423 96425 96440 96446 96450 96521 96522 96523 96542 96549 96567 96570 96571 96900 96902 96904 96910 96912 96913 96920 96921 96922 96931 96932 96933 96934 96935 96936 96999 97001 97002 97003 97004 97005 97006 97010 97012 97014 97016 97018 97022 97024 97026 97028 97032 97033 97034 97035 97036 97039 97110 97112 97113 97116 97124 97139 97140 97150 97530 97532 97533 97535 HEALTH AND BEHAVIOR INTERVENTION, EA 15 MINUTES; F HEALTH AND BEHAVIOR INTERVENTION, EA 15 MINUTES; F IV INFUSION HYDRATION INITIAL 31 MIN-1 HOUR IV INFUSION HYDRATION EACH ADDITIONAL HOUR IV INFUSION THERAPY/PROPHYLAXIS /DX 1ST >1 HOUR IV INFUSION THERAPY PROPHYLAXIS/DX EA HOUR IV INFUSION THER PROPH ADDL SEQUENTIAL TO 1 HR IV NFS THERAPY PROPHYLAXIS/DX CONCURRENT NFS SUBCUTANEOUS INFUSION INITIAL 1 HR W/PUMP SET-UP SUBCUTANEOUS INFUSION EACH ADDITIONAL HOUR SUBQ INFUSION ADDITIONAL PUMP INFUSION SITE THERAPEUTIC PROPHYLACTIC/DX INJECTION SUBQ/IM THERAPEUTIC PROPHYLACTIC/DX NJX INTRA-ARTERIAL THER PROPH/DX NJX IV PUSH SINGLE/1ST SBST/DRUG THER PROPH/DX NJX EA SEQL IV PUSH SBST/DRUG SUBCUTANEOUS INFUSION EACH ADDITIONAL IV PUSH UNLISTED THERAPEUTIC PROPH/DX IV/IA NJX/NFS CHEMOTHERAPY ADMINISTRATION, SUBQ/IM, NON-HORMONAL CHEMOTHERAPY ADMINISTRATION, SUBQ/IM, HORMONAL ANT CHEMOTHERAPY ADMINISTRATION, INTRALESIONAL; UP TO CHEMOTHERAPY ADMINISTRATION, INTRALESIONAL; GREATE CHEMOTHERAPY ADMINISTRATION, IV; PUSH TECHNIQUE; I CHEMOTHERAPY ADMINISTRATION, IV; PUSH TECHNIQUE; E CHEMOTHERAPY, IV; INFUSION, UP TO 1 HR; SINGLE/INI CHEMOTHERAPY, IV; INFUSION, EACH ADD'L HR; 1-8 HR CHEMOTHERAPY, IV; INFUSION, INITIATION OF PROLONGE CHEMOTHERAPY, IV; INFUSION, EACH SEQUENTIAL INFUSI CHEMOTHERAPY ADMINISTRATION, INTRA-ARTERIAL; PUSH CHEMOTHERAPY ADMINISTRATION, INTRA-ARTERIAL; INFUS CHEMOTHERAPY ADMINISTRATION, INTRA-ARTERIAL; INFUS CHEMOTHERAPY ADMINISTRATION, INTRA-ARTERIAL; INFUS CHEMOTHERAPY ADMINISTRATION INTO PLEURAL CAVITY, R CHEMOTX ADMN PRTL CAVITY PORT/CATH CHEMOTHERAPY ADMINISTRATION, CNS, REQUIRING, W/LUM REFILLING AND MAINTENANCE OF PORTABLE PUMP REFILLING AND MAINTENANCE OF PORTABLE PUMP, SYSTEM IRRIGATION OF IMPLANTED VENOUS ACCESS DEVICE FOR D CHEMOTHERAPY INJECTION, SUBARACHNOID/INTRAVENTRICU UNLISTED CHEMOTHERAPY PROC EXTERNAL PHOTODYNAMIC THERAPY, EACH PHOTOTHERAPY E ENDOSCOPIC PHOTODYNAMIC THERAPY; 1ST 30 MIN ENDOSCOPIC PHOTODYNAMIC THERAPY; EACH ADDL 15 MIN ACTINOTHERAPY (ULTRAVIOLET LIGHT) MICROSCOPIC EXAM, HAIR PLUCKED/CLIPPED, EXAMINER Whole body photography PHOTOCHEMOTHERAPY; TAR AND UVB/PETROLATUM AND UVB PHOTOCHEMOTHERAPY; PSORALENS AND ULTRAVIOLET A (PU PHOTOCHEMOTHERAPY, 4-8 HR, DIRECT SUPERVISION, PHY LASER TX, INFLAMMATORY SKIN DISEASE (PSORIASIS); T LASER TX, INFLAMMATORY SKIN DISEASE (PSORIASIS); 2 LASER TX, INFLAMMATORY SKIN DISEASE (PSORIASIS); G REFLECTANCE CONFOCAL MICROSCOPY (RCM) FOR CELLULAR AND SUB-CELLULAR IMAGING O REFLECTANCE CONFOCAL MICROSCOPY (RCM) FOR CELLULAR AND SUB-CELLULAR IMAGING O REFLECTANCE CONFOCAL MICROSCOPY (RCM) FOR CELLULAR AND SUB-CELLULAR IMAGING O REFLECTANCE CONFOCAL MICROSCOPY (RCM) FOR CELLULAR AND SUB-CELLULAR IMAGING O REFLECTANCE CONFOCAL MICROSCOPY (RCM) FOR CELLULAR AND SUB-CELLULAR IMAGING O REFLECTANCE CONFOCAL MICROSCOPY (RCM) FOR CELLULAR AND SUB-CELLULAR IMAGING O UNLISTED SPECIAL DERMATOLOGICAL SERVICE/PROC PHYSICAL THERAPY EVAL PHYSICAL THERAPY RE-EVAL OCCUPATIONAL THERAPY EVAL OCCUPATIONAL THERAPY RE-EVAL ATHLETIC TRAINING EVALUATION ATHLETIC TRAINING RE-EVALUATION APPLICATION, MODALITY TO 1 OR MORE AREAS; HOT/COLD APPLICATION, MODALITY TO 1OR MORE AREAS; TRACTION, APPLICATION, MODALITY TO 1OR MORE AREAS; ELECTRICA APPLICATION, MODALITY TO 1OR MORE AREAS; VASOPNEUM APPLICATION, MODALITY TO 1OR MORE AREAS; PARAFFIN APPLICATION, MODALITY TO 1 OR MORE AREAS; WHIRLPOO APPLICATION, MODALITY TO 1 OR MORE AREAS; DIATHERM APPLICATION, MODALITY TO 1 OR MORE AREAS; INFRARED APPLICATION, MODALITY TO 1 OR MORE AREAS; ULTRAVIO APPLICATION, MODALITY TO 1 OR MORE AREAS; ELECTRIC APPLICATION, MODALITY TO 1 OR MORE AREAS; IONTOPHO APPLICATION, MODALITY TO 1 OR MORE AREAS; CONTRAST APPLICATION, MODALITY TO 1 OR MORE AREAS; ULTRASOU APPLICATION, MODALITY TO 1 OR MORE AREAS; HUBBARD UNLISTED MODALITY (SPECIFY TYPE AND TIME IF CONSTA THERAPEUTIC PROC, 1 OR MORE AREAS, EACH 15 MIN; TH THERAPEUTIC PROC, 1 OR MORE AREAS, EACH 15 MIN; NE THERAPEUTIC PROC, 1 OR MORE AREAS, EACH 15 MIN; AQ THERAPEUTIC PROC, 1 OR MORE AREAS, EACH 15 MIN; GA THERAPEUTIC PROC, 1 OR MORE AREAS, EACH 15 MIN; MA UNLISTED THERAPEUTIC PROCEDURE (SPECIFY) MANUAL THERAPY TECHNIQUES, 1 OR MORE REGIONS, EACH THERAPEUTIC PROC(S), GROUP, (2 OR MORE INDIVIDUALS THERAPEUT ACTVITY DIRECT PT CONTACT EACH 15 MIN DEVELOPMENT OF COGNITIVE SKILLS EACH 15 MINUTES SENSORY INTEGRATIVE TECHNIQUES EACH 15 MINUTES SELF-CARE/HOME MGMT TRAINING EACH 15 MINUTES 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N Y N Y N N N Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y OUTPT ASC 13X AND 83X & POS 22 OR 24 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 97537 97542 97545 97546 97597 97598 97602 97605 97606 97607 97608 97610 97750 97755 97760 97761 97762 97799 97802 97803 97804 97810 97811 97813 97814 98925 98926 98927 98928 98929 98940 98941 98942 98943 98960 98961 98962 98966 98967 98968 98969 99000 99001 99002 99024 99026 99027 99050 99051 99053 99056 99058 99060 99070 99071 99075 99078 99080 99082 99090 99091 99100 99116 99135 99140 99143 99144 99145 99148 99149 99150 99170 99172 99173 99174 99175 99177 99183 99184 99188 99190 99191 99192 99195 99199 99201 99202 99203 99204 99205 99211 COMMUNITY/WORK REINTEGRATION TRAINJ EA 15 MIN WHEELCHAIR MANAGEMENT/PROPULSION TRAIN, EACH 15 MI WORK HARDENING/CONDITIONING; INITIAL 2 HOURS WORK HARDENING/CONDITIONING; ADDL HR DEBRIDEMENT OPEN WOUND 20 SQ CM< DEBRIDEMENT OPEN WOUND ADDL 20 SQ CM REMOVAL DEVITAL TISSUE, WOUND; NON-SELECTIVE DEBRI NEGATIVE PRESSURE WOUND THERAPY DME </= 50 SQ CM NEGATIVE PRESSURE WOUND THERAPY DME >50 SQ CM NEG PRESSURE WOUND THERAPY NON DME </= 50 SQ CM NEG PRESSURE WOUND THERAPY NON DME >50 SQ CM LOW FREQUENCY NON-THERMAL ULTRASOUND PER DAY PHYSICAL PERFORMANCE TEST, W/WRITTEN REPORT, EACH ASSTV TECHNOL ASSMT DIR CNTCT W/REPRT EA 15 MIN ORTHOTIC MGMT AND TRAINING; EACH 15 MINUTES PROSTHETIC TRAINING; UPPER AND/OR LOWER EXTREMITY; CHECKOUT FOR ORTHOTIC/PROSTHETIC USE. ESTABLISHED UNLISTED PHYSICAL MEDICINE/REHABILITATION SERVICE/ MEDICAL NUTRITION THERAPY; INITIAL ASSESSMENT AND MEDICAL NUTRITION THERAPY; RE-ASSESSMENT AND INTER MEDICAL NUTRITION THERAPY; GROUP (2 OR MORE IND),E ACUPUNCTURE, WITHOUT ELECTRICAL STIMULATION, INITI ACUPUNCTURE, WITHOUT ELECTRICAL STIMULATION, EACH ACUPUNCTURE, WITH ELECTRICAL STIMULATION, INITIAL ACUPUNCTURE, WITH ELECTRICAL STIMULATION, EACH ADD OSTEOPATHIC MANIPULATIVE TREATMENT (OMT); 1-2 BODY OSTEOPATHIC MANIPULATIVE TREATMENT (OMT); 3-4 BODY OSTEOPATHIC MANIPULATIVE TREATMENT (OMT); 5-6 BODY OSTEOPATHIC MANIPULATIVE TREATMENT (OMT); 7-8 BODY OSTEOPATHIC MANIPULATIVE TREATMENT (OMT); 9-10 BOD CHIROPRACTIC MANIPULATIVE TREATMENT (CMT); SPINAL, CHIROPRACTIC MANIPULATIVE TREATMENT (CMT); SPINAL, CHIROPRACTIC MANIPULATIVE TREATMENT (CMT); SPINAL, CHIROPRACTIC MANIPULATIVE TREATMENT (CMT); EXTRASP EDUCATION/TRAINING FOR PT SELF-MGMT ; EACH 30 MINU EDUCATION/TRAINING FOR PT SELF-MGMT ; EACH 30 MINU EDUCATION/TRAINING FOR PT SELF-MGMT ; EACH 30 MINU NONPHYSICIAN TELEPHONE ASSESSMENT 5-10 MIN NONPHYSICIAN TELEPHONE ASSESSMENT 11-20 MIN NONPHYSICIAN TELEPHONE ASSESSMENT 21-30 MIN NONPHYSICIAN ONLINE ASSESSMENT AND MANAGEMENT HANDLG&/OR CONVEY OF SPEC FOR TR OFFICE TO LAB HANDLG&/OR CONVEY OF SPEC FOR TR FROM PT TO LAB HANDLE/CONVEY/ANY OTH SVC DEVICE FIT PHYS/QHP POSTOPERATIVE FOLLOW-UP VISIT, INCLUDED SURGICAL P HOSPITAL MANDATED ON CALL SERVICE; IN-HOSPITAL, EA HOSPITAL MANDATED ON CALL SERVICE; OUT-OF-HOSPITAL SERVICES AFTER POSTED OFFICE HOURS, AND BASIC SER SERVICES PROVIDED IN THE OFFICE DURING REGULARLY S Service provided between 10:00 pm and 8:00 am at NON-OFFICE MEDICAL SERVICES, PATIENT REQUEST, NORM OFFICE SERVICES, EMERGENCY BASIS SERVICES PROVIDED ON AN EMERGENCY BASIS; IN ADDITI SUPPLIES&MATERIALS ABOVE/BEYOND PROV BY PHYS/QHP EDUCATIONAL SUPPLIES PRV BY THE PHYS AT COST MEDICAL TESTIMONY PHYS/QHP EDUCATION SVCS RENDERED PTS GRP SETTING SPECIAL REPORTS/INSURANCE FORMS UNUSUAL TRAVEL ANALYSIS, STORED COMPUTER CLINICAL/DATA COLLJ&INTERPJ PHYS/QHP PHYSIO COMPUTR DATA 30 MI ANESTHESIA, PATIENT, EXTREME AGE UNDER 1 YR, AND ANESTHESIA W/HYPOTHERMIA ANESTHESIA W/HYPOTENSION ANESTHESIA, EMERGENCY CONDITIONS MODERATE SEDATJ SAME PHYS/QHP <5 YRS INIT 30 MIN MODERATE SEDATJ SAME PHYS/QHP 5/>YRS INIT 30 MIN MODERATE SEDATJ SAME PHYS/QHP EACH ADDL 15 MIN MOD SEDATJ DIFF PHYS/QHP <5 YRS INIT 30 MIN MODERATE SEDATJ DIFF PHYS/QHP 5/>YRS INIT 30 MIN MODERATE SEDATJ DIFF PHYS/QHP EA ADDL 15 MIN ANOGENITAL XM MAGNIFY CHILD/SUSPECT TRAUMA W IMG VISUAL FUNCTION SCREENING, AUTOMATED, SEMI-AUTOMAT SCREENING, VISUAL ACUITY, QUANTITATIVE, BILAT INSTRUMENT BASED OCULAR SCREENING BILATERAL INDUCTION, VOMITING, POISON INSTRUMENT-BASED OCULAR SCREENING (EG, PHOTOSCREENING, AUTOMATED-REFRACTION) PHYS/QHP ATTN&SUPVJ HYPRBARIC OXYGEN TX /SESSION INITIAT SELECTIVE HEAD/BODY HYPOTHERMIA NEONATE APPLICATION TOPICAL FLUORIDE VARNISH BY PHS/QHP ASSEMBLY AND OPERATION, PUMP W/OXYGENATOR/HEAT EXC ASSEMBLY AND OPERATION, PUMP W/OXYGENATOR/HEAT EXC ASSEMBLY AND OPERATION, PUMP W/OXYGENATOR/HEAT EXC PHLEBOTOMY, THERAPEUTIC (SEP PROC) UNLISTED PROC, SPECIAL SERVICE/REPORT OFFICE OUTPATIENT NEW 10 MINUTES OFFICE OUTPATIENT NEW 20 MINUTES OFFICE OUTPATIENT NEW 30 MINUTES OFFICE OUTPATIENT NEW 45 MINUTES OFFICE OUTPATIENT NEW 60 MINUTES OFFICE OUTPATIENT VISIT 5 MINUTES 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2004 1/1/2005 1/1/2005 1/1/2015 1/1/2015 1/1/2014 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2005 1/1/2005 1/1/2005 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2016 1/1/2004 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N Y N N N N N N N N N N N N Y Y Y N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N Diagnosis validation Diagnosis validation Diagnosis validation CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT 99212 99213 99214 99215 99217 99218 99219 99220 99221 99222 99223 99224 99225 99226 99231 99232 99233 99234 99235 99236 99238 99239 99241 99242 99243 99244 99245 99251 99252 99253 99254 99255 99281 99282 99283 99284 99285 99288 99291 99292 99304 99305 99306 99307 99308 99309 99310 99315 99316 99318 99324 99325 99326 99327 99328 99334 99335 99336 99337 99339 99340 99341 99342 99343 99344 99345 99347 99348 99349 99350 99354 99355 99356 99357 99358 99359 99360 99363 99364 99366 99367 99368 99374 99375 99377 99378 99379 99380 99381 99382 99383 OFFICE OUTPATIENT VISIT 10 MINUTES OFFICE OUTPATIENT VISIT 15 MINUTES OFFICE OUTPATIENT VISIT 25 MINUTES OFFICE OUTPATIENT VISIT 40 MINUTES OBSERVATION CARE DISCHARGE MANAGEMENT INITIAL OBSERVATION CARE/DAY 30 MINUTES INITIAL OBSERVATION CARE/DAY 50 MINUTES INITIAL OBSERVATION CARE/DAY 70 MINUTES INITIAL HOSPITAL CARE/DAY 30 MINUTES INITIAL HOSPITAL CARE/DAY 50 MINUTES INITIAL HOSPITAL CARE/DAY 70 MINUTES SBSQ OBSERVATION CARE/DAY 15 MINUTES SBSQ OBSERVATION CARE/DAY 25 MINUTES SBSQ OBSERVATION CARE/DAY 35 MINUTES SBSQ HOSPITAL CARE/DAY 15 MINUTES SBSQ HOSPITAL CARE/DAY 25 MINUTES SBSQ HOSPITAL CARE/DAY 35 MINUTES OBSERVATION/INPATIENT HOSPITAL CARE 40 MINUTES OBSERVATION/INPATIENT HOSPITAL CARE 50 MINUTES OBSERVATION/INPATIENT HOSPITAL CARE 55 MINUTES HOSPITAL DISCHARGE DAY MANAGEMENT; UP TO 30 MIN HOSPITAL DISCHARGE DAY MANAGEMENT; OVER 30 MIN OFFICE CONSULTATION NEW/ESTAB PATIENT 15 MIN OFFICE CONSULTATION NEW/ESTAB PATIENT 30 MIN OFFICE CONSULTATION NEW/ESTAB PATIENT 40 MIN OFFICE CONSULTATION NEW/ESTAB PATIENT 60 MIN OFFICE CONSULTATION NEW/ESTAB PATIENT 80 MIN INITL INPATIENT CONSULT NEW/ESTAB PT 20 MIN INITL INPATIENT CONSULT NEW/ESTAB PT 40 MIN INITL INPATIENT CONSULT NEW/ESTAB PT 55 MIN INITL INPATIENT CONSULT NEW/ESTAB PT 80 MIN INITIAL INPATIENT CONSULT NEW/ESTAB PT 110 MIN EMERGENCY DEPARTMENT VISIT LIMITED/MINOR PROB EMERGENCY DEPARTMENT VISIT LOW/MODER SEVERITY EMERGENCY DEPARTMENT VISIT MODERATE SEVERITY EMERGENCY DEPARTMENT VISIT HIGH/URGENT SEVERITY EMERGENCY DEPT VISIT HIGH SEVERITY&THREAT FUNCJ PHYS/QHP DIRECTION EMERGENCY MEDICAL SYSTEMS CRITICAL CARE, EVALUATION AND MANAGEMENT CRITICAL CARE, EVALUATION AND MANAGEMENT, ADDL 30 INITIAL NURSING FACILITY CARE/DAY 25 MINUTES INITIAL NURSING FACILITY CARE/DAY 35 MINUTES INITIAL NURSING FACILITY CARE/DAY 45 MINUTES SBSQ NURSING FACILITY CARE/DAY E/M STABLE 10 MIN SBSQ NURSING FACIL CARE/DAY MINOR COMPLJ 15 MIN SBSQ NURSING FACIL CARE/DAY NEW PROBLEM 25 MIN SBSQ NURS FACIL CARE/DAY UNSTABL/NEW PROB 35 MIN NURSING FACILITY DISCHARGE DAY MANAGEMENT; 30 MIN NURSING FACILITY DISCHARGE DAY MANAGEMENT; MORE TH E/M ANNUAL NURSING FACILITY ASSESS STABLE 30 MIN DOMICIL/REST HOME NEW PT VISIT LOW SEVER 20 MIN DOMICIL/REST HOME NEW PT VISIT MOD SEVER 30 MIN DOMICIL/REST HOME NEW PT HI-MOD SEVER 45 MINUTES DOMICIL/REST HOME NEW PT VISIT HI SEVER 60 MIN DOM/R-HOME E/M NEW PT SIGNIF NEW PROB 75 MINUTES DOM/R-HOME E/M EST PT SELF-LMTD/MINOR 15 MINUTES DOM/R-HOME E/M EST PT LW MOD SEVERITY 25 MINUTES DOM/R-HOME E/M EST PT MOD HI SEVERITY 40 MINUTES DOM/R-HOME E/M EST PT SIGNIF NEW PROB 60 MINUTES IND PHYSICIAN SUPERVISION OF A PATIENT IN HOME, DO IND PHYSICIAN SUPERVISION OF A PATIENT IN HOME, DO HOME VISIT NEW PATIENT LOW SEVERITY 20 MINUTES HOME VISIT NEW PATIENT MOD SEVERITY 30 MINUTES HOME VST NEW PATIENT MOD-HI SEVERITY 45 MINUTES HOME VISIT NEW PATIENT HI SEVERITY 60 MINUTES HOME VISIT NEW PT UNSTABL/SIGNIF NEW PROB 75 MIN HOME VISIT EST PT SELF LIMITED/MINOR 15 MINUTES HOME VISIT EST PT LOW-MOD SEVERITY 25 MINUTES HOME VISIT EST PT MOD-HI SEVERITY 40 MINUTES HOME VST EST PT UNSTABLE/SIGNIF NEW PROB 60 MINS PROLNG SVC OFFICE O/P DIR CONTACT 1ST HR PROLONGED PHYSICIAN SERVICE, OFFICE/OP W/DIRECT CO PROLONGED PHYSICIAN SERVICE, INPT W/DIRECT CONTACT PROLONGED SVC I/P REQ UNIT/FLOOR TIME EA 30 MIN PROLNG E/M SVC BEFORE&/AFTER DIR PT CARE 1ST HR PROLNG E/M BEFORE&/AFTER DIR CARE EA 30 MIN PHYS STANDBY SVC PROLNG PHYS ATTN EA 30 MINUTES Anticoag mgmt, init Anticoag mgmt, subseq TEAM CONFERENCE FACE-TO-FACE NONPHYSICIAN TEAM CONFERENCE NON-FACE-TO-FACE PHYSICIAN TEAM CONFERENCE NON-FACE-TO-FACE NONPHYSICIAN SUPVJ PT HOME HEALTH AGENCY MO 15-29 MINUTES SUPERVISION PT HOME HEALTH AGENCY MONTH 30 MIN/> SUPERVISION HOSPICE PATIENT/MONTH 15-29 MIN SUPERVISION HOSPICE PATIENT/MONTH 30 MINUTES/> SUPERVISION NURS FACILITY PATIENT MO 15-29 MIN SUPERVISION NURS FACILITY PATIENT MONTH 30 MIN/> INITIAL COMPREHENSIVE PREVENTIVE MEDICINE E AND M INITIAL COMPREHENSIVE PREVENTIVE MEDICINE E AND M INITIAL COMPREHENSIVE PREVENTIVE MEDICINE E AND M 1/1/2004 1/1/2004 1/1/2003 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N N N N POS 22 or 23 POS 22 or 23 POS 22 or 23 CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CPT CUST DRG DRG DRG DRG DRG DRG DRG DRG 99384 99385 99386 99387 99391 99392 99393 99394 99395 99396 99397 99401 99402 99403 99404 99406 99407 99408 99409 99411 99412 99415 99416 99420 99429 99441 99442 99443 99444 99446 99447 99448 99449 99450 99455 99456 99460 99461 99462 99463 99464 99465 99466 99467 99468 99469 99471 99472 99475 99476 99477 99478 99479 99480 99485 99486 99487 99489 99490 99495 99496 99497 99498 99499 99500 99501 99502 99503 99504 99505 99506 99507 99509 99510 99511 99512 99600 99601 99602 99605 99606 99607 SS001 001 002 003 004 005 006 007 008 INITIAL COMPREHENSIVE PREVENTIVE MEDICINE E AND M INITIAL COMPREHENSIVE PREVENTIVE MEDICINE E AND M INITIAL COMPREHENSIVE PREVENTIVE MEDICINE E AND M INITIAL COMPREHENSIVE PREVENTIVE MEDICINE E AND M PERIODIC COMPREHENSIVE PREVENTIVE MEDICINE E AND M PERIODIC COMPREHENSIVE PREVENTIVE MEDICINE EANDM W PERIODIC COMPREHENSIVE PREVENTIVE MEDICINE EANDM W PERIODIC COMPREHENSIVE PREVENTIVE MEDICINE EANDM W PERIODIC COMPREHENSIVE PREVENTIVE MEDICINE EANDM W PERIODIC COMPREHENSIVE PREVENTIVE MEDICINE EANDM W PERIODIC COMPREHENSIVE PREVENTIVE MEDICINE EANDM W PREVENTIVE MEDICINE COUNSELING, INDIV; 15 MIN PREVENTIVE MEDICINE COUNSELING, INDIV; 30 MIN PREVENTIVE MEDICINE COUNSELING, INDIV; 45 MIN PREVENTIVE MEDICINE COUNSELING, INDIV; 60 MIN TOBACCO USE CESSATION INTERMEDIATE 3-10 MINUTES TOBACCO USE CESSATION INTENSIVE >10 MINUTES ALCOHOL/SUBSTANCE SCREEN & INTERVEN 15-30 MIN ALCOHOL/SUBSTANCE SCREEN & INTERVEN >30 MIN PREVENTIVE MEDICINE COUNSELING, GROUP; 30 MIN PREVENTIVE MEDICINE COUNSELING, GROUP; 60 MIN PROLONGED CLINICAL STAFF SERVICE (THE SERVICE BEYOND THE TYPICAL SERVICE TIME) DUR PROLONGED CLINICAL STAFF SERVICE (THE SERVICE BEYOND THE TYPICAL SERVICE TIME) DUR ADMINISTRATION AND INTERPRETATION HEALTH RISK ASSE UNLISTED PREVENTIVE MEDICINE SERVICE PHYS/QHP TELEPHONE EVALUATION 5-10 MIN PHYS/QHP TELEPHONE EVALUATION 11-20 MIN PHYS/QHP TELEPHONE EVALUATION 21-30 MIN PHYS/QHP ONLINE EVALUATION & MANAGEMENT SERVICE INTERPROF PHONE/INTERNET ASSESS/MANAGE 5-10 INTERPROF PHONE/INTERNET ASSESS/MANAGE 11-20 INTERPROF PHONE/INTERNET ASSESS/MANAGE 21-30 INTERPROF PHONE/INTERNET ASSESS/MANAGE31/> BASIC LIFE/DISABILITY EXAM WORK/MEDICAL DISABILITY EXAM, TREATING PHYSICIAN WORK/MEDICAL DISABILITY EXAM, NON-TREATING PHYSICI 1ST HOSP/BIRTHING CENTER CARE PER DAY NML NB 1ST CARE PR DAY NML NB XCPT HOSP/BIRTHING CENTER SUBQ HOSPITAL CARE PER DAY E/M NORMAL NEWBORN 1ST HOSP/BIRTHING CENTER NB ADMIT&DSCHG SM DATE ATTN AT DELIVERY 1ST STABILIZATION OF NEWBORN DELIVERY/BIRTHING ROOM RESUSCITATION CRITICAL CARE INTERFACILITY TRANSPORT 30-74 MIN CRITICAL CARE INTERFACILITY TRANSPORT EA 30 MIN 1ST INPATIENT CRITICAL CARE PR DAY AGE 28 DAYS/< SUBQ I/P CRITICAL CARE PR DAY AGE 28 DAYS/< INITIAL PED CRITICAL CARE 29 D THRU 24 MO SUBSEQUENT PED CRITICAL CARE 29 D THRU 24 MO INITIAL PED CRITICAL CARE 2 THRU 5 YEARS SUBSEQUENT PED CRITICAL CARE 2 THRU 5 YEARS INITIAL HOSP NEONATE 28 D/< NOT CRITICALLY ILL SUBSEQUENT INTENSIVE CARE INFANT < 1500 GRAMS SUBSEQUENT INTENSIVE CARE INFANT 1500-2500 GRAMS SUBSEQUENT INTENSIVE CARE INFANT 2501-5000 GRAMS SUPERVISION INTERFACILITY TRANSPORT INIT 30 MIN SUPERVISION INTERFACILITY TRANSPORT ADDL 30 MIN CMPLX CHRON CARE MGMT W/O PT VST 1ST HR PER MO CMPLX CHRON CARE MGMT EA ADDL 30 MIN PER MONTH CHRON CARE MANAGEMENT SRVC 20 MIN PER MONTH TRANSITIONAL CARE MANAGE SERVICE 14 DAY DISCHRGE TRANSITIONAL CARE MANAGE SERVICE 7 DAY DISCHARGE ADVANCE CARE PLANNING FIRST 30 MINS ADVANCE CARE PLANNING EA ADDL 30 MINS UNLISTED EVALUATION AND MANAGEMENT SERVICE HOME VISIT, PRENAT MONITOR ASSESS, FETAL HEART RAT HOME VISIT, POSTNATAL ASSESSMENT, FOLLOW-UP CARE HOME VISIT, NEWBORN CARE, ASSESSMENT HOME VISIT, RESPIRATORY THERAPY CARE (BRONCHODILAT HOME VISIT, MECHANICAL VENTILATION CARE HOME VISIT, STOMA CARE AND MAINTENANCE, COLOSTOMY, HOME VISIT, IM INJECTIONS HOME VISIT, CARE AND MAINTENANCE CATHETER(S) (THER HOME VISIT, ASSISTANCE W/ACTIVITIES DAILY LIVING A HOME VISIT INDIVIDUAL, FAMILY, MARRIAGE COUNSELING HOME VISIT, FECAL IMPACTION MANAGEMENT AND ENEMA A HOME VISIT, HEMODIALYSIS UNLISTED HOME VISIT SERVICE/PROCEDURE HOME INFUSION/SPECIALTY DRUG ADMINISTRATION, PER V HOME INFUSION/SPECIALTY DRUG ADMINISTRATION, PER V MEDICATION THERAPY 1ST 15 MIN NEW PATIENT MEDICATION THERAPY F2F 1ST 15 MIN ESTABLISHED PT MEDICATION THERAPY F2F EA ADDITIONAL 15 MIN SPECIAL SERVICES Heart transplant or implant of heart assist system w MCC Heart transplant or implant of heart assist system w/o MCC ECMO or trach w MV 96+ hrs or PDX exc face, mouth & neck w maj OR Trach w MV 96+ hrs or PDX exc face, mouth & neck w/o maj OR Liver transplant w MCC or intestinal transplant Liver transplant w/o MCC Lung transplant Simultaneous pancreas/kidney transplant 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2009 1/1/2008 1/1/2009 1/1/2009 1/1/2009 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2015 1/1/2013 1/1/2013 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2006 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y Y N Y Y Y N N N N Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG 009 010 011 012 013 014 015 016 017 018 019 020 021 022 023 024 025 026 027 028 029 030 031 032 033 034 035 036 037 038 039 040 041 042 043 044 045 046 047 048 049 050 051 052 053 054 055 056 057 058 059 060 061 062 063 064 065 066 067 068 069 070 071 072 073 074 075 076 077 078 079 080 081 082 083 084 085 086 087 088 089 090 091 092 093 094 095 096 097 098 099 SPINAL DISORDERS AND INJURIES Pancreas transplant Tracheostomy for face,mouth & neck diagnoses w MCC Tracheostomy for face,mouth & neck diagnoses w CC Tracheostomy for face,mouth & neck diagnoses w/o CC/MCC Allogeneic Bone Marrow Transplant Autologous Bone Marrow Transplant Autologous Bone Marrow Transplant W CC/MCC Autologous Bone Marrow Transplant W/O CC/MCC CRANIAL AND PERIPHERAL NERVE DISORDERS W CC CRANIAL AND PERIPHERAL NERVE DISORDERS W/O CC Intracranial vascular procedures w PDX hemorrhage w MCC Intracranial vascular procedures w PDX hemorrhage w CC Intracranial vascular procedures w PDX hemorrhage w/o CC/MCC Cranio w major dev impl/acute complex CNS PDX w MCC or chemo implant Cranio w major dev impl/acute complex CNS PDX w/o MCC Craniotomy & endovascular intracranial procedures w MCC Craniotomy & endovascular intracranial procedures w CC Craniotomy & endovascular intracranial procedures w/o CC/MCC Spinal procedures w MCC Spinal procedures w CC or spinal neurostimulators Spinal procedures w/o CC/MCC Ventricular shunt procedures w MCC Ventricular shunt procedures w CC Ventricular shunt procedures w/o CC/MCC Carotid artery stent procedure w MCC Carotid artery stent procedure w CC Carotid artery stent procedure w/o CC/MCC Extracranial procedures w MCC Extracranial procedures w CC Extracranial procedures w/o CC/MCC Periph/cranial nerve & other nerv syst proc w MCC Periph/cranial nerve & other nerv syst proc w CC or periph neurostim Periph/cranial nerve & other nerv syst proc w/o CC/MCC HYPHEMA ACUTE MAJOR EYE INFECTIONS Neurological eye disorders OTHER DISORDERS OF THE EYE AGE >17 W CC OTHER DISORDERS OF THE EYE AGE >17 W/O CC OTHER DISORDERS OF THE EYE AGE 0-17 MAJOR HEAD AND NECK PROCEDURES SIALOADENECTOMY SALIVARY GLAND PROCEDURES EXCEPT SIALOADENECTOMY Spinal disorders & injuries w CC/MCC Spinal disorders & injuries w/o CC/MCC Nervous system neoplasms w MCC Nervous system neoplasms w/o MCC Degenerative nervous system disorders w MCC Degenerative nervous system disorders w/o MCC Multiple sclerosis & cerebellar ataxia w MCC Multiple sclerosis & cerebellar ataxia w CC Multiple sclerosis & cerebellar ataxia w/o CC/MCC Acute ischemic stroke w use of thrombolytic agent w MCC Acute ischemic stroke w use of thrombolytic agent w CC Acute ischemic stroke w use of thrombolytic agent w/o CC/MCC Intracranial hemorrhage or cerebral infarction w MCC Intracranial hemorrhage or cerebral infarction w CC Intracranial hemorrhage or cerebral infarction w/o CC/MCC Nonspecific cva & precerebral occlusion w/o infarct w MCC Nonspecific cva & precerebral occlusion w/o infarct w/o MCC TRANSIENT ISCHEMIA Nonspecific cerebrovascular disorders w MCC Nonspecific cerebrovascular disorders w CC Nonspecific cerebrovascular disorders w/o CC/MCC Cranial & peripheral nerve disorders w MCC Cranial & peripheral nerve disorders w/o MCC Viral meningitis w CC/MCC Viral meningitis w/o CC/MCC Hypertensive encephalopathy w MCC Hypertensive encephalopathy w CC Hypertensive encephalopathy w/o CC/MCC Nontraumatic stupor & coma w MCC Nontraumatic stupor & coma w/o MCC Traumatic stupor & coma, coma >1 hr w MCC Traumatic stupor & coma, coma >1 hr w CC Traumatic stupor & coma, coma >1 hr w/o CC/MCC Traumatic stupor & coma, coma <1 hr w MCC Traumatic stupor & coma, coma <1 hr w CC Traumatic stupor & coma, coma <1 hr w/o CC/MCC Concussion w MCC Concussion w CC Concussion w/o CC/MCC Other disorders of nervous system w MCC Other disorders of nervous system w CC Other disorders of nervous system w/o CC/MCC Bacterial & tuberculous infections of nervous system w MCC Bacterial & tuberculous infections of nervous system w CC Bacterial & tuberculous infections of nervous system w/o CC/MCC Non-bacterial infect of nervous sys exc viral meningitis w MCC Non-bacterial infect of nervous sys exc viral meningitis w CC Non-bacterial infect of nervous sys exc viral meningitis w/o CC/MCC 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 10/1/2011 10/1/2011 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG 100 101 102 103 104 105 106 107 108 109 110 111 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 Seizures w MCC Seizures w/o MCC Headaches w MCC Headaches w/o MCC CARDIAC VALVE AND OTH MAJOR CARDIOTHORACIC PROC W CARDIAC VALVE AND OTH MAJOR CARDIOTHORACIC PROC W/ CORONARY BYPASS W PTCA CORONARY BYPASS W CARDIAC CATH OTHER CARDIOTHORACIC PROCEDURES CORONARY BYPASS W/O PTCA OR CARDIAC CATH MAJOR CARDIOVASCULAR PROCEDURES W CC MAJOR CARDIOVASCULAR PROCEDURES W/O CC Orbital procedures w CC/MCC Orbital procedures w/o CC/MCC Extraocular procedures except orbit Intraocular procedures w CC/MCC Intraocular procedures w/o CC/MCC CARDIAC PACEMAKER DEVICE REPLACEMENT VEIN LIGATION AND STRIPPING OTHER CIRCULATORY SYSTEM O.R. PROCEDURES Acute major eye infections w CC/MCC Acute major eye infections w/o CC/MCC NEUROLOGICAL EYE DISORDERS Other disorders of the eye w MCC Other disorders of the eye w/o MCC ACUTE AND SUBACUTE ENDOCARDITIS HEART FAILURE AND SHOCK DEEP VEIN THROMBOPHLEBITIS Major head & neck procedures w CC/MCC or major device Major head & neck procedures w/o CC/MCC Cranial/facial procedures w CC/MCC Cranial/facial procedures w/o CC/MCC Other ear, nose, mouth & throat OR procedures w CC/MCC Other ear, nose, mouth & throat OR procedures w/o CC/MCC Sinus & mastoid procedures w CC/MCC Sinus & mastoid procedures w/o CC/MCC Mouth procedures w CC/MCC Mouth procedures w/o CC/MCC Salivary gland procedures ANGINA PECTORIS SYNCOPE AND COLLAPSE W CC SYNCOPE AND COLLAPSE W/O CC CHEST PAIN Other circulatory system diagnoses w CC OTHER CIRCULATORY SYSTEM DIAGNOSES W/O CC Ear, nose, mouth & throat malignancy w MCC Ear, nose, mouth & throat malignancy w CC Ear, nose, mouth & throat malignancy w/o CC/MCC Dysequilibrium Epistaxis w MCC Epistaxis w/o MCC Otitis media & URI w MCC Otitis media & URI w/o MCC Other ear, nose, mouth & throat diagnoses w MCC Other ear, nose, mouth & throat diagnoses w CC Other ear, nose, mouth & throat diagnoses w/o CC/MCC Dental & Oral Diseases w MCC Dental & Oral Diseases w CC Dental & Oral Diseases w/o CC/MCC HERNIA PROCEDURES EXCEPT INGUINAL AND FEMORAL AGE INGUINAL AND FEMORAL HERNIA PROCEDURES AGE >17 W C INGUINAL AND FEMORAL HERNIA PROCEDURES AGE >17 W/O Major chest procedures w MCC Major chest procedures w CC Major chest procedures w/o CC/MCC Other resp system OR procedures w MCC Other resp system OR procedures w CC Other resp system OR procedures w/o CC/MCC MOUTH PROCEDURES W/O CC OTHER DIGESTIVE SYSTEM O.R. PROCEDURES W CC OTHER DIGESTIVE SYSTEM O.R. PROCEDURES W/O CC DIGESTIVE MALIGNANCY W CC DIGESTIVE MALIGNANCY W/O CC G.I. HEMORRHAGE W CC Pulmonary embolism w MCC Pulmonary embolism w/o MCC Respiratory infections & inflammations w MCC Respiratory infections & inflammations w CC Respiratory infections & inflammations w/o CC/MCC Respiratory neoplasms w MCC Respiratory neoplasms w CC Respiratory neoplasms w/o CC/MCC Major chest trauma w MCC Major chest trauma w CC Major chest trauma w/o CC/MCC Pleural effusion w MCC Pleural effusion w CC Pleural effusion w/o CC/MCC Pulmonary edema & respiratory failure Chronic obstructive pulmonary disease w MCC Chronic obstructive pulmonary disease w CC 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 Chronic obstructive pulmonary disease w/o CC/MCC Simple pneumonia & pleurisy w MCC Simple pneumonia & pleurisy w CC Simple pneumonia & pleurisy w/o CC/MCC Interstitial lung disease w MCC Interstitial lung disease w CC Interstitial lung disease w/o CC/MCC Pneumothorax w MCC Pneumothorax w CC Pneumothorax w/o CC/MCC Bronchitis & asthma w CC/MCC Bronchitis & asthma w/o CC/MCC Respiratory signs & symptoms Other respiratory system diagnoses w MCC Other respiratory system diagnoses w/o MCC Respiratory system diagnosis w ventilator support 96+ hours Respiratory system diagnosis w ventilator support <96 hours MAJOR JOINT AND LIMB REATTACHMENT PROCEDURES OF LO HIP AND FEMUR PROCEDURES EXCEPT MAJOR JOINT AGE >1 HIP AND FEMUR PROCEDURES EXCEPT MAJOR JOINT AGE >1 HIP AND FEMUR PROCEDURES EXCEPT MAJOR JOINT AGE 0AMPUTATION FOR MUSCULOSKELETAL SYSTEM AND CONN TIS OTHER HEART ASSIST SYSTEM IMPLANT Cardiac valve & oth maj cardiothoracic proc w card cath w MCC Cardiac valve & oth maj cardiothoracic proc w card cath w CC Cardiac valve & oth maj cardiothoracic proc w card cath w/o CC/MCC Cardiac valve & oth maj cardiothoracic proc w/o card cath w MCC Cardiac valve & oth maj cardiothoracic proc w/o card cath w CC Cardiac valve & oth maj cardiothoracic proc w/o card cath w/o CC/MCC Cardiac defib implant w cardiac cath w AMI/HF/shock w MCC Cardiac defib implant w cardiac cath w AMI/HF/shock w/o MCC Cardiac defib implant w cardiac cath w/o AMI/HF/shock w MCC Cardiac defib implant w cardiac cath w/o AMI/HF/shock w/o MCC Cardiac defibrillator implant w/o cardiac cath w MCC Cardiac defibrillator implant w/o cardiac cath w/o MCC Other cardiothoracic procedures w MCC Other cardiothoracic procedures w CC Other cardiothoracic procedures w/o CC/MCC Coronary bypass w PTCA w MCC Coronary bypass w PTCA w/o MCC Coronary bypass w cardiac cath w MCC Coronary bypass w cardiac cath w/o MCC Coronary bypass w/o cardiac cath w MCC Coronary bypass w/o cardiac cath w/o MCC Major cardiovasc procedures w MCC or thoracic aortic anuerysm repair Major cardiovasc procedures w/o MCC Amputation for circ sys disorders exc upper limb & toe w MCC Amputation for circ sys disorders exc upper limb & toe w CC Amputation for circ sys disorders exc upper limb & toe w/o CC/MCC Permanent cardiac pacemaker implant w MCC Permanent cardiac pacemaker implant w CC Permanent cardiac pacemaker implant w/o CC/MCC AICD generator procedures Perc cardiovasc proc w drug-eluting stent w MCC or 4+ vessels/stents Perc cardiovasc proc w drug-eluting stent w/o MCC Perc cardiovasc proc w non-drug-eluting stent w MCC or 4+ ves/stents Perc cardiovasc proc w non-drug-eluting stent w/o MCC Perc cardiovasc proc w/o coronary artery stent w MCC Perc cardiovasc proc w/o coronary artery stent w/o MCC Other vascular procedures w MCC Other vascular procedures w CC Other vascular procedures w/o CC/MCC Upper limb & toe amputation for circ system disorders w MCC Upper limb & toe amputation for circ system disorders w CC Upper limb & toe amputation for circ system disorders w/o CC/MCC Cardiac pacemaker device replacement w MCC Cardiac pacemaker device replacement w/o MCC Cardiac pacemaker revision except device replacement w MCC Cardiac pacemaker revision except device replacement w CC Cardiac pacemaker revision except device replacement w/o CC/MCC Vein ligation & stripping Other circulatory system OR procedures AICD lead procedures SKIN GRAFT AND/OR DEBRID EXCEPT FOR SKIN ULCER OR PERIANAL AND PILONIDAL PROCEDURES SKIN, SUBCUTANEOUS TISSUE AND BREAST PLASTIC PROCE OTHER SKIN, SUBCUT TISS AND BREAST PROC W CC OTHER SKIN, SUBCUT TISS AND BREAST PROC W/O CC SKIN ULCERS MAJOR SKIN DISORDERS W CC MAJOR SKIN DISORDERS W/O CC MALIGNANT BREAST DISORDERS W CC MALIGNANT BREAST DISORDERS W/O CC NON-MALIGANT BREAST DISORDERS CELLULITIS AGE >17 W CC CELLULITIS AGE >17 W/O CC CELLULITIS AGE 0-17 Acute myocardial infarction, discharged alive w MCC Acute myocardial infarction, discharged alive w CC Acute myocardia infarction, discharged alive w/o CC/MCC Acute myocardial infarction, expired w MCC 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326 327 328 329 330 331 332 333 334 335 336 337 338 339 340 341 342 343 344 345 346 347 348 349 350 351 352 353 354 355 356 357 358 359 360 361 362 363 364 365 366 367 368 369 370 371 372 373 374 Acute myocardial infarction, expired w CC Acute myocardial infarction, expired w/o CC/MCC Circulatory disorders except AMI, w card cath w MCC Circulatory disorders except AMI, w card cath w/o MCC Acute & subacute endocarditis w MCC Acute & subacute endocarditis w CC Acute & subacute endocarditis w/o CC/MCC Heart failure & shock w MCC Heart failure & shock w CC Heart failure & shock w/o CC/MCC Deep vein thrombophlebitis w CC/MCC Deep vein thrombophlebitis w/o CC/MCC Cardiac arrest, unexplained w MCC Cardiac arrest, unexplained w CC Cardiac arrest, unexplained w/o CC/MCC Peripheral vascular disorders w MCC Peripheral vascular disorders w CC Peripheral vascular disorders w/o CC/MCC Atherosclerosis w MCC Atherosclerosis w/o MCC Hypertension w MCC Hypertension w/o MCC Cardiac congenital & valvular disorders w MCC Cardiac congenital & valvular disorders w/o MCC Cardiac arrhythmia & conduction disorders w MCC Cardiac arrhythmia & conduction disorders w CC Cardiac arrhythmia & conduction disorders w/o CC/MCC Angina pectoris Syncope & collapse Chest pain Other circulatory system diagnoses w MCC OTHER CIRCULATORY SYSTEM DIAGNOSES W CC Other circulatory system diagnoses w/o CC/MCC ADMIT FOR RENAL DIALYSIS KIDNEY AND URINARY TRACT NEOPLASMS W CC KIDNEY AND URINARY TRACT NEOPLASMS W/O CC KIDNEY AND URINARY TRACT INFECTIONS AGE >17 W CC KIDNEY AND URINARY TRACT INFECTIONS AGE >17 W/O CC KIDNEY AND URINARY TRACT INFECTIONS AGE 0-17 URINARY STONES W CC, AND/OR ESW LITHOTRIPSY URINARY STONES W/O CC KIDNEY AND URINARY TRACT SIGNS AND SYMPTOMS AGE >1 Stomach, esophageal & duodenal proc w MCC Stomach, esophageal & duodenal proc w CC Stomach, esophageal & duodenal proc w/o CC/MCC Major small & large bowel procedures w MCC Major small & large bowel procedures w CC Major small & large bowel procedures w/o CC/MCC Rectal resection w MCC Rectal resection w CC Rectal resection w/o CC/MCC Peritoneal adhesiolysis w MCC Peritoneal adhesiolysis w CC Peritoneal adhesiolysis w/o CC/MCC Appendectomy w complicated principal diag w MCC Appendectomy w complicated principal diag w CC Appendectomy w complicated principal diag w/o CC/MCC Appendectomy w/o complicated principal diag w MCC Appendectomy w/o complicated principal diag w CC Appendectomy w/o complicated principal diag w/o CC/MCC Minor small & large bowel procedures w MCC Minor small & large bowel procedures w CC Minor small & large bowel procedures w/o CC/MCC Anal & stomal procedures w MCC Anal & stomal procedures w CC Anal & stomal procedures w/o CC/MCC Inguinal & femoral hernia procedures w MCC Inguinal & femoral hernia procedures w CC Inguinal & femoral hernia procedures w/o CC/MCC Hernia procedures except inguinal & femoral w MCC Hernia procedures except inguinal & femoral w CC Hernia procedures except inguinal & femoral w/o CC/MCC Other digestive system OR procedures w MCC Other digestive system OR procedures w CC Other digestive system OR procedures w/o CC/MCC UTERINE AND ADNEXA PROC FOR NON-MALIGNANCY W/O CC VAGINA, CERVIX AND VULVA PROCEDURES LAPAROSCOPY AND INCISIONAL TUBAL INTERRUPTION ENDOSCOPIC TUBAL INTERRUPTION D AND C, CONIZATION AND RADIO-IMPLANT, FOR MALIGNA D AND C, CONIZATION EXCEPT FOR MALIGNANCY OTHER FEMALE REPRODUCTIVE SYSTEM O.R. PROCEDURES Malignancy, female reproductive system w CC MALIGNANCY, FEMALE REPRODUCTIVE SYSTEM W/O CC Major esophageal disorders w MCC Major esophageal disorders w CC Major esophageal disorders w/o CC/MCC Major gastrointestinal disorders & peritoneal infections w MCC Major gastrointestinal disorders & peritoneal infections w CC Major gastrointestinal disorders & peritoneal infections w/o CC/MCC Digestive malignancy w MCC 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG 375 376 377 378 379 380 381 382 383 384 385 386 387 388 389 390 391 392 393 394 395 396 397 398 399 401 402 403 404 405 406 407 408 409 410 411 412 413 414 415 416 417 418 419 420 421 422 423 424 425 426 427 428 429 430 431 432 433 434 435 436 437 438 439 440 441 442 443 444 445 446 447 448 449 450 451 452 453 454 455 456 457 458 459 460 461 462 463 464 465 466 Digestive malignancy w CC Digestive malignancy w/o CC/MCC G.I. hemorrhage w MCC G.I. hemorrhage w CC G.I. hemorrhage w/o CC/MCC Complicated peptic ulcer w MCC Complicated peptic ulcer w CC Complicated peptic ulcer w/o CC/MCC Uncomplicated peptic ulcer w MCC Uncomplicated peptic ulcer w/o MCC Inflammatory bowel disease w MCC Inflammatory bowel disease w CC Inflammatory bowel disease w/o CC/MCC G.I. obstruction w MCC G.I. obstruction w CC G.I. obstruction w/o CC/MCC Esophagitis, gastroent & misc digest disorders w MCC Esophagitis, gastroent & misc digest disorders w/o MCC Other digestive system diagnoses w MCC Other digestive system diagnoses w CC Other digestive system diagnoses w/o CC/MCC RED BLOOD CELL DISORDERS AGE 0-17 COAGULATION DISORDERS RETICULOENDOTHELIAL AND IMMUNITY DISORDERS W CC RETICULOENDOTHELIAL AND IMMUNITY DISORDERS W/O CC LYMPHOMA AND NON-ACUTE LEUKEMIA W OTHER O.R. PROC LYMPHOMA AND NON-ACUTE LEUKEMIA W OTHER O.R. PROC LYMPHOMA AND NON-ACUTE LEUKEMIA W CC LYMPHOMA AND NON-ACUTE LEUKEMIA W/O CC Pancreas, liver & shunt procedures w MCC Pancreas, liver & shunt procedures w CC Pancreas, liver & shunt procedures w/o CC/MCC Biliary tract proc except only cholecyst w or w/o c.d.e. w MCC Biliary tract proc except only cholecyst w or w/o c.d.e. w CC Biliary tract proc except only cholecyst w or w/o c.d.e. w/o CC/MCC Cholecystectomy w c.d.e. w MCC Cholecystectomy w c.d.e. w CC Cholecystectomy w c.d.e. w/o CC/MCC Cholecystectomy except by laparoscope w/o c.d.e. w MCC Cholecystectomy except by laparoscope w/o c.d.e. w CC Cholecystectomy except by laparoscope w/o c.d.e. w/o CC/MCC Laparoscopic cholecystectomy w/o c.d.e. w MCC Laparoscopic cholecystectomy w/o c.d.e. w CC Laparoscopic cholecystectomy w/o c.d.e. w/o CC/MCC Hepatobiliary diagnostic procedures w MCC Hepatobiliary diagnostic procedures w CC Hepatobiliary diagnostic procedures w/o CC/MCC Other hepatobiliary or pancreas OR procedures w MCC Other hepatobiliary or pancreas OR procedures w CC Other hepatobiliary or pancreas OR procedures w/o CC/MCC Depressive neuroses Neuroses except depressive DISORDERS OF PERSONALITY AND IMPULSE CONTROL ORGANIC DISTURBANCES AND MENTAL RETARDATION Psychoses CHILDHOOD MENTAL DISORDERS Cirrhosis & alcoholic hepatitis w MCC Cirrhosis & alcoholic hepatitis w CC Cirrhosis & alcoholic hepatitis w/o CC/MCC Malignancy of hepatobiliary system or pancreas w MCC Malignancy of hepatobiliary system or pancreas w CC Malignancy of hepatobiliary system or pancreas w/o CC/MCC Disorders of pancreas except malignancy w MCC Disorders of pancreas except malignancy w CC Disorders of pancreas except malignancy w/o CC/MCC Disorders of liver except malig,cirr,alc hepa w MCC Disorders of liver except malig,cirr,alc hepa w CC Disorders of liver except malig,cirr,alc hepa w/o CC/MCC Disorders of the biliary tract w MCC Disorders of the biliary tract w CC Disorders of the biliary tract w/o CC/MCC ALLERGIC REACTIONS AGE >17 ALLERGIC REACTIONS AGE 0-17 POISONING AND TOXIC EFFECTS OF DRUGS AGE >17 W CC POISONING AND TOXIC EFFECTS OF DRUGS AGE >17 W/O C POISONING AND TOXIC EFFECTS OF DRUGS AGE 0-17 COMPLICATIONS OF TREATMENT W CC Combined anterior/posterior spinal fusion w MCC Combined anterior/posterior spinal fusion w CC Combined anterior/posterior spinal fusion w/o CC/MCC Spinal fus exc cerv w spinal curv/malig/infec or 9+ fus w MCC Spinal fus exc cerv w spinal curv/malig/infec or 9+ fus w CC Spinal fus exc cerv w spinal curv/malig/infec or 9+ fus w/o CC/MCC Spinal fusion except cervical w MCC Spinal fusion except cervical w/o MCC Bilateral or multiple major joint procs of lower extremity w MCC Bilateral or multiple major joint procs of lower extremity w/o MCC Wnd debrid & skn grft exc hand, for musculo-conn tiss dis w MCC Wnd debrid & skn grft exc hand, for musculo-conn tiss dis w CC Wnd debrid & skn grft exc hand, for musculo-conn tiss dis w/o CC/MCC Revision of hip or knee replacement w MCC 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG 467 468 469 470 471 472 473 474 475 476 477 478 479 480 481 482 483 484 485 486 487 488 489 490 491 492 493 494 495 496 497 498 499 500 501 502 503 504 505 506 507 508 509 510 511 512 513 514 515 516 517 518 519 520 521 522 523 524 525 526 527 528 529 530 531 532 533 534 535 536 537 538 539 540 541 542 543 544 545 546 547 548 549 550 551 552 553 554 555 556 557 Revision of hip or knee replacement w CC Revision of hip or knee replacement w/o CC/MCC Major joint replacement or reattachment of lower extremity w MCC Major joint replacement or reattachment of lower extremity w/o MCC Cervical spinal fusion w MCC Cervical spinal fusion w CC Cervical spinal fusion w/o CC/MCC Amputation for musculoskeletal sys & conn tissue dis w MCC Amputation for musculoskeletal sys & conn tissue dis w CC Amputation for musculoskeletal sys & conn tissue dis w/o CC/MCC Biopsies of musculoskeletal system & connective tissue w MCC Biopsies of musculoskeletal system & connective tissue w CC Biopsies of musculoskeletal system & connective tissue w/o CC/MCC Hip & femur procedures except major joint w MCC Hip & femur procedures except major joint w CC Hip & femur procedures except major joint w/o CC/MCC Major joint & limb reattachment proc of upper extremity w CC/MCC Major joint & limb reattachment proc of upper extremity w/o CC/MCC Knee procedures w pdx of infection w MCC Knee procedures w pdx of infection w CC Knee procedures w pdx of infection w/o CC/MCC Knee procedures w/o pdx of infection w CC/MCC Knee procedures w/o pdx of infection w/o CC/MCC Back & neck proc exc spinal fusion w CC/MCC or disc device/neurostim Back & neck proc exc spinal fusion w/o CC/MCC Lower extrem & humer proc except hip,foot,femur w MCC Lower extrem & humer proc except hip,foot,femur w CC Lower extrem & humer proc except hip,foot,femur w/o CC/MCC Local excision & removal int fix devices exc hip & femur w MCC Local excision & removal int fix devices exc hip & femur w CC Local excision & removal int fix devices exc hip & femur w/o CC/MCC Local excision & removal int fix devices of hip & femur w CC/MCC Local excision & removal int fix devices of hip & femur w/o CC/MCC Soft tissue procedures w MCC Soft tissue procedures w CC Soft tissue procedures w/o CC/MCC Foot procedures w MCC Foot procedures w CC Foot procedures w/o CC/MCC Major thumb or joint procedures Major shoulder or elbow joint procedures w CC/MCC Major shoulder or elbow joint procedures w/o CC/MCC Arthroscopy Shoulder,elbow or forearm proc,exc major joint proc w MCC Shoulder,elbow or forearm proc,exc major joint proc w CC Shoulder,elbow or forearm proc,exc major joint proc w/o CC/MCC Hand or wrist proc, except major thumb or joint proc w CC/MCC Hand or wrist proc, except major thumb or joint proc w/o CC/MCC Other musculoskelet sys & conn tiss OR proc w MCC Other musculoskelet sys & conn tiss OR proc w CC Other musculoskelet sys & conn tiss OR proc w/o CC/MCC PERC CARDIO PROC W/O CORONARY ARTERY STENT OR AMI CERVICAL SPINAL FUSION W CC CERVICAL SPINAL FUSION W/O CC ALCOHOL/DRUG ABUSE OR DEPENDENCE W CC ALC/DRUG ABUSE OR DEPEND W REHABILITATION THERAPY ALC/DRUG ABUSE OR DEPEND W/O REHABILITATION THERAP Transient ischemia Other heart assist system implant PERCUTANEOUS CARDIOVASCULAR PROC WITH STENT PERCUTNEOUS CARDIOVASULAR PROC W DRUG ELUTING STEN INTRACRANIAL VASCULAR PROC W PDX HEMORRHAGE VENTRICULAR SHUNT PROCEDURES W CC VENTRICULAR SHUNT PROCEDURES W/O CC SPINAL PROCEDURES W CC SPINAL PROCEDURES W/O CC Fractures of femur w MCC Fractures of femur w/o MCC Fractures of hip & pelvis w MCC Fractures of hip & pelvis w/o MCC Sprains, strains, & dislocations of hip, pelvis & thigh w CC/MCC Sprains, strains, & dislocations of hip, pelvis & thigh w/o CC/MCC Osteomyelitis w MCC Osteomyelitis w CC Osteomyelitis w/o CC/MCC Pathological fractures & musculoskelet & conn tiss malig w MCC Pathological fractures & musculoskelet & conn tiss malig w CC Pathological fractures & musculoskelet & conn tiss malig w/o CC/MCC Connective tissue disorders w MCC Connective tissue disorders w CC Connective tissue disorders w/o CC/MCC Septic arthritis w MCC Septic arthritis w CC Septic arthritis w/o CC/MCC Medical back problems w MCC Medical back problems w/o MCC Bone diseases & arthropathies w MCC Bone diseases & arthropathies w/o MCC Signs & symptoms of musculoskeletal system & conn tissue w MCC Signs & symptoms of musculoskeletal system & conn tissue w/o MCC Tendonitis, myositis & bursitis w MCC 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG 558 559 560 561 562 563 564 565 566 567 568 569 570 571 572 573 574 575 576 577 578 579 580 581 582 583 584 585 588 589 591 592 593 594 595 596 597 598 599 600 601 602 603 604 605 606 607 608 609 611 612 613 614 615 616 617 618 619 620 621 622 623 624 625 626 627 628 629 630 631 633 634 636 637 638 639 640 641 642 643 644 645 650 651 652 653 654 655 656 657 658 Tendonitis, myositis & bursitis w/o MCC Aftercare, musculoskeletal system & connective tissue w MCC Aftercare, musculoskeletal system & connective tissue w CC Aftercare, musculoskeletal system & connective tissue w/o CC/MCC Fx, sprn, strn & disl except femur, hip, pelvis & thigh w MCC Fx, sprn, strn & disl except femur, hip, pelvis & thigh w/o MCC Other musculoskeletal sys & connective tissue diagnoses w MCC Other musculoskeletal sys & connective tissue diagnoses w CC Other musculoskeletal sys & connective tissue diagnoses w/o CC/MCC STOMACH, ESOPHAGEAL & DUODENAL PROC AGE LESS THAN STOMACH, ESOPHAGEAL & DUODENAL PROC AGE LESS THAN MAJOR SMALL & LARGE BOWEL PROC W CC W/O MAJOR GI Skin Debridement W MCC Skin Debridement W CC Skin Debridement W/O CC/MCC Skin graft &/or debrid for skn ulcer or cellulitis w MCC Skin graft &/or debrid for skn ulcer or cellulitis w CC Skin graft &/or debrid for skn ulcer or cellulitis w/o CC/MCC Skin graft &/or debrid exc for skin ulcer or cellulitis w MCC Skin graft &/or debrid exc for skin ulcer or cellulitis w CC Skin graft &/or debrid exc for skin ulcer or cellulitis w/o CC/MCC Other skin, subcut tiss & breast proc w MCC Other skin, subcut tiss & breast proc w CC Other skin, subcut tiss & breast proc w/o CC/MCC Mastectomy for malignancy w CC/MCC Mastectomy for malignancy w/o CC/MCC Breast biopsy, local excision & other breast procedures w CC/MCC Breast biopsy, local excision & other breast procedures w/o CC/MCC NEONATE BWT <1500G W MAJOR PROCEDURE NEONATE BWT <500G OR GA <24 WEEKS NEONATE BIRTHWT 500-749G W/O MAJOR PROCEDURE Skin ulcers w MCC Skin ulcers w CC Skin ulcers w/o CC/MCC Major skin disorders w MCC Major skin disorders w/o MCC Malignant breast disorders w MCC Malignant breast disorders w CC Malignant breast disorders w/o CC/MCC Non-malignant breast disorders w CC/MCC Non-malignant breast disorders w/o CC/MCC Cellulitis w MCC Cellulitis w/o MCC Trauma to the skin, subcut tiss & breast w MCC Trauma to the skin, subcut tiss & breast w/o MCC Minor skin disorders w MCC Minor skin disorders w/o MCC NEONATE BWT 1250-1499G W OR W/O OTHER SIGNIFICANT CONDITION NEONATE BWT 1500-2499G W MAJOR PROCEDURE NEONATE BIRTHWT 1500-1999G W MAJOR ANOMALY NEONATE BWT 1500-1999G W RESP DIST SYND/OTH MAJ RESP COND NEONATE BIRTHWT 1500-1999G W CONGENITAL/PERINATAL INFECTION Adrenal & pituitary procedures w CC/MCC Adrenal & pituitary procedures w/o CC/MCC Amputat of lower limb for endocrine,nutrit,& metabol dis w MCC Amputat of lower limb for endocrine,nutrit,& metabol dis w CC Amputat of lower limb for endocrine,nutrit,& metabol dis w/o CC/MCC OR procedures for obesity w MCC OR procedures for obesity w CC OR procedures for obesity w/o CC/MCC Skin grafts & wound debrid for endoc, nutrit & metab dis w MCC Skin grafts & wound debrid for endoc, nutrit & metab dis w CC Skin grafts & wound debrid for endoc, nutrit & metab dis w/o CC/MCC Thyroid, parathyroid & thyroglossal procedures w MCC Thyroid, parathyroid & thyroglossal procedures w CC Thyroid, parathyroid & thyroglossal procedures w/o CC/MCC Other endocrine, nutrit & metab OR proc w MCC Other endocrine, nutrit & metab OR proc w CC Other endocrine, nutrit & metab OR proc w/o CC/MCC NEONATE BIRTHWT >2499G W OTHER MAJOR PROCEDURE NEONATE BIRTHWT >2499G W MAJOR ANOMALY NEONATE, BIRTHWT >2499G W RESP DIST SYND/OTH MAJ RESP COND NEONATE BIRTHWT >2499G W CONGENITAL/PERINATAL INFECTION Diabetes w MCC Diabetes w CC Diabetes w/o CC/MCC Nutritional & misc metabolic disorders w MCC Nutritional & misc metabolic disorders w/o MCC Inborn errors of metabolism Endocrine disorders w MCC Endocrine disorders w CC Endocrine disorders w/o CC/MCC SPLENECTOMY OTHER PROCEDURES OF BLOOD & BLOOD-FORMING ORGANS Kidney transplant Major bladder procedures w MCC Major bladder procedures w CC Major bladder procedures w/o CC/MCC Kidney & ureter procedures for neoplasm w MCC Kidney & ureter procedures for neoplasm w CC Kidney & ureter procedures for neoplasm w/o CC/MCC 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 10/1/2006 10/1/2006 10/1/2006 10/1/2011 10/1/2011 10/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 6/1/2012 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 9/1/2012 9/1/2012 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 9/1/2012 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG 659 660 661 662 663 664 665 666 667 668 669 670 671 672 673 674 675 680 681 682 683 684 685 686 687 688 689 690 691 692 693 694 695 696 697 698 699 700 707 708 709 710 711 712 713 714 715 716 717 718 720 721 722 723 724 725 726 727 728 729 730 734 735 736 737 738 739 740 741 742 743 744 745 746 747 748 749 750 751 752 753 754 755 756 757 758 759 760 761 765 766 Kidney & ureter procedures for non-neoplasm w MCC Kidney & ureter procedures for non-neoplasm w CC Kidney & ureter procedures for non-neoplasm w/o CC/MCC Minor bladder procedures w MCC Minor bladder procedures w CC Minor bladder procedures w/o CC/MCC Prostatectomy w MCC Prostatectomy w CC Prostatectomy w/o CC/MCC Transurethral procedures w MCC Transurethral procedures w CC Transurethral procedures w/o CC/MCC Urethral procedures w CC/MCC Urethral procedures w/o CC/MCC Other kidney & urinary tract procedures w MCC Other kidney & urinary tract procedures w CC Other kidney & urinary tract procedures w/o CC/MCC MAJOR O.R. PROCEDURES FOR LYMPHATIC/HEMATOPOIETIC/OTHER NEOPLASMS OTHER O.R. PROCEDURES FOR LYMPHATIC/HEMATOPOIETIC/OTHER NEOPLASMS Renal failure w MCC Renal failure w CC Renal failure w/o CC/MCC Admit for renal dialysis Kidney & urinary tract neoplasms w MCC Kidney & urinary tract neoplasms w CC Kidney & urinary tract neoplasms w/o CC/MCC Kidney & urinary tract infections w MCC Kidney & urinary tract infections w/o MCC Urinary stones w esw lithotripsy w CC/MCC Urinary stones w esw lithotripsy w/o CC/MCC Urinary stones w/o esw lithotripsy w MCC Urinary stones w/o esw lithotripsy w/o MCC Kidney & urinary tract signs & symptoms w MCC Kidney & urinary tract signs & symptoms w/o MCC Urethral stricture Other kidney & urinary tract diagnoses w MCC Other kidney & urinary tract diagnoses w CC Other kidney & urinary tract diagnoses w/o CC/MCC Major male pelvic procedures w CC/MCC Major male pelvic procedures w/o CC/MCC Penis procedures w CC/MCC Penis procedures w/o CC/MCC Testes procedures w CC/MCC Testes procedures w/o CC/MCC Transurethral prostatectomy w CC/MCC Transurethral prostatectomy w/o CC/MCC Other male reproductive system OR proc for malignancy w CC/MCC Other male reproductive system OR proc for malignancy w/o CC/MCC Other male reproductive system OR proc exc malignancy w CC/MCC Other male reproductive system OR proc exc malignancy w/o CC/MCC SEPTICEMIA & DISSEMINATED INFECTIONS POST-OPERATIVE, POST-TRAUMATIC, OTHER DEVICE INFECTIONS Malignancy, male reproductive system w MCC Malignancy, male reproductive system w CC Malignancy, male reproductive system w/o CC/MCC Benign prostatic hypertrophy w MCC Benign prostatic hypertrophy w/o MCC Inflammation of the male reproductive system w MCC Inflammation of the male reproductive system w/o MCC Other male reproductive system diagnoses w CC/MCC Other male reproductive system diagnoses w/o CC/MCC Pelvic evisceration, rad hysterectomy & rad vulvectomy w CC/MCC Pelvic evisceration, rad hysterectomy & rad vulvectomy w/o CC/MCC Uterine & adnexa proc for ovarian or adnexal malignancy w MCC Uterine & adnexa proc for ovarian or adnexal malignancy w CC Uterine & adnexa proc for ovarian or adnexal malignancy w/o CC/MCC Uterine,adnexa proc for non-ovarian/adnexal malig w MCC Uterine,adnexa proc for non-ovarian/adnexal malig w CC Uterine,adnexa proc for non-ovarian/adnexal malig w/o CC/MCC Uterine & adnexa proc for non-malignancy w CC/MCC Uterine & adnexa proc for non-malignancy w/o CC/MCC D&C, conization, laparascopy & tubal interruption w CC/MCC D&C, conization, laparascopy & tubal interruption w/o CC/MCC Vagina, cervix & vulva procedures w CC/MCC Vagina, cervix & vulva procedures w/o CC/MCC Female reproductive system reconstructive procedures Other female reproductive system OR procedures w CC/MCC Other female reproductive system OR procedures w/o CC/MCC MAJOR DEPRESSIVE DISORDERS & OTHER/UNSPECIFIED PSYCHOSES Disorders of personality & impulse control BIPOLAR DISORDERS Malignancy, female reproductive system w MCC MALIGNANCY, FEMALE REPRODUCTIVE SYSTEM W CC Malignancy, female reproductive system w/o CC/MCC Infections, female reproductive system w MCC Infections, female reproductive system w CC Infections, female reproductive system w/o CC/MCC Menstrual & other female reproductive system disorders w CC/MCC Menstrual & other female reproductive system disorders w/o CC/MCC Cesarean section w CC/MCC Cesarean section w/o CC/MCC 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 1/1/2011 9/1/2012 1/1/2011 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG 767 768 769 770 772 773 774 775 776 777 778 779 780 781 782 789 790 791 792 793 794 795 799 800 801 802 803 804 808 809 810 811 812 813 814 815 816 820 821 822 823 824 825 826 827 828 829 830 834 835 836 837 838 839 840 841 842 843 844 845 846 847 848 849 850 853 854 855 856 857 858 860 861 862 863 864 865 866 867 868 869 870 871 872 876 880 881 882 883 884 885 Vaginal delivery w sterilization &/or D&C Vaginal delivery w OR proc except steril &/or D&C Postpartum & post abortion diagnoses w OR procedure Abortion w D&C, aspiration curettage or hysterotomy ALCOHOL & DRUG DEPENDENCE W REHAB OR REHAB/DETOX THERAPY OPIOID ABUSE & DEPENDENCE Vaginal delivery w complicating diagnoses Vaginal delivery w/o complicating diagnoses Postpartum & post abortion diagnoses w/o OR procedure Ectopic pregnancy Threatened abortion Abortion w/o D&C False labor Other antepartum diagnoses w medical complications Other antepartum diagnoses w/o medical complications Neonates, died or transferred to another acute care facility Extreme immaturity or respiratory distress syndrome, neonate Prematurity w major problems Prematurity w/o major problems Full term neonate w major problems Neonate w other significant problems Normal newborn Splenectomy w MCC Splenectomy w CC Splenectomy w/o CC/MCC Other OR proc of the blood & blood forming organs w MCC Other OR proc of the blood & blood forming organs w CC Other OR proc of the blood & blood forming organs w/o CC/MCC Major hematol/immun diag exc sickle cell crisis & coagul w MCC Major hematol/immun diag exc sickle cell crisis & coagul w CC Major hematol/immun diag exc sickle cell crisis & coagul w/o CC/MCC Red blood cell disorders w MCC Red blood cell disorders w/o MCC Coagulation disorders Reticuloendothelial & immunity disorders w MCC Reticuloendothelial & immunity disorders w CC Reticuloendothelial & immunity disorders w/o CC/MCC Lymphoma & leukemia w major OR procedure w MCC Lymphoma & leukemia w major OR procedure w CC Lymphoma & leukemia w major OR procedure w/o CC/MCC Lymphoma & non-acute leukemia w other OR proc w MCC Lymphoma & non-acute leukemia w other OR proc w CC Lymphoma & non-acute leukemia w other OR proc w/o CC/MCC Myeloprolif disord or poorly diff neopl w maj OR proc w MCC Myeloprolif disord or poorly diff neopl w maj OR proc w CC Myeloprolif disord or poorly diff neopl w maj OR proc w/o CC/MCC Myeloprolif disord or poorly diff neopl w other OR proc w CC/MCC Myeloprolif disord or poorly diff neopl w other OR proc w/o CC/MCC Acute leukemia w/o major OR procedure w MCC Acute leukemia w/o major OR procedure w CC Acute leukemia w/o major OR procedure w/o CC/MCC Chemo w acute leukemia as sdx or w high dose chemo agent w MCC Chemo w acute leukemia as sdx w CC or high dose chemo agent Chemo w acute leukemia as sdx w/o CC/MCC Lymphoma & non-acute leukemia w MCC Lymphoma & non-acute leukemia w CC Lymphoma & non-acute leukemia w/o CC/MCC Other myeloprolif dis or poorly diff neopl diag w MCC Other myeloprolif dis or poorly diff neopl diag w CC Other myeloprolif dis or poorly diff neopl diag w/o CC/MCC Chemotherapy w/o acute leukemia as secondary diagnosis w MCC Chemotherapy w/o acute leukemia as secondary diagnosis w CC Chemotherapy w/o acute leukemia as secondary diagnosis w/o CC/MCC Radiotherapy EXTENSIVE 3RD DEGREE BURNS W SKIN GRAFT Infectious & parasitic diseases w OR procedure w MCC Infectious & parasitic diseases w OR procedure w CC Infectious & parasitic diseases w OR procedure w/o CC/MCC Postoperative or post-traumatic infections w OR proc w MCC Postoperative or post-traumatic infections w OR proc w CC Postoperative or post-traumatic infections w OR proc w/o CC/MCC REHABILITATION SIGNS, SYMPTOMS & OTHER FACTORS INFLUENCING HEALTH STATUS Postoperative & post-traumatic infections w MCC Postoperative & post-traumatic infections w/o MCC Fever Viral illness w MCC Viral illness w/o MCC Other infectious & parasitic diseases diagnoses w MCC Other infectious & parasitic diseases diagnoses w CC Other infectious & parasitic diseases diagnoses w/o CC/MCC Septicemia or severe sepsis w MV 96+ hours Septicemia or severe sepsis w/o MV 96+ hours w MCC Septicemia or severe sepsis w/o MV 96+ hours w/o MCC OR procedure w principal diagnoses of mental illness Acute adjustment reaction & psychosocial dysfunction DEPRESSIVE NEUROSES NEUROSES EXCEPT DEPRESSIVE DISORDERS OF PERSONALITY & IMPULSE CONTROL Organic disturbances & mental retardation PSYCHOSES 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2004 1/1/2004 9/1/2012 1/1/2011 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG DRG HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC 886 887 890 892 893 894 895 896 897 901 902 903 904 905 906 907 908 909 910 911 912 913 914 915 916 917 918 919 920 921 922 923 927 928 929 930 933 934 935 939 940 941 945 946 947 948 949 950 951 952 955 956 957 958 959 963 964 965 969 970 974 975 976 977 981 982 983 984 985 986 987 988 989 998 999 3210F 3250F 3775F 3776F 6010F 6015F A0021 A0080 A0090 A0100 A0110 A0120 A0130 A0140 A0160 A0170 Behavioral & developmental disorders Other mental disorder diagnoses HIV W MULTIPLE MAJOR HIV RELATED CONDITIONS HIV W MAJOR HIV RELATED CONDITION HIV W MULTIPLE SIGNIFICANT HIV RELATED CONDITIONS Alcohol/drug abuse or dependence, left ama Alcohol/drug abuse or dependence w rehabilitation therapy Alcohol/drug abuse or dependence w/o rehabilitation therapy w MCC Alcohol/drug abuse or dependence w/o rehabilitation therapy w/o MCC Wound debridements for injuries w MCC Wound debridements for injuries w CC Wound debridements for injuries w/o CC/MCC Skin grafts for injuries w CC/MCC Skin grafts for injuries w/o CC/MCC Hand procedures for injuries Other OR procedures for injuries w MCC Other OR procedures for injuries w CC Other OR procedures for injuries w/o CC/MCC Craniotomy for multiple significant trauma EXTENSIVE ABDOMINAL/THORACIC PROCEDURES FOR MULT SIGNIFICANT TRAUMA MUSCULOSKELETAL & OTHER PROCEDURES FOR MULTIPLE SIGNIFICANT TRAUMA Traumatic injury w MCC Traumatic injury w/o MCC Allergic reactions w MCC Allergic reactions w/o MCC Poisoning & toxic effects of drugs w MCC Poisoning & toxic effects of drugs w/o MCC Complications of treatment w MCC Complications of treatment w CC Complications of treatment w/o CC/MCC Other injury, poisoning & toxic effect diag w MCC Other injury, poisoning & toxic effect diag w/o MCC Extensive burns or full thickness burns w MV 96+ hrs w skin graft Full thickness burn w skin graft or inhal inj w CC/MCC Full thickness burn w skin graft or inhal inj w/o CC/MCC MULTIPLE SIGNIFICANT TRAUMA W/O O.R. PROCEDURE Extensive burns or full thickness burns w MV 96+ hrs w/o skin graft Full thickness burn w/o skin grft or inhal inj Non-extensive burns OR proc w diagnoses of other contact w health services w MCC OR proc w diagnoses of other contact w health services w CC OR proc w diagnoses of other contact w health services w/o CC/MCC Rehabilitation w CC/MCC Rehabilitation w/o CC/MCC Signs & symptoms w MCC Signs & symptoms w/o MCC Aftercare w CC/MCC Aftercare w/o CC/MCC Other factors influencing health status NONEXTENSIVE PROCEDURE UNRELATED TO PRINCIPAL DIAGNOSIS CRANIOTOMY FOR MULTIPLE SIGNIFICANT TRAUMA Limb reattachment, hip & femur proc for multiple significant trauma Other OR procedures for multiple significant trauma w MCC Other OR procedures for multiple significant trauma w CC Other OR procedures for multiple significant trauma w/o CC/MCC Other multiple significant trauma w MCC Other multiple significant trauma w CC Other multiple significant trauma w/o CC/MCC HIV w extensive OR procedure w MCC HIV w extensive OR procedure w/o MCC HIV w major related condition w MCC HIV w major related condition w CC HIV w major related condition w/o CC/MCC HIV w or w/o other related condition Extensive OR procedure unrelated to principal diagnosis w MCC Extensive OR procedure unrelated to principal diagnosis w CC Extensive OR procedure unrelated to principal diagnosis w/o CC/MCC Prostatic OR procedure unrelated to principal diagnosis w MCC Prostatic OR procedure unrelated to principal diagnosis w CC Prostatic OR procedure unrelated to principal diagnosis w/o CC/MCC Non-extensive OR proc unrelated to principal diagnosis w MCC Non-extensive OR proc unrelated to principal diagnosis w CC Non-extensive OR proc unrelated to principal diagnosis w/o CC/MCC Principal diagnosis invalid as discharge diagnosis Ungroupable GROUP A STREP TEST PERFORMED (PHAR) SPECIMEN SITE OTHER THAN ANATOMIC LOCATION OF PRIM ADENOMA(S)/NEOPLASM DETECTED SCRNG CLNSCPY ADENOMA(S)/NEOPLASM NOT DETECTED SCRNG CLNSCPY DYSPHAGIA SCREENING CONDUCTED PRIOR TO ORDER FOR OR RECEIPT OF ANY FOODS, FLUID PATIENT RECEIVING OR ELIGIBLE TO RECEIVE FOODS, FLUIDS, OR MEDICATION BY MOUTH (ST OUTSIDE STATE AMBULANCE SERVICE NONINTEREST ESCORT IN NON ER INTEREST ESCORT IN NON ER NONEMERGENCY TRANSPORT TAXI NONEMERGENCY TRANSPORT BUS NON ER TRANSPORT MINI-BUS NON ER TRANSPORT WHEELCHAIR VAN NON EMERGENCY TRANSPORT AIR NON ER TRANSPORT CASE WORKER TRANSPORT PARKING FEES/TOLLS 1/1/2011 1/1/2011 9/1/2012 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 9/1/2012 9/1/2012 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2011 10/1/2009 4/1/2014 4/1/2014 1/1/2011 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth all inpatient confinements require auth HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC A0180 A0190 A0200 A0210 A0225 A0380 A0382 A0384 A0390 A0392 A0394 A0396 A0398 A0420 A0422 A0424 A0425 A0426 A0427 A0428 A0429 A0430 A0431 A0432 A0433 A0434 A0435 A0436 A0888 A0998 A0999 A4206 A4207 A4208 A4209 A4210 A4211 A4212 A4213 A4215 A4216 A4217 A4218 A4220 A4221 A4222 A4223 A4230 A4231 A4232 A4233 A4234 A4235 A4236 A4244 A4245 A4246 A4247 A4248 A4250 A4252 A4253 A4255 A4256 A4258 A4259 A4261 A4263 A4264 A4265 A4266 A4267 A4268 A4269 A4270 A4280 A4281 A4282 A4283 A4284 A4285 A4286 A4290 A4300 A4301 A4305 A4306 A4310 A4311 A4312 A4313 NON ER TRANSPORT LODGING RECIPIENT NON ER TRANSPORT MEALS RECIPIENT NON ER TRANSPORT LODGING ESCORT NON ER TRANSPORT MEALS ESCORT NEONATAL EMERGENCY TRANSPORT ONE WAY BASIC LIFE SUPPORT MILEAGE BASIC LIFE SUPPORT ROUTINE SUPPLIES BASIC LIFE SUPPORT DEFIBRILLATION SUPPLIES ADVANCED LIFE SUPPORT MILEAGE ADVANCED LIFE SUPPORT DEFIBRILLATION SUPPLIES ADVANCED LIFE SUPPORT IV DRUG THERAPY SUPPLIES ADVANCED LIFE SUPPORT ESOPHAGEAL INTUBATION SUPPLI ADVANCED LIFE SUPPORT ROUTINE DISPOSABLE SUPPLIES AMBULANCE WAITING TIME IN 1/2 HOUR INCREMENTS AMBULANCE OXYGEN LIFE SUSTAINING EXTRA AMBULANCE ATTENDANT GROUND MILEAGE ALS 1 ALS1 EMERGENCY BLS BLS-EMERGENCY FIXED WING AIR TRANSPORT ROTARY WING AIR TRANSPORT PI VOLUNTEER AMBULANCE COMP ALS 2 SPECIALTY CARE TRANSPORT FIXED WING AIR MILEAGLE ROTARY WING AIR MILEAGE NON COVERED AMBULANCE MILEAGE AMBULANCE RESPONSE AND TREATMENT,NO TRANSPORT UNLISTED AMBULANCE SERVICE SYRINGE WITH NEEDLE, STERILE 1CC, EACH SYRINGE WITH NEEDLE, STERILE 2CC, EACH SYRINGE WITH NEEDLE, STERILE 3CC, EACH SYRINGE WITH NEEDLE, STERILE 5CC OR GREATER, EACH NEEDLE-FREE INJECTION DEVICE, EACH SUPPLIES FOR SELF ADMINISTERED INJECTIONS NONCORING NEEDLE OR STYLET WITH OR WITHOUT CATHETE SYRINGE, STERILE, 20 CC OR GREATER, EACH NEEDLES ONLY, STERILE, ANY SIZE, EACH STERILE WATER/SALINE, 10 ML STERILE WATER/SALINE, 500 ML Sterile saline or water, metered dose dispenser, 1 REFILL KIT FOR IMPLANTABLE INFUSION PUMP SUPPLIES FOR MAINTENANCE OF DRUG INFUSION CATHETER INFUSION SUPPLIES FOR EXTERNAL DRUG INFUSION PUMP, INFUSION SUPPLIES NOT USED WITH EXTERNAL INFUSION INFUSION SET FOR EXTERNAL INSULIN PUMP, NON NEEDLE INFUSION SET FOR EXTERNAL INSULIN PUMP, NEEDLE TYP SYRINGE WITH NEEDLE FOR EXTERNAL INSULIN PUMP, STE REPLACEMENT BATTERY, ALKALINE - OTHER THAN J CELL Replacement battery, alkaline, j cell, for use wit Replacement battery, lithium, for use with medical Replacement battery, silver oxide, for use with me ALCOHOL OR PEROXIDE, PER PINT ALCOHOL WIPES, PER BOX BETADINE OR PHISOHEX SOLUTION, PER PINT BETADINE OR IODINE SWABS/WIPES, PER BOX CHLORHEXIDINE CONTAINING ANTISEPTIC, 1 ML URINE TEST OR REAGENT STRIPS OR TABLETS (100 TABLE BLOOD KETONE TEST OR REAGENT STRIP EACH BLOOD GLUCOSE TEST OR REAGENT STRIPS FOR HOME BLOO PLATFORMS FOR HOME BLOOD GLUCOSE MONITOR, 50 PER B NORMAL, LOW, HIGH CALIBRATOR SOLUTION/CHIPS SPRING-POWERED DEVICE FOR LANCET, EACH LANCETS, PER BOX OF 100 CERVICAL CAP FOR CONTRACEPTIVE USE PERMANENT, LONG TERM, NON-DISSOLVABLE LACRIMAL DUC PERM IMPL CONTRACEPTIVE TUBAL OCCL DEV & DEL SYS PARAFFIN, PER POUND DIAPHRAGM FOR CONTRACEPTIVE USE CONTRACEPTIVE SUPPLY, CONDOM, MALE, EACH CONTRACEPTIVE SUPPLY, CONDOM, FEMALE, EACH CONTRACEPTIVE SUPPLY, SPERMICIDE (E.G., FOAM, GEL) DISPOSABLE ENDOSCOPE SHEATH, EACH ADHESIVE SKIN SUPPORT ATTACHMENT FOR USE WITH EXTE TUBING FOR BREAST PUMP, REPLACEMENT ADAPTER FOR BREAST PUMP, REPLACEMENT CAP FOR BREAST PUMP BOTTLE, REPLACEMENT BREAST SHIELD AND SPLASH PROTECTOR FOR USE WITH BR POLYCARBONATE BOTTLE FOR USE WITH BREAST PUMP, REP LOCKING RING FOR BREAST PUMP, REPLACEMENT SACRAL NERVE STIMULATION TEST LEAD, EACH IMPLANTABLE ACCESS CATHETER, (E,G., VENOUS, ARTERI IMPLANTABLE ACCESS TOTAL CATHETER, PORT/RESERVOIR DISPOSABLE DRUG DELIVERY SYSTEM, FLOW RATE OF 50 M DISPOSABLE DRUG DELIVERY SYSTEM, FLOW RATE OF 5 ML INSERTION TRAY WITHOUT DRAINAGE BAG AND WITHOUT CA INSERTION TRAY WITHOUT DRAINAGE BAG WITH INDWELLIN INSERTION TRAY WITHOUT DRAINAGE BAG WITH INDWELLIN INSERTION TRAY WITHOUT DRAINAGE BAG WITH INDWELLIN 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2013 1/1/2013 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC A4314 A4315 A4316 A4320 A4321 A4322 A4326 A4327 A4328 A4330 A4331 A4332 A4333 A4334 A4335 A4336 A4337 A4338 A4340 A4344 A4346 A4349 A4351 A4352 A4353 A4354 A4355 A4356 A4357 A4358 A4360 A4361 A4362 A4363 A4364 A4366 A4367 A4368 A4369 A4371 A4372 A4373 A4375 A4376 A4377 A4378 A4379 A4380 A4381 A4382 A4383 A4384 A4385 A4387 A4388 A4389 A4390 A4391 A4392 A4393 A4394 A4395 A4396 A4397 A4398 A4399 A4400 A4402 A4404 A4405 A4406 A4407 A4408 A4409 A4410 A4411 A4412 A4413 A4414 A4415 A4416 A4417 A4418 A4419 A4420 A4421 A4422 A4423 A4424 A4425 A4426 INSERTION TRAY WITH DRAINAGE BAG WITH INDWELLING C INSERTION TRAY WITH DRAINAGE BAG WITH INDWELLING C INSERTION TRAY WITH DRAINAGE BAG WITH INDWELLING C IRRIGATION TRAY WITH BULB OR PISTON SYRINGE, ANY P THERAPEUTIC AGENT FOR URINARY CATHETER IRRIGATION IRRIGATION SYRINGE, BULB OR PISTON, EACH MALE EXTERNAL CATHETER SPECIALTY TYPE WITH INTEGRA FEMALE EXTERNAL URINARY COLLECTION DEVICE MEATAL FEMALE EXTERNAL URINARY COLLECTION DEVICE POUCH, PERIANAL FECAL COLLECTION POUCH WITH ADHESIVE, EAC EXTENSION DRAINAGE TUBING, ANY TYPE, ANY LENGTH, W LUBRICANT, INDIVIDUAL STERILE PACKET, EACH URINARY CATHETER ANCHORING DEVICE, ADHESIVE SKIN A URINARY CATHETER ANCHORING DEVICE, LEG STRAP, EACH INCONTINENCE SUPPLY; MISCELLANEOUS INCONTINENCE SUPPLY URETHRAL INSERT ANY TYPE EA INCONTINENCE SUPPLY, RECTAL INSERT, ANY TYPE, EACH INDWELLING CATHETER; FOLEY TYPE, TWO-WAY LATEX WIT INDWELLING CATHETER; SPECIALTY TYPE, EG COUDE, M INDWELLING CATHETER, FOLEY TYPE, TWO-WAY, ALL SILI INDWELLING CATHETER; FOLEY TYPE, THREE WAY FOR CON MALE EXTERNAL CATHETER, WITH OR WITHOUT ADHESIVE, INTERMITTENT URINARY CATHETER; STRAIGHT TIP, WITH INTERMITTENT URINARY CATHETER; COUDE (CURVED) TIP, INTERMITTENT URINARY CATHETER, WITH INSERTION SUPP INSERTION TRAY WITH DRAINAGE BAG BUT WITHOUT CATHE IRRIGATION TUBING SET FOR CONTINUOUS BLADDER IRRIG EXTERNAL URETHRAL CLAMP OR COMPRESSION DEVICE (NOT BEDSIDE DRAINAGE BAG, DAY OR NIGHT, WITH OR WITHOU URINARY DRAINAGE BAG, LEG OR ABDOMEN, VINYL, WITH DISPSBL EXT URETHRAL CLAMP/COMP DEV PAD POUCH EA OSTOMY FACEPLATE, EACH SKIN BARRIER; SOLID, 4 X 4 OR EQUIVALENT; EACH OSTOMY CLAMP, ANY TYPE, REPLACEMENT ONLY, EACH ADHESIVE, LIQUID OR EQUAL, ANY TYPE, PER OZ OSTOMY VENT, ANY TYPE, EACH OSTOMY BELT, EACH OSTOMY FILTER, ANY TYPE, EACH OSTOMY SKIN BARRIER, LIQUID (SPRAY, BRUSH, ETC), P OSTOMY SKIN BARRIER, POWDER, PER OZ OSTOMY SKIN BARRIER, SOLID 4X4 OR EQUIVALENT, WIT OSTOMY SKIN BARRIER, WITH FLANGE (SOLID, FLEXIBLE OSTOMY POUCH, DRAINABLE, WITH FACEPLATE ATTACHED, OSTOMY POUCH, DRAINABLE, WITH FACEPLATE ATTACHED, OSTOMY POUCH, DRAINABLE, FOR USE ON FACEPLATE, PLA OSTOMY POUCH, DRAINABLE, FOR USE ON FACEPLATE, RUB OSTOMY POUCH, URINARY, WITH FACEPLATE ATTACHED, PL OSTOMY POUCH, URINARY, WITH FACEPLATE ATTACHED, RU OSTOMY POUCH, URINARY, FOR USE ON FACEPLATE, PLAST OSTOMY POUCH, URINARY, FOR USE ON FACEPLATE, HEAVY OSTOMY POUCH, URINARY, FOR USE ON FACEPLATE, RUBBE OSTOMY FACEPLATE EQUIVALENT, SILICONE RING, EACH OSTOMY SKIN BARRIER, SOLID 4X4 OR EQUIVALENT, EXTE OSTOMY POUCH, CLOSED, WITH BARRIER ATTACHED, WITH OSTOMY POUCH, DRAINABLE, WITH EXTENDED WEAR BARRIE OSTOMY POUCH, DRAINABLE, WITH BARRIER ATTACHED, WI OSTOMY POUCH, DRAINABLE, WITH EXTENDED WEAR BARRIE OSTOMY POUCH, URINARY, WITH EXTENDED WEAR BARRIER OSTOMY POUCH, URINARY, WITH STANDARD WEAR BARRIER OSTOMY POUCH, URINARY, WITH EXTENDED WEAR BARRIER OSTOMY DEODORANT FOR USE IN OSTOMY POUCH, LIQUID, OSTOMY DEODORANT FOR USE IN OSTOMY POUCH, SOLID, P OSTOMY BELT WITH PERISTOMAL HERNIA SUPPORT IRRIGATION SUPPLY; SLEEVE, EACH OSTOMY IRRIGATION SUPPLY; BAG, EACH OSTOMY IRRIGATION SUPPLY; CONE/CATH W/WO BRUSH OSTOMY IRRIGATION SET LUBRICANT, PER OUNCE OSTOMY RING, EACH OSTOMY SKIN BARRIER, NON-PECTIN BASED, PASTE, PER OSTOMY SKIN BARRIER, PECTIN-BASED, PASTE, PER OUNC OSTOMY SKIN BARRIER, WITH FLANGE (SOLID, FLEXIBLE, OSTOMY SKIN BARRIER, WTIH FLANGE (SOLID, FLEXIBLE OSTOMY SKIN BARRIER, WITH FLANGE (SOLID, FLEXIBLE OSTOMY SKIN BARRIER, WITH FLANGE (SOLID, FLEXIBLE OSTOMY SKIN BARRIER, WITH FLANGE (SOLID, 4X4 OR EQ OSTOMY POUCH, DRAINABLE, HIGH OUTPUT, FOR USE ON A OSTOMY POUCH, DRAINABLE, HIGH OUTPUT, FOR USE ON A OSTOMY SKIN BARRIER, WITH FLANGE (SOLID, FLEXIBLE OSTOMY SKIN BARRIER, WITH FLANGE (SOLID, FLEXIBLE OSTOMY POUCH, CLOSED, WITH BARRIER ATTACHED, WITH OSTOMY POUCH, CLOSED, WITH BARRIER ATTACHED, WITH OSTOMY POUCH, CLOSED; WITHOUT BARRIER ATTACHED, WI OSTOMY POUCH, CLOSED; FOR USE ON BARRIER WITH NONOSTOMY POUCH, CLOSED; FOR USE ON BARRIER WITH LOCK OSTOMY SUPPLY; MISCELLANEOUS OSTOMY ABSORBENT MATERIAL (SHEET/PAD/CRYSTAL PACKE OSTOMY POUCH, CLOSED; FOR USE ON BARRIER WITH LOCK OSTOMY POUCH, DRAINABLE, WITH BARRIER ATTACHED, WI OSTOMY POUCH, DRAINABLE; FOR USE ON BARRIER WITH N OSTOMY POUCH, DRAINABLE; FOR USE ON BARRIER WITH L 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC A4427 A4428 A4429 A4430 A4431 A4432 A4433 A4434 A4435 A4450 A4452 A4455 A4456 A4458 A4459 A4461 A4463 A4465 A4466 A4470 A4480 A4481 A4483 A4490 A4495 A4500 A4510 A4520 A4550 A4554 A4555 A4556 A4557 A4558 A4559 A4561 A4562 A4565 A4566 A4570 A4575 A4580 A4590 A4595 A4600 A4601 A4602 A4604 A4605 A4606 A4608 A4611 A4612 A4613 A4614 A4615 A4616 A4617 A4618 A4619 A4620 A4623 A4624 A4625 A4626 A4627 A4628 A4629 A4630 A4633 A4634 A4635 A4636 A4637 A4638 A4639 A4640 A4641 A4642 A4648 A4649 A4650 A4651 A4652 A4653 A4657 A4660 A4663 A4670 A4671 A4672 OSTOMY POUCH, DRAINABLE; FOR USE ON BARRIER WITH L OSTOMY POUCH, URINARY, WITH EXTENDED WEAR BARRIER OSTOMY POUCH, URINARY, WITH BARRIER ATTACHED, WITH OSTOMY POUCH, URINARY, WITH EXTENDED WEAR BARRIER OSTOMY POUCH, URINARY; WITH BARRIER ATTACHED, WITH OSTOMY POUCH, URINARY; FOR USE ON BARRIER WITH NON OSTOMY POUCH, URINARY; FOR USE ON BARRIER WITH LOC OSTOMY POUCH, URINARY; FOR USE ON BARRIER WITH LOC OST POUCH DRAIN HI OP EXT WEAR BARR W/WO FLTR EA TAPE, NON-WATERPROOF, PER 18 SQUARE INCHES TAPE, WATERPROOF, PER 18 SQUARE INCHES ADHESIVE REMOVER OR SOLVENT (FOR TAPE, CEMENT OR O ADHESIVE REMOVER WIPES ANY TYPE EACH ENEMA BAG WITH TUBING, REUSABLE MANUAL PUMP-OPERATED ENEMA SYS REUSABLE ANY TYPE SURGICAL DRESS HOLD NON-REUSE SURGICAL DRESSING HOLDER, REUSE NON-ELASTIC BINDER FOR EXTREMITY GARMENT BELT SLEEVE OR OTH COVERING ELASTIC EACH GRAVLEE JET WASHER VABRA ASPIRATOR TRACHEOSTOMA FILTER, ANY TYPE, ANY SIZE, EACH MOISTURE EXCHANGER, DISPOSABLE, FOR USE WITH INVAS SURGICAL STOCKINGS ABOVE KNEE LENGTH, EACH SURGICAL STOCKINGS THIGH LENGTH, EACH SURGICAL STOCKINGS BELOW KNEE LENGTH, EACH SURGICAL STOCKINGS FULL LENGTH, EACH INCONTINENCE GARMENT, ANY TYPE, (E.G. BRIEF, DIAPE SURGICAL TRAYS DISPOSABLE UNDERPADS, ALL SIZES, (E.G., CHUXS) ELECTRD/TRANSDUCR E-STIM DVC USED CA TX RPL ONLY ELECTRODES, (E.G., APNEA MONITOR), PER PAIR LEAD WIRES, (E.G., APNEA MONITOR), PER PAIR CONDUCTIVE PASTE OR GEL COUPLING GEL OR PASTE PESSARY, RUBBER, ANY TYPE PESSARY, NON RUBBER, ANY TYPE SLINGS SHOULDER SLING/VEST ABDUCTION RESTRAINER PREFAB SPLINT TOPICAL HYPERBARIC OXYGEN CHAMBER, DISPOSABLE CAST SUPPLIES (E.G. PLASTER) SPECIAL CASTING MATERIAL (E.G. FIBERGLASS) ELECTRICAL STIMULATOR SUPPLIES, 2 LEAD, PER MONTH, Sleeve, inter limb comp dev LITHIUM ION BATT RECHARG NONPROS USE REPLACEMENT REPL BA EXT INFUS PUMP OWND PATIENT LI 1.5 V EA TUBING WITH INTEGRATED HEATING ELEMENT FOR USE WITH TRACHEAL SUCTION CATHETER, CLOSED SYSTEM, EACH OXYGEN PROBE FOR USE WITH OXIMETER DEVICE, REPLACE TRANSTRACHEAL OXYGEN CATHETER, EACH BATTERY, HEAVY DUTY; REPLACEMENT FOR PATIENT OWNED BATTERY CABLES; REPLACEMENT FOR PATIENT-OWNED VENT BATTERY CHARGER; REPLACEMENT FOR PATIENT-OWNED VEN PEAK EXPIRATORY FLOW RATE METER, HAND HELD CANNULA, NASAL TUBING (OXYGEN), PER FOOT MOUTH PIECE BREATHING CIRCUITS FACE TENT VARIABLE CONCENTRATION MASK TRACHEOSTOMY, INNER CANNULA TRACHEAL SUCTION CATHETER, ANY TYPE OTHER THAN CLO TRACHEOSTOMY CARE KIT FOR NEW TRACHEOSTOMY TRACHEOSTOMY CLEANING BRUSH, EACH SPACER, BAG OR RESERVOIR, WITH OR WITHOUT MASK, FO OROPHARYNGEAL SUCTION CATHETER, EACH TRACHEOSTOMY CARE KIT FOR ESTABLISHED TRACHEOSTOMY REPLACEMENT BATTERIES. MEDICALLY NECESSARY T.E.N. REPLACEMENT BULB/LAMP FOR ULTRAVIOLET LIGHT THERAP REPLACEMENT BULB FOR THERAPEUTIC LIGHT BOX, TABLET UNDERARM PAD, CRUTCH, REPLACEMENT, EACH REPLACEMENT, HANDGRIP, CANE, CRUTCH, OR WALKER, EA REPLACEMENT, TIP, CANE, CRUTCH, WALKER, EACH. REPLACEMENT BATTERY FOR PATIENT-OWNED EAR PULSE GE REPLACEMENT PAD FOR INFRARED HEATING PAD SYSTEM, E REPLACEMENT PAD FOR USE WITH MEDICALLY NECESSARY SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF SATUMOMAB PENDETIDE, RADIOPHARMACEUTICAL TISSUE MARKER IMPLANTABLE ANY TYPE EACH SURGICAL SUPPLY; MISCELLANEOUS IMPLANTABLE RADIATION DOSIMETER EACH CALIBRATED MICROCAPILLARY TUBE, EACH MICROCAPILLARY TUBE SEALANT PERITONEAL DIALYSIS CATHETER ANCHORING DEVICE, BEL SYRINGE, WITH OR WITHOUT NEEDLE, EACH SPHYGMOMANOMETER/BLOOD PRESSURE APPARATUS WITH CUF BLOOD PRESSURE CUFF ONLY AUTOMATIC BLOOD PRESSURE MONITOR DISPOSABLE CYCLER SET USED WITH CYCLER DIALYSIS MA DRAINAGE EXTENSION LINE, STERILE, FOR DIALYSIS, EA 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2015 1/1/2007 1/1/2007 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2015 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC A4673 A4674 A4680 A4690 A4706 A4707 A4708 A4709 A4714 A4719 A4720 A4721 A4722 A4723 A4724 A4725 A4726 A4728 A4730 A4736 A4737 A4740 A4750 A4755 A4760 A4765 A4766 A4770 A4771 A4772 A4773 A4774 A4802 A4860 A4870 A4890 A4911 A4913 A4918 A4927 A4928 A4929 A4930 A4931 A4932 A5051 A5052 A5053 A5054 A5055 A5056 A5057 A5061 A5062 A5063 A5071 A5072 A5073 A5081 A5082 A5083 A5093 A5102 A5105 A5112 A5113 A5114 A5120 A5121 A5122 A5126 A5131 A5200 A5500 A5501 A5503 A5504 A5505 A5506 A5507 A5508 A5510 A5512 A5513 A6000 A6010 A6011 A6021 A6022 A6023 A6024 EXTENSION LINE WITH EASY LOCK CONNECTORS, USED WIT CHEMICALS/ANTISEPTICS SOLUTION USED TO CLEAN/STERI ACTIVATED CARBON FILTER FOR HEMODIALYSIS, EACH DIALYZER (ARTIFICIAL KIDNEYS), ALL TYPES, ALL SIZE BICARBONATE CONCENTRATE, SOLUTION, FOR HEMODIALYSI BICARBONATE CONCENTRATE, POWDER, FOR HEMODIALYSIS, ACETATE CONCENTRATE SOLUTION, FOR HEMODIALYSIS, PE ACID CONCENTRATE, SOLUTION, FOR HEMODIALYSIS, PER TREATED WATER (DEIONIZED, DISTILLED, OR REVERSE OS Y SET TUBING FOR PERITONEAL DIALYSIS DIALYSATE SOLUTION, ANY CONCENTRATION OF DEXTROSE, DIALYSATE SOLUTION, ANY CONCENTRATION OF DEXTROSE, DIALYSATE SOLUTION, ANY CONCENTRATION OF DEXTROSE, DIALYSATE SOLUTION, ANY CONCENTRATION OF DEXTROSE, DIALYSATE SOLUTION, ANY CONCENTRATION OF DEXTROSE, DIALYSATE SOLUTION, ANY CONCENTRATION OF DEXTROSE, DIALYSATE SOLUTION, ANY CONCENTRATION OF DEXTROSE, DIALYSATE SOLUTION, NON-DEXTROSE CONTAINING, 500 M FISTULA CANNULATION SET FOR HEMODIALYSIS, EACH TOPICAL ANESTHETIC, FOR DIALYSIS, PER GRAM INJECTABLE ANESTHETIC, FOR DIALYSIS, PER 10 ML SHUNT ACCESSORY, FOR HEMODIALYSIS, ANY TYPE, EACH BLOOD TUBING, ARTERIAL OR VENOUS, FOR HEMODIALYSIS BLOOD TUBING, ARTERIAL AND VENOUS COMBINED, FOR HE DIALYSATE SOLUTION TEST KIT, FOR PERITONEAL DIALYS DIALYSATE CONCENTRATE, POWDER, ADDITIVE FOR PERITO DIALYSATE CONCENTRATE, SOLUTION, ADDITIVE FOR PERI BLOOD COLLECTION TUBE, VACUUM, FOR DIALYSIS, PER 5 SERUM CLOTTING TIME TUBE, FOR DIALYSIS, PER 50 BLOOD GLUCOSE TEST STRIPS, FOR DIALYSIS, PER 50 OCCULT BLOOD TEST STRIPS, FOR DIALYSIS, PER 50 AMMONIA TEST STRIPS, FOR DIALYSIS, PER 50 PROTAMINE SULFATE, FOR HEMODIALYSIS, PER 50 MG DISPOSABLE CATHETER TIPS FOR PERITONEAL DIALYSIS, PLUMBING AND/OR ELECTRICAL WORK FOR HOME HEMODIALY CONTRACTS, REPAIR AND MAINTENANCE, FOR HEMODIALYSI DRAIN BAG/BOTTLE, FOR DIALYSIS, EACH MISCELLANEOUS DIALYSIS SUPPLIES, NOT OTHERWISE SPE VENOUS PRESSURE CLAMP, FOR HEMODIALYSIS, EACH GLOVES, NON-STERILE, PER 100 SURGICAL MASK, PER 20 TOURNIQUET FOR DIALYSIS, EACH GLOVES, STERILE, PER PAIR ORAL THERMOMETER, REUSABLE, ANY TYPE, EACH RECTAL THERMOMETER, REUSABLE, ANY TYPE, EACH OSTOMY POUCH, CLOSED; WITH BARRIER ATTACHED (1 PIE OSTOMY POUCH, CLOSED; WITHOUT BARRIER ATTACHED (1 OSTOMY POUCH, CLOSED; FOR USE ON FACEPLATE, EACH OSTOMY POUCH, CLOSED; FOR USE ON BARRIER WITH FLAN STOMA CAP OST POUCH DRAINABLE EXT WEAR BARRIER W/FILTER EA OST POUCH DRAINABL EXT WEAR BARR CONVXTY FLTR EA OSTOMY POUCH, DRAINABLE; WITH BARRIER ATTACHED, (1 OSTOMY POUCH, DRAINABLE; WITHOUT BARRIER ATTACHED OSTOMY POUCH, DRAINABLE; FOR USE ON BARRIER WITH F OSTOMY POUCH, URINARY; WITH BARRIER ATTACHED (1 PI OSTOMY POUCH, URINARY; WITHOUT BARRIER ATTACHED (1 OSTOMY POUCH, URINARY; FOR USE ON BARRIER WITH FLA STOMA PLUG OR SEAL ANY TYPE CONTINENT DEVICE; CATHETER FOR CONTINENT STOMA CONTINENT DEVICE STOMA ABSORPTIVE COVER STOMA OSTOMY ACCESSORY; CONVEX INSERT BEDSIDE DRAINAGE BOTTLE WITH OR WITHOUT TUBING, RI URINARY SUSPENSORY; WITH LEG BAG, WITH OR WITHOUT URINARY DRAINAGE BAG LEG OR ABDOMEN LATEX EACH LEG STRAP; LATEX, REPLACEMENT ONLY, PER SET LEG STRAP; FOAM OR FABRIC, REPLACEMENT ONLY, PER SKIN BARRIER, WIPES OR SWABS, EACH SKIN BARRIER; SOLID, 6 X 6 OR EQUIVALENT, EACH SKIN BARRIER; SOLID, 8 X 8 OR EQUIVALENT, EACH ADHESIVE OR NON-ADHESIVE; DISK OR FOAM PAD APPLIANCE CLEANER, INCONTINENCE AND OSTOMY APPLIAN PERCUTANEOUS CATHETER/TUBE ANCHORING DEVICE, ADHES FOR DIABETICS ONLY, FITTING (INCLUDING FOLLOW-UP), FOR DIABETICS ONLY, FITTING (INCLUDING FOLLOW-UP), FOR DIABETICS ONLY, MODIFICATION (INCLUDING FITTIN FOR DIABETICS ONLY, MODIFICATION (INCLUDING FITTIN FOR DIABETICS ONLY, MODIFICATION (INCLUDING FITTIN FOR DIABETICS ONLY, MODIFICATION (INCLUDING FITTIN FOR DIABETICS ONLY, NOT OTHERWISE SPECIFIED MODIFI FOR DIABETICS ONLY, DELUXE FEATURE OF OFF-THE-SHEL FOR DIABETICS ONLY, DIRECT FORMED, COMPRESSION MOL For diabetics only, multiple density insert, direc FOR DIABETICS ONLY,MULTIPLE DENSITY INSERT,CUSTOM NON-CONTACT WOUND WARMING WOUND COVER FOR USE WITH COLLAGEN BASED WOUND FILLER, DRY FORM, PER GRAM OF COLLEGEN BASED WOUND FILLR GEL/PASTE STERL PER G COLLAGEN DRESSING, PAD SIZE 16 SQ. IN. OR LESS, EA COLLAGEN DRESSING, PAD SIZE MORE THAN 16 SQ. IN. B COLLAGEN DRESSING, PAD SIZE MORE THAN 48 SQ. IN., COLLAGEN DRESSING WOUND FILLER, PER 6 INCHES 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC A6025 A6154 A6196 A6197 A6198 A6199 A6203 A6204 A6205 A6206 A6207 A6208 A6209 A6210 A6211 A6212 A6213 A6214 A6215 A6216 A6217 A6218 A6219 A6220 A6221 A6222 A6223 A6224 A6228 A6229 A6230 A6231 A6232 A6233 A6234 A6235 A6236 A6237 A6238 A6239 A6240 A6241 A6242 A6243 A6244 A6245 A6246 A6247 A6248 A6250 A6251 A6252 A6253 A6254 A6255 A6256 A6257 A6258 A6259 A6260 A6261 A6262 A6266 A6402 A6403 A6404 A6407 A6410 A6411 A6412 A6413 A6441 A6442 A6443 A6444 A6445 A6446 A6447 A6448 A6449 A6450 A6451 A6452 A6453 A6454 A6455 A6456 A6457 A6501 A6502 A6503 GEL SHEET FOR DERMAL OR EPIDERMAL APPLICATION, (E. WOUND POUCH, EACH ALGINATE OR OTHER FIBER GELLING DRESSING, WOUND CO ALGINATE OR OTHER FIBER GELLING DRESSING, WOUND CO ALGINATE OR OTHER FIBER GELLING DRESSING, WOUND CO ALGINATE OR OTHER FIBER GELLING DRESSING, WOUND FI COMPOSITE DRESSING, PAD SIZE 16 SQ. IN. OR LESS, W COMPOSITE DRESSING, PAD SIZE MORE THAN 16 SQ. IN. COMPOSITE DRESSING, PAD SIZE MORE THAN 48 SQ. IN., CONTACT LAYER, 16 SQ. IN. OR LESS, EACH DRESSING CONTACT LAYER, MORE THAN 16 SQ. IN. BUT LESS THAN CONTACT LAYER, MORE THAN 48 SQ. IN., EACH DRESSING FOAM DRESSING, WOUND COVER, PAD SIZE 16 SQ. IN. OR FOAM DRESSING, WOUND COVER, PAD SIZE MORE THAN 16 FOAM DRESSING, WOUND COVER, PAD SIZE MORE THAN 48 FOAM DRESSING, WOUND COVER, PAD SIZE 16 SQ. IN. OR FOAM DRESSING, WOUND COVER, PAD SIZE MORE THAN 16 FOAM DRESSING, WOUND COVER, PAD SIZE MORE THAN 48 FOAM DRESSING, WOUND FILLER, PER GRAM GAUZE, NON-IMPREGNATED, NON-STERILE, PAD SIZE 16 S GAUZE, NON-IMPREGNATED, NON-STERILE, PAD SIZE MORE GAUZE, NON-IMPREGNATED, NON-STERILE, PAD SIZE MORE GAUZE, NON-IMPREGNATED, PAD SIZE 16 SQ. IN. OR LES GAUZE, NON-IMPREGNATED, PAD SIZE MORE THAN 16 SQ. GAUZE, NON-IMPREGNATED, PAD SIZE MORE THAN 48 SQ. GAUZE, IMPREGNATED WITH OTHER THAN WATER, NORMAL S GAUZE, IMPREGNATED WITH OTHER THAN WATER, NORMAL S GAUZE, IMPREGNATED WITH OTHER THAN WATER, NORMAL S GAUZE, IMPREGNATED, WATER OR NORMAL SALINE, PAD SI GAUZE, IMPREGNATED, WATER OR NORMAL SALINE, PAD SI GAUZE, IMPREGNATED, WATER OR NORMAL SALINE, PAD SI GAUZE, IMPREGNATED, HYDROGEL, FOR DIRECT WOUND CON GAUZE, IMPREGNATED, HYDROGEL, FOR DIRECT WOUND CON GAUZE, IMPREGNATED, HYDROGEL FOR DIRECT WOUND CONT HYDROCOLLOID DRESSING, WOUND COVER, PAD SIZE 16 SQ HYDROCOLLOID DRESSING, WOUND COVER, PAD SIZE MORE HYDROCOLLOID DRESSING, WOUND COVER, PAD SIZE MORE HYDROCOLLOID DRESSING, WOUND COVER, PAD SIZE 16 SQ HYDROCOLLOID DRESSING, WOUND COVER, PAD SIZE MORE HYDROCOLLOID DRESSING, WOUND COVER, PAD SIZE MORE HYDROCOLLOID DRESSING, WOUND FILLER, PASTE, PER FL HYDROCOLLOID DRESSING, WOUND FILLER, DRY FORM, PER HYDROGEL DRESSING, WOUND COVER, PAD SIZE 16 SQ. IN HYDROGEL DRESSING, WOUND COVER, PAD SIZE MORE THAN HYDROGEL DRESSING, WOUND COVER, PAD SIZE MORE THAN HYDROGEL DRESSING, WOUND COVER, PAD SIZE 16 SQ. IN HYDROGEL DRESSING, WOUND COVER, PAD SIZE MORE THAN HYDROGEL DRESSING, WOUND COVER, PAD SIZE MORE THAN HYDROGEL DRESSING WOUND FILLER GEL PER FL OZ SKIN SEALANTS, PROTECTANTS, MOISTURIZERS, OINTMENT SPECIALTY ABSORPTIVE DRESSING, WOUND COVER, PAD SI SPECIALTY ABSORPTIVE DRESSING, WOUND COVER, PAD SI SPECIALTY ABSORPTIVE DRESSING, WOUND COVER, PAD SI SPECIALTY ABSORPTIVE DRESSING, WOUND COVER, PAD SI SPECIALTY ABSORPTIVE DRESSING, WOUND COVER, PAD SI SPECIALTY ABSORPTIVE DRESSING, WOUND COVER, PAD SI TRANSPARENT FILM, 16 SQ. IN. OR LESS, EACH DRESSIN TRANSPARENT FILM, MORE THAN 16 SQ. IN. BUT LESS TH TRANSPARENT FILM, MORE THAN 48 SQ. IN., EACH DRESS WOUND CLEANSERS ANY TYPE ANY SIZE WOUND FILLER GEL/PASTE PER FL OZ NOS WOUND FILLER DRY FORM PER G NOT OTHERWISE SPEC GAUZE, IMPREGNATED, OTHER THAN WATER, NORMAL SALIN GAUZE, NON-IMPREGNATED, STERILE, PAD SIZE 16 SQ. I GAUZE, NON-IMPREGNATED, STERILE, PAD SIZE MORE THA GAUZE, NON-IMPREGNATED, STERILE, PAD SIZE MORE THA PACKING STRIPS, NON-IMPREGNATED, UP TO 2 INCHES IN EYE PAD, STERILE, EACH EYE PAD, NON-STERILE, EACH EYE PATCH, OCCLUSIVE, EACH ADHESIVE BANDAGE FIRST-AID TYPE ANY SIZE EACH PADDING BANDAGE, NON-ELASTIC, NON-WOVEN/NON-KNITTE CONFORMING BANDAGE, NON-ELASTIC, KNITTED/WOVEN, NO CONFORMING BANDAGE, NON-ELASTIC, KNITTED/WOVEN, NO CONFORMING BANDAGE, NON-ELASTIC, KNITTED/WOVEN, NO CONFORMING BANDAGE, NON-ELASTIC, KNITTED/WOVEN, ST CONFORMING BANDAGE, NON-ELASTIC, KNITTED/WOVEN, ST CONFORMING BANDAGE, NON-ELASTIC, KNITTED/WOVEN, ST LIGHT COMPRESSION BANDAGE, ELASTIC, KNITTED/WOVEN, LIGHT COMPRESSION BANDAGE, ELASTIC, KNITTED/WOVEN, LIGHT COMPRESSION BANDAGE, ELASTIC, KNITTED/WOVEN, MODERATE COMPRESSION BANDAGE, ELASTIC, KNITTED/WOV HIGH COMPRESSION BANDAGE, ELASTIC, KNITTED/WOVEN, SELF-ADHERENT BANDAGE, ELASTIC, NON-KNITTED/NON-WO SELF-ADHERENT BANDAGE, ELASTIC, NON-KNITTED/NON-WO SELF-ADHERENT BANDAGE, ELASTIC, NON-KNITTED/NON-WO ZINC PASTE IMPREGNATED BANDAGE, NON-ELASTIC, KNITT TUBULAR DRESSING W/WO ELEASTIC, ANY WIDTH, PER LIN COMPRESSION BURN GARMENT, BODYSUIT (HEAD TO FOOT), COMPRESSION BURN GARMENT, CHIN STRAP, CUSTOM FABRI COMPRESSION BURN GARMENT, FACIAL HOOD, CUSTOM FABR 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC A6504 A6505 A6506 A6507 A6508 A6509 A6510 A6511 A6512 A6513 A6530 A6531 A6532 A6533 A6534 A6535 A6536 A6537 A6538 A6539 A6540 A6541 A6544 A6545 A6549 A6550 A7000 A7001 A7002 A7003 A7004 A7005 A7006 A7007 A7008 A7009 A7010 A7011 A7012 A7013 A7014 A7015 A7016 A7017 A7018 A7020 A7025 A7026 A7027 A7028 A7029 A7030 A7031 A7032 A7033 A7034 A7035 A7036 A7037 A7038 A7039 A7040 A7041 A7044 A7045 A7046 A7047 A7048 A7501 A7502 A7503 A7504 A7505 A7506 A7507 A7508 A7509 A7520 A7521 A7522 A7523 A7524 A7525 A7526 A7527 A8000 A8001 A8002 A8003 A8004 A9150 COMPRESSION BURN GARMENT, GLOVE TO WRIST, CUSTOM F COMPRESSION BURN GARMENT, GLOVE TO ELBOW, CUSTOM F COMPRESSION BURN GARMENT, GLOVE TO AXILLA, CUSTOM COMPRESSION BURN GARMENT, FOOT TO KNEE LENGTH, CUS COMPRESSION BURN GARMENT, FOOT TO THIGH LENGTH, CU COMPRESSION BURN GARMENT, UPPER TRUNK TO WAIST INC COMPRESSION BURN GARMENT, TRUNK, INCLUDING ARMS DO COMPRESSION BURN GARMENT, LOWER TRUNK INCLUDING LE COMPRESSION BURN GARMENT, NOT OTHERWISE CLASSIFIED COMPRESSION BURN MASK,FACE AND/OR NEC, PLASTICS Gradient compression stocking, below knee, GRADIENT COMPRESSION STOCKING, BELOW KNEE GRADIENT COMPRESSION STOCKING, BELOW KNEE GRADIENT COMPRESSION STOCKING, THIGH LENGTH GRADIENT COMPRESSION STOCKING, THIGH LENGTH GRADIENT COMPRESSION STOCKING, THIGH LENGTH GRADIENT COMPRESSION STOCKING, FULL LENGTH/CHAP ST GRADIENT COMPRESSION STOCKING, FULL LENGTH/CHAP ST GRADIENT COMPRESSION STOCKING, FULL LENGTH/CHAP ST GRADIENT COMPRESSION STOCKING, WAIST LENGTH, 18-30 GRADIENT COMPRESSION STOCKING,WAIST LENGTH, 30-40GRADIENT COMPRESSION STOCKING, WAIST LEGNTH, 40-50 GRADIENT COMPRESSION STOCKING, GARTER BELT GRADIENT COMPRS WRAP NONELAST BK 30-50 MM HG EA GRADIENT COMPRESSION STOCKING/SLEEVE NOS DRESSING SET FOR NEGATIVE PRESSURE WOUND THERAPY E CANISTER, DISPOSABLE, USED WITH SUCTION PUMP, EACH CANISTER, NON-DISPOSABLE, USED WITH SUCTION PUMP, TUBING, USED WITH SUCTION PUMP, EACH ADMINISTRATION SET, WITH SMALL VOLUME NONFILTERED SMALL VOLUME NONFILTERED PNEUMATIC NEBULIZER, DISP ADMINISTRATION SET, WITH SMALL VOLUME NONFILTERED ADMINISTRATION SET, WITH SMALL VOLUME FILTERED PNE LARGE VOLUME NEBULIZER, DISPOSABLE, UNFILLED, USED LARGE VOLUME NEBULIZER, DISPOSABLE, PREFILLED, USE RESERVOIR BOTTLE, NON-DISPOSABLE, USED WITH LARGE CORRUGATED TUBING, DISPOSABLE, USED WITH LARGE VOL CORRUGATED TUBING, NON-DISPOSABLE, USED WITH LARGE WATER COLLECTION DEVICE, USED WITH LARGE VOLUME NE FILTER DISPOSABL W/AREOSOL COMPRESS/US GENERATOR FILTER, NONDISPOSABLE, USED WITH AEROSOL COMPRESSO AEROSOL MASK, USED WITH DME NEBULIZER DOME AND MOUTHPIECE, USED WITH SMALL VOLUME ULTRAS NEBULIZER, DURABLE, GLASS OR AUTOCLAVABLE PLASTIC, WATER, DISTILLED, USED WITH LARGE VOLUME NEBULIZER INTERFACE COUGH STIMULAT DEVC REPLACEMENT ONLY HIGH FREQUENCY CHEST WALL OSCILLATION SYSTEM VEST, HIGH FREQUENCY CHEST WALL OSCILLATION SYSTEM HOSE, COMB ORAL/NASAL MASK USED W/CPAP DEVICE EACH ORAL CUSHION COMB ORAL/NASAL MASK REPL ONLY EACH NASAL PILLOWS COMB ORAL/NASL MASK REPL ONLY PAIR FULL FACE MASK USED WITH POSITIVE AIRWAY PRESSURE FACE MASK INTERFACE, REPLACEMENT FOR FULL FACE MAS REPLACEMENT CUSHION FOR NASAL APPLICATION DEVICE, REPLACEMENT PILLOWS FOR NASAL APPLICATION DEVICE, NASAL INTERFACE (MASK OR CANNULA TYPE) USED WITH P HEADGEAR USED WITH POSITIVE AIRWAY PRESSURE DEVICE CHINSTRAP USED WITH POSITIVE AIRWAY PRESSURE DEVIC TUBING USED WITH POSITIVE AIRWAY PRESSURE DEVICE FILTER, DISPOSABLE, USED WITH POSITIVE AIRWAY PRES FILTER, NON DISPOSABLE, USED WITH POSITIVE AIRWAY ONE WAY CHEST DRAIN VALVE WATER SEAL DRAINAGE CONTAINER AND TUBING FOR USE W ORAL INTERFACE USED WITH POSITIVE AIRWAY PRESSURE EXHALATION PORT WITH OR WITHOUT SWIVEL USED WITH A WATER CHAMBER FOR HUMIDIFIER, USED WITH POSITIVE A ORAL INTERFACE USED RESPIRATORY SUCTION PUMP EA VACUUM DRAINAGE COLLECTION UNIT & TUBING KIT EA TRACHEOSTOMA VALVE, INCLUDING DIAPHRAGM, EACH REPLACEMENT DIAPHRAGM/FACEPLATE FOR TRACHEOSTOMA V FILTER HOLDER OR FILTER CAP, REUSABLE, FOR USE IN FILTER FOR USE IN A TRACHEOSTOMA HEAT AND MOISTURE HOUSING, REUSABLE WITHOUT ADHESIVE, FOR USE IN A H ADHESIVE DISC FOR USE IN A HEAT AND MOISTURE EXCHA FILTER HOLDER AND INTEGRATED FILTER WITHOUT ADHESI HOUSING AND INTEGRATED ADHESIVE, FOR USE IN A TRAC FILTER HOLDER AND INTEGRATED FILTER HOUSING, AND A TRACHEOSTOMY/LARYNGECTOMY TUBE, NON-CUFFED, POLYVI TRACHEOSTOMY/LARYNGECTOMY TUBE, CUFFED, POLYVINYLC TRACHEOSTOMY/LARYNGECTOMY TUBE, STAINLESS STEEL OR TRACHEOSTOMY SHOWER PROTECTOR, EACH TRACHEOSTOMA STENT/STUD/BUTTON, EACH TRACHEOSTOMY MASK, EACH TRACHEOSTOMY TUBE COLLAR/HOLDER, EACH TRACHEOSTOMY/LARYNGECTOMY TUBE PLUG/STOP, EACH Soft protect helmet prefab Hard protect helmet prefab Soft protect helmet custom Hard protect helmet custom Repl soft interface, helmet NON-PRESCRIPTION DRUGS 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2009 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N Y N N N Y Y Y Y N Y N N Y N N N N N N N N N N N N N N N N N N N N N Y Y N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC A9152 A9153 A9155 A9180 A9270 A9272 A9273 A9274 A9275 A9276 A9277 A9278 A9279 A9280 A9282 A9283 A9284 A9300 A9500 A9501 A9502 A9503 A9504 A9505 A9507 A9508 A9509 A9510 A9512 A9516 A9517 A9520 A9521 A9524 A9526 A9527 A9528 A9529 A9530 A9531 A9532 A9537 A9538 A9539 A9540 A9541 A9547 A9552 A9555 A9558 A9560 A9561 A9562 A9567 A9568 A9569 A9570 A9571 A9572 A9575 A9576 A9577 A9578 A9579 A9580 A9581 A9582 A9583 A9584 A9585 A9586 A9599 A9600 A9604 A9606 A9698 A9699 A9700 A9900 A9901 A9999 B4034 B4035 B4036 B4081 B4082 B4083 B4087 B4088 B4100 B4102 SINGLE VITAMIN/MINERAL/TRACE ELEMENT, ORAL, PER DO MULTIPLE VITAMINS, WITH OR WITHOUT MINERALS AND TR ARTFICIAL SALIVA 30 ML PEDICULOSIS (LICE INFESTATION) TREATMENT, TOPICAL, NON-COVERED ITEM OR SERVICE WND SUCT DISPBL DSG ALL ACC & CMPNT ANY TYP EA HOT WATER BOTTLE ICE CAP OR COLLAR ANY TYPE EXTERNAL AMB INSULIN DEL SYSTEM DISPOSABLE EA HOME GLUCOSE DISPOSABLE MONITOR, INCLUDES TEST STRIPS. SENSOR;INVSV DISP INTRSTL CONT GLU MON SYS 1U=1D TRANSMITTER; EXT INTERSTITIAL CONT GLU MON SYS RECEIVER MON; EXT INTERSTITIAL CONT GLU MON SYS Monitoring feature/deviceNOC ALERT OR ALARM DEVICE, NOT OTHERWISE CLASSIFIED WIG, ANY TYPE, EACH FOOT PRESSURE OFF LOAD/SUPP DEVICE ANY TYPE EACH SPIROMETER NONELECTRONIC INCL ALL ACCESSORIES EXERCISE EQUIPMENT TECHNETIUM TC-99M SESTAMIBI DX PER STUDY DOSE TECHNETIUM TC-99M TEBOROXIME DX PER STUDY DOSE SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A IODINE I-123 SODIUM IODIDE DX PER MILLICURIE SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL THERAPEUTIC IMAGING TECHNETIUM TC-99M TILMANOCEPT DX TO 0.5 MCI SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC IMAGING A Iodine I-125 sodium iodide SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC AGENT, ISUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC AGENT, ISUPPLY OF RADIOPHARMACEUTICAL THERAPEUTIC AGENT, I SUPPLY OF RADIOPHARMACEUTICAL DIAGNOSTIC AGENT, ISUPPLY OF RADIOPHARMACEUTICAL THERAPEUTIC AGENT, I Technetium tc-99m mebrofenin, diagnostic, per stud TECHMETIUM TC-99M MEBROFENIN, DIAGNOSTIC, PER STUD TECHNETIUM 99M PENTETATE DIAG PER STUDY DOSE UP TO 25 MILLICURIES TECHNETIUM TC-99M MACROAGGREGATED ALBUMIN, DIAG, PER STUDY DOES, UP TO 10 MILL TECHNETIUM TC-99M SULFUR COLLOID, DIAGNOSTIC, PER Indium in-111 oxyquinoline, diagnostic, per 0.5 mi Fluorodeoxyglucose f-s8 fdg, diagnostic, per study RUBIDIUM RB-82 DIAGNOSTIC PER STUDY DOSE UP TO 60 MILLICURIES XENON XE-133 GAS, DIAGNOSTIC, PER 10 MILLICURIES COBALT TC-99M LABELED RED BLOOD CELLS, DIAGNOSTIC, Technetium tc-99m oxidronate, diagnostic, per stud Technetium tc-99m oxidronate, diagnostic TECHNETIUM TC-99M PENTETATE, DIAGNOSTIC, AEROSOL, PER STUDY DOSE, UP TO 75 MILLCU Technetium tc99m arcitumomab TECHNETIUM TC-99M EXAMETAZIME AUTOLG WBC DX DOSE INDIUM IN-111 AUTOLOGOUS WBC DX PER STUDY DOSE INDIUM IN-111 AUTOLOGOUS PLATELETS DX STUDY DOSE INDIUM IN-111 PENTETREOTIDE DX DOSE TO 6 MCI INJECTION GADOTERATE MEGLUMINE 0.1 ML INJECTION GADOTERIDOL PROHANCE MULTIPACK PER ML INJ GADOBENATE DIMEGLUMINE MULTIHANCE PER ML INJ GADOBENATE DIMEGLUMINE MXHANCE MXPACK PER ML INJECTION, GADOLINIUM-BASED MAGNETIC RESONANCE CON SODIUM FLUORIDE F-18 DX PER STUDY DOSE TO 30 MCI INJECTION GADOXETATE DISODIUM 1 ML IODINE I-123 IOBENGUANE DX STUDY DOSE TO 15 MCI INJECTION GADOFOSVESET TRISODIUM 1 ML IODINE I-123 IOFLUPANE DX-STUDY DOSE UP 5 MCI INJECTION GADOBUTROL 0.1 ML FLORBETAPIR F18 DX PER STUDY DOSE UP TO 10 MCI RADIOPHRM DX BETA-AMYLOID PET IMAG PER STDY DOSE SUPPLY OF THERAPEUTIC RADIOPHARMACEUTICAL, STRONTI SAMARIUM SM-153 LEXIDRONAM TX DOSE TO 150 MCI RADIUM RA-223 DICHLORIDE THERAPEUTIC PER UCI NON-RADIOACTIVE CONTRAST IMAGING MATERIAL, NOT OTHERWISE CLASSIFIED, PER STUDY SUPPLY OF RADIOPHARMACEUTICAL THERAPEUTIC IMAGING SUPPLY OF INJECTABLE CONTRAST MATERIAL FOR USE IN MISCELLANEOUS DME SUPPLY, ACCESSORY, AND/OR SERVIC DME DELIVERY, SET UP, AND/OR DISPENSING SERVICE CO MISCELLANEOUS DME SUPPLY OR ACCESSORY, NOT OTHERWI ENTERAL FEEDING SUPPLY KIT; SYRINGE FED PER DAY ENTERAL FEEDING SUPPLY KIT; PUMP FED PER DAY ENTERAL FEEDING SUPPLY KIT; GRAVITY FED PER DAY NASOGASTRIC TUBING WITH STYLET NASOGASTRIC TUBING WITHOUT STYLET STOMACH TUBE - LEVINE TYPE GASTROSTOMY/J-TUBE STANDARD ANY MATERIAL/TYPE EA GASTROSTOMY/J-TUBE LOW-PROFILE ANY MAT/TYPE EACH FOOD THICKENER, ADMINISTERED ORALLY, PER OUNCE ENTERAL FORMULA, FOR ADULTS, USED TO REPLACE FLUID 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2012 1/1/2011 1/1/2008 1/1/2013 1/1/2008 1/1/2008 1/1/2008 1/1/2007 1/1/2004 1/1/2007 1/1/2008 1/1/2009 1/1/2004 1/1/2010 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2014 1/1/2007 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2014 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2009 1/1/2010 1/1/2010 1/1/2010 1/1/2012 1/1/2012 1/1/2013 1/1/2014 1/1/2004 1/1/2010 1/1/2015 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y Y Y Y Y Y Y HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC B4103 B4104 B4149 B4150 B4152 B4153 B4154 B4155 B4157 B4158 B4159 B4160 B4161 B4162 B4164 B4168 B4172 B4176 B4178 B4180 B4185 B4189 B4193 B4197 B4199 B4216 B4220 B4222 B4224 B5000 B5100 B5200 B9000 B9002 B9004 B9006 B9998 B9999 C1713 C1714 C1715 C1716 C1717 C1719 C1721 C1722 C1724 C1725 C1726 C1727 C1728 C1729 C1730 C1731 C1732 C1733 C1749 C1750 C1751 C1752 C1753 C1754 C1755 C1756 C1757 C1758 C1759 C1760 C1762 C1763 C1764 C1765 C1766 C1767 C1768 C1769 C1770 C1771 C1772 C1773 C1776 C1777 C1778 C1779 C1780 C1781 C1782 C1783 C1784 C1785 C1786 ENTERAL FORMULA, FOR PEDIATRICS, USED TO REPLACE F ADDITIVE FOR ENTERAL FORMULA (E.G. FIBER) ENTERAL FORMULA, BLENDERIZED NATURAL FOODS WITH IN ENTERAL FORMULA, NUTRITIONALLY COMPLETE WITH INTAC ENTERAL FORMULA, NUTRITIONALLY COMPLETE, CALORICAL ENTERAL FORMULA, NUTRITIONALLY COMPLETE, HYDROLYZE ENTERAL FORMULA, NUTRITIONALLY COMPLETE, FOR SPECI ENTERAL FORMULA, NUTRITIONALLY INCOMPLETE/MODULAR ENTERAL FORMULA, NUTRITIONALLY COMPLETE, FOR SPECI ENTERAL FORMULA, FOR PEDIATRICS, NUTRITIONALLY COM ENTERAL FORMULA, FOR PEDIATRICS, NUTRITIONALLY COM ENTERAL FORMULA, FOR PEDIATRICS, NUTRITIONALLY COM ENTERAL FORMULA, FOR PEDIATRICS, HYDROLYZED/AMINO ENTERAL FORMULA, FOR PEDIATRICS, SPECIAL METABOLIC PARENTERAL NUTRITION SOLUTION: CARBOHYDRATES (DEX PARENTERAL NUTRITION SOLUTION; AMINO ACID, 3.5 PER PARENTERAL NUTRITION SOLUTION; AMINO ACID, 5.5 PER PARENTERAL NUTRITION SOLUTION; AMINO ACID, 7 PER C PARENTERAL NUTRITION SOLUTION: AMINO ACID, GREATER PARENTERAL NUTRITION SOLUTION; CARBOHYDRATES (DEXT Parenteral nutrition solution, per 10 grams lipids PARENTERAL NUTRITION SOLUTION; COMPOUNDED AMINO AC PARENTERAL NUTRITION SOLUTION; COMPOUNDED AMINO AC PARENTERAL NUTRITION SOLUTION; COMPOUNDED AMINO AC PARENTERAL NUTRITION SOLUTION; COMPOUNDED AMINO AC PARENTERAL NUTRITION; ADDITIVES (VITAMINS, TRACE E PARENTERAL NUTRITION SUPPLY KIT; PREMIX, PER DAY PARENTERAL NUTRITION SUPPLY KIT; HOME MIX, PER DAY PARENTERAL NUTRITION ADMINISTRATION KIT, PER DAY PARENTERAL NUTRITION SOLUTION: COMPOUNDED AMINO A PARENTERAL NUTRITION SOLUTION: COMPOUNDED AMINO AC PARENTERAL NUTRITION SOLUTION: COMPOUNDED AMINO A ENTERAL NUTRITION INFUSION PUMP - WITHOUT ALARM ENTERAL NUTRITION INFUSION PUMP - WITH ALARM PARENTERAL NUTRITION INFUSION PUMP, PORTABLE PARENTERAL NUTRITION INFUSION PUMP, STATIONARY NOC FOR ENTERAL SUPPLIES NOC FOR PARENTERAL SUPPLIES ANCHOR/SCREW FOR OPPOSING BONE-TO-BONE OR SOFT TIS CATHETER, TRANSLUMINAL ATHERECTOMY, DIRECTIONAL BRACHYTHERAPY NEEDLE BRACHYTHERAPY SOURCE, GOLD 198, PER SOURCE BRACHYTHERAPY SOURCE, HIGH DOSE RATE IRIDIUM 192, BRACHYTHERAPY SOURCE, NON-HIGH DOSE RATE IRIDIUM 1 CARDIOVERTER-DEFIBRILLATOR, DUAL CHAMBER (IMPLANTA CARDIOVERTER-DEFIBRILLATOR, SINGLE CHAMBER (IMPLAN CATHETER, TRANSLUMINAL ATHERECTOMY, ROTATIONAL CATHETER, TRANSLUMINAL ANGIOPLASTY, NON-LASER (MAY CATHETER, BALLOON DILATATION, NON-VASCULAR CATHETER, BALLOON TISSUE DISSECTOR, NON-VASCULAR ( CATHETER, BRACHYTHERAPY SEED ADMINISTRATION CATHETER, DRAINAGE CATHETER, ELECTROPHYSIOLOGY, DIAGNOSTIC, OTHER THA CATHETER, ELECTROPHYSIOLOGY, DIAGNOSTIC, OTHER THA CATHETER, ELECTROPHYSIOLOGY, DIAGNOSTIC/ABLATION, CATHETER, ELECTROPHYSIOLOGY, DIAGNOSTIC/ABLATION, ENDO RETRO IMAG/ILLUMINATION COLONOSCOPE DEVICE CATHETER, HEMODIALYSIS, LONG-TERM CATHETER, INFUSION, INSERTED PERIPHERALLY, CENTRAL CATHETER, HEMODIALYSIS, SHORT-TERM CATHETER, INTRAVASCULAR ULTRASOUND CATHETER, INTRADISCAL CATHETER, INTRASPINAL CATHETER, PACING, TRANSESOPHAGEAL CATHETER, THROMBECTOMY/EMBOLECTOMY CATHETER, URETERAL CATHETER, INTRACARDIAC ECHOCARDIOGRAPHY CLOSURE DEVICE, VASCULAR (IMPLANTABLE/INSERTABLE) CONNECTIVE TISSUE, HUMAN (INCLUDES FASCIA LATA) CONNECTIVE TISSUE, NON-HUMAN (INCLUDES SYNTHETIC) EVENT RECORDER, CARDIAC (IMPLANTABLE) ADHESION BARRIER INTRODUCER/SHEATH, GUIDING, INTRACARDIAC ELECTROPH GENERATOR, NEUROSTIMULATOR (IMPLANTABLE) GRAFT, VASCULAR GUIDE WIRE IMAGING COIL, MAGNETIC RESONANCE (INSERTABLE) REPAIR DEVICE, URINARY, INCONTINENCE, WITH SLING G INFUSION PUMP, PROGRAMMABLE (IMPLANTABLE) RETRIEVAL DEVICE, INSERTABLE (USED TO RETRIEVE FRA JOINT DEVICE (IMPLANTABLE) LEAD, CARDIOVERTER-DEFIBRILLATOR, ENDOCARDIAL SING LEAD, NEUROSTIMULATOR (IMPLANTABLE) LEAD, PACEMAKER, TRANSVENOUS VDD SINGLE PASS LENS, INTRAOCULAR (NEW TECHNOLOGY) MESH (IMPLANTABLE) MORCELLATOR OCULAR IMPLANT, AQUEOUS DRAINAGE ASSIST DEVICE OCULAR DEVICE, INTRAOPERATIVE, DETACHED RETINA PACEMAKER, DUAL CHAMBER, RATE-RESPONSIVE (IMPLANTA PACEMAKER, SINGLE CHAMBER, RATE-RESPONSIVE (IMPLAN 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 10/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC C1787 C1788 C1789 C1813 C1814 C1815 C1816 C1817 C1818 C1819 C1820 C1821 C1822 C1830 C1840 C1841 C1874 C1875 C1876 C1877 C1878 C1880 C1881 C1882 C1883 C1884 C1885 C1886 C1887 C1888 C1891 C1892 C1893 C1894 C1895 C1896 C1897 C1898 C1899 C1900 C2613 C2614 C2615 C2616 C2617 C2618 C2619 C2620 C2621 C2622 C2623 C2624 C2625 C2626 C2627 C2628 C2629 C2630 C2631 C2639 C2644 C2645 C5271 C5272 C5273 C5274 C5275 C5276 C5277 C5278 C8900 C8901 C8902 C8903 C8904 C8905 C8906 C8907 C8908 C8909 C8910 C8911 C8912 C8913 C8914 C8918 C8919 C8920 C8921 C8922 C8923 PATIENT PROGRAMMER, NEUROSTIMULATOR PORT, INDWELLING (IMPLANTABLE) PROSTHESIS, BREAST (IMPLANTABLE) PROSTHESIS, PENILE, INFLATABLE RETINAL TAMPONADE DEVICE, SILICONE OIL PROSTHESIS, URINARY SPHINCTER (IMPLANTABLE) RECEIVER AND/OR TRANSMITTER, NEUROSTIMULATOR (IMPL SEPTAL DEFECT IMPLANT SYSTEM, INTRACARDIAC INTEGRATED KERATOPROSTHESIS SURGICAL TISSUE LOCALIZATION AND EXCISION DEVICE ( GENERATOR NEURO RECHG BAT SYSTEM Interspinous implant GENERATOR, NEUROSTIMULATOR (IMPLANTABLE), HIGH FREQUENCY, WITH RECHARGEABLE B POWERED BONE MARROW BIOPSY NEEDLE LENS INTRAOCULAR TELESCOPIC RETINAL PROSTH INCL ALL INTRL & EXTERNL CMPNT STENT, COATED/COVERED, WITH DELIVERY SYSTEM STENT, COATED/COVERED, WITHOUT DELIVERY SYSTEM STENT, NON-COATED/NON-COVERED, WITH DELIVERY SYSTE STENT, NON-COATED/NON-COVERED, WITHOUT DELIVERY SY MATERIAL FOR VOCAL CORD MEDIALIZATION, SYNTHETIC ( VENA CAVA FILTER DIALYSIS ACCESS SYSTEM (IMPLANTABLE) CARDIOVERTER-DEFIBRILLATOR, OTHER THAN SINGLE OR D ADAPTOR/EXTENSION, PACING LEAD OR NEUROSTIMULATOR EMBOLIZATION PROTECTIVE SYSTEM CATHETER, TRANSLUMINAL ANGIOPLASTY, LASER CATH EXTRAVASCULAR TISSUE ABLAT MODAL INSERTABLE CATHETER, GUIDING (MAY INCLUDE INFUSION/PERFUSION CATHETER, ABLATION, NON-CARDIAC, ENDOVASCULAR (IMP INFUSION PUMP, NON-PROGRAMMABLE, PERMANENT (IMPLAN INTRODUCER/SHEATH, GUIDING, INTRACARDIAC ELECTROPH INTRODUCER/SHEATH, GUIDING, INTRACARDIAC ELECTROPH INTRODUCER/SHEATH, OTHE THAN GUIDING, INTRACARDIAC LEAD, CARDIOVERTER-DEFIBRILLATOR, ENDOCARDIAL DUAL LEAD, CARDIOVERTER-DEFIBRILLATOR, OTHER THAN ENDOC LEAD, NEUROSTIMULATOR TEST KIT (IMPLANTABLE) LEAD, PACEMAKER, OTHER THAN TRANSVENOUS VDD SINGLE LEAD, PACEMAKER/CARDIOVERTER-DEFIBRILLATOR COMBINA LEAD, LEFT VENTRICULAR CORONARY VENOUS SYSTEM LUNG BIOPSY PLUG WITH DELIVERY SYSTEM PROBE, PERCUTANEOUS LUMBAR DISCECTOMY SEALANT, PULMONARY, LIQUID BRACHYTHERAPY SOURCE, YTTRIUM-90, PER SOURCE STENT, NON-CORONARY, TEMPORARY, WITHOUT DELIVERY S PROBE/NEEDLE CRYOABLATION PACEMAKER, DUAL CHAMBER, NON RATE-RESPONSIVE (IMPL PACEMAKER, SINGLE CHAMBER, NON RATE-RESPONSIVE (IM PACEMAKER, OTHER THAN SINGLE OR DUAL CHAMBER (IMPL PROSTHESIS, PENILE, NON-INFLATABLE CATHETER, TRANSLUMINAL ANGIOPLASTY, DRUG-COATED, NON-LASER IMPL WIRELESS PULM ARTERY PRESS SENSOR DEL CATH STENT, NON-CORONARY, TEMPORARY, WITH DELIVERY SYST INFUSION PUMP, NON-PROGRAMMABLE, TEMPORARY (IMPLAN CATHETER, SUPRAPUBIC/CYSTOSCOPIC CATHETER, OCCLUSION INTRDUCR/SHTH OTH THAN GUID OTH THAN IC EEG LASR CATHETER, ELECTROPHYSIOLOGY, DIAGNOSTIC/ABLATION, REPAIR DEVICE, URINARY, INCONTINENCE, WITHOUT SLIN BRACHYTHERAPY SOURCE, NON-STRANDED, IODINE-125, BRACHYTHERAPY SRC CESIUM-131 CHLORID SOL PER MCI BRACHYTHERAPY PLANAR SOURCE, PALLADIUM-103, PER SQUARE MILLIMETER APPL SKN GRFT TRUNK ARM LEG 100 CM; 1ST 25 CM/< APPL SG TRNK ARMS LEGS AREA 100 CM; EA ADD 25 CM APPL SG TRUNK ARM LEG AREA >/=100 CM;1ST 100 CM APPL SG TRNK ARM LEG AREA>/=100 CM;EA ADD 100 CM APPL SG F-S-N-H-F-G-M-D A TO 100 CM; 1ST 25 CM/< APPL SG F-S-N-H-F-G-M-D A TO 100 CM;EA ADD 25 CM APP SG F/N/HF/G A >/=100 CM;1ST 100 CM/1% A CHLD APP SG F/N/HF/G A >/=100 CM;EA ADD 100 CM/1% CHL MAGNETIC RESONANCE ANGIOGRAPHY WITH CONTRAST, ABDO MAGNETIC RESONANCE ANGIOGRAPHY WITHOUT CONTRAST, A MAGNETIC RESONANCE ANGIOGRAPHY WITHOUT CONTRAST FO MAGNETIC RESONANCE IMAGING WITH CONTRAST, BREAST; MAGNETIC RESONANCE IMAGING WITHOUT CONTRAST, BREAS MAGNETIC RESONANCE IMAGING WITHOUT CONTRAST FOLLOW MAGNETIC RESONANCE IMAGING WITH CONTRAST, BREAST; MAGNETIC RESONANCE IMAGING WITHOUT CONTRAST, BREAS MAGNETIC RESONANCE IMAGING WITHOUT CONTRAST FOLLOW MAGNETIC RESONANCE ANGIOGRAPHY WITH CONTRAST, CHES MAGNETIC RESONANCE ANGIOGRAPHY WITHOUT CONTRAST, C MAGNETIC RESONANCE ANGIOGRAPHY WITHOUT CONTRAST FO MAGNETIC RESONANCE ANGIOGRAPHY WITH CONTRAST, LOWE MAGNETIC RESONANCE ANGIOGRAPHY WITHOUT CONTRAST, L MAGNETIC RESONANCE ANGIOGRAPHY WITHOUT CONTRAST FO MAGNETIC RESONANCE ANGIOGRAPHY WITH CONTRAST, PELV MAGNETIC RESONANCE ANGIOGRAPHY WITHOUT CONTRAST, P MAGNETIC RESONANCE ANGIOGRAPHY WITHOUT CONTRAST FO TTE W OR W/O CONT CONG CARDIAC ANOMALIES; COMPLETE TTE W OR W/O CONT CONG CARDIAC ANOM; F-UP/LIMIT TTE REAL TIME IMAGE DOC 2D W/WO M-MODE; COMPLETE 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2007 1/1/2016 10/1/2011 10/1/2011 10/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 4/1/2013 1/1/2004 1/1/2004 1/1/2008 7/1/2014 1/1/2016 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2008 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC C8924 C8925 C8926 C8927 C8928 C8929 C8930 C8931 C8932 C8933 C8934 C8935 C8936 C8957 C9025 C9026 C9027 C9113 C9121 C9132 C9136 C9248 C9250 C9254 C9257 C9273 C9278 C9290 C9293 C9349 C9352 C9353 C9354 C9356 C9358 C9359 C9360 C9361 C9362 C9363 C9364 C9399 C9442 C9443 C9444 C9446 C9447 C9458 C9459 C9460 C9497 C9600 C9601 C9602 C9603 C9604 C9605 C9606 C9607 C9608 C9716 C9726 C9727 C9728 C9737 C9739 C9740 C9741 C9742 C9743 C9800 C9898 C9899 D0120 D0140 D0145 D0150 D0160 D0170 D0171 D0180 D0190 D0191 D0210 D0220 D0230 D0240 D0250 D0260 D0270 D0272 TTE REAL TIME IMAGE DOC 2D; FOLLOW-UP/LIMITED TEE REAL TIME IMAGE DOC 2D; PROBE PLCMT ACQ I&R TEE CONG CARDIAC ANOMALIES; PROBE PLCMT ACQ I&R TEE MONITORING ASSESS CARDIAC PUMPIMG FUNCTION TTE DURING REST AND CV STRESS TEST WITH I&R TTE CMPL SPEC DOPPLER & COLOR FLOW DOPPLER ECHO TTE CMPL DUR REST & CVST W/I&R W/PHYS SUP MR ANGIOGRAPHY W/CONTRAST SPINAL CANAL CONTENTS MR ANGIOGRAPHY W/O CONTRST SPINAL CANAL CONTENTS MR ANGIO NO CONTRST FLW W/CONTRST SP CANAL CNTN MR ANGIOGRAPHY WITH CONTRAST UPPER EXTREMITY MR ANGIOGRAPHY WITHOUT CONTRAST UPPER EXTREMITY MR ANGIO W/O CONTRST FOLLOWED W/CONTRST UP EXT IV INFUSION FOR THERAPY, PROLONGED, REQ PUMP INJECTION, RAMUCIRUMAB INJECTION, VEDOLIZUMAB INJECTION PEMBROLIZUMAB 1 MG INJECTION, PANTOPRAZOLE SODIUM, PER VIAL INJECTION, ARGATROBAN, PER 5 MG PROTHROMBIN CMPLX CONC KCENTRA I.U. FCT IX ACTV INJECTION FACTOR VIII FC FUSION PROTEIN PER I.U. INJECTION CLEVIDIPINE BUTYRATE 1 MG HUMAN PLASMA FIBRIN SEALANT, VAPOR-HEATED, SOLVENT INJECTION LACOSAMIDE 1 MG INJECTION BEVACIZUMAB 0.25 MG SIPULEUCEL-T INCLUDING LEUKAPHERESIS PER INFUS NJECTION INCOBOTULINUMTOXINA 1 UNIT INJECTION, BUPIVACAINE LIPOSOME, 1MG INJECTION GLUCARPIDASE 10 UNITS FORTADERM & FORTADERM ANTIMICROBL TYP PER SQ CM MICROPOROUS COLLAGEN IMPLANTABLE TUBE PER CM LEN MICROPOROUS COLLAGEN IMPLANTABLE SLIT TUBE CM ACELLULAR PERICARDIAL TISSUE MATRIX OF NON-HUMAN ORIGIN (VERITAS), PER SQUARE CE TENDON POROUS MATRIX COLLAGEN & GAG PER SQ CM DERMAL SUBSTITUTE, NATIVE, NONDENATURED COLLAGEN, POROUS PURIFIED COLL MATRIX B VOID FILLR -0.5 CC DERMAL SUBSTITUTE, NATIVE, NON DENATURED COLLAGEN, COLLAGEN MATRIX NERVE WRAP (NEUROMEND COLLAGEN NER POROUS PURIFIED COLLAGEN MATRIX BONE VOID FILLER ( SKIN SUBSTITUE (INTEGRA MESHED BILAYER WOULD MATRI PORCINE IMPLANT (PERMACOL), PER SQUARE CM UNCLASSIFIED DRUGS OR BIOLOGICALS INJECTION BELINOSTAT 10 MG INJECTION DALBAVANCIN 10 MG INJECTION ORITAVANCIN 10 MG INJECTION TEDIZOLID PHOSPHATE 1 MG INJECTION PHENYLEPHRINE AND KETOROLAC 4 ML VIAL FLORBETABEN F18, DIAGNOSTIC, PER STUDY DOSE, UP TO 8.1 MILLICURIES FLUTEMETAMOL F18, DIAGNOSTIC, PER STUDY DOSE, UP TO 5 MILLICURIES INJECTION, CANGRELOR, 1 MG LOXAPINE INHALATION POWDER 10 MG PC TRNSCTH PLCMT RX ELUT IC STENTS; 1 MAJ CA/BR PC TRNSCTH PLCMT RX-ELUT IC STNT;EA ADD BR MCA PC TL COR ATHERECT W/RX ELUT IC STENT; 1 MCA/BR PERQ TL COR ATHERECT; EA ADD BR MAJ CORONARY ART PC TL REV OF/THRU CABG COMB DE IC STNT; 1 VES PC TL REV OF/THRU CABG; EA ADD BR SUBTEND BP GFT PERQ TL REV AC TOTAL/SUBTOTAL OCCLUSION 1 VES PC TL REV CHRN TOT OCCL CA CA BR/CABG; 1 VES PC TL REV CHRN TOT OCCL; EA ADD CA CA BR/BP GFT CREATIONS OF THERMAL ANAL LESIONS BY RADIOFREQUENC PLCMT & REMV AA INTO BRST IORT ADD-ON BRST PROC INSERTION OF IMPLANTS INTO THE SOFT PALATE; MINIMU PLCMT INTERSTITIAL DEV NOT ABD PELV PROS RP THOR LAPAROSCOPY SURG ESOPHAGEAL SPHINCTER AUG W/DEVC CYSTURETHRSCPY INSRT TRANSPROSTAT IMPL; 1-3 IMPL CYSTURETHRSCPY INSRT TRANSPROSTAT IMPL; 4/> IMPL RHC W/IMPLANTATION WIRELESS PRESS SENS PULM ART LARYNGSCOP FLX FIBROPT INJ VOCAL CORD TX INCL DX INJECTION/IMPLANTATION OF BULKING OR SPACER MATERIAL (ANY TYPE) WITH OR WITHOUT DERM INJ FACL LDS PRVS RADIESSE/SCULPTRA FILLER RADIOLABELED PROD PROV DURING A HOSPITAL IP STAY IMPL PROS DEVC PAYBLE IP WHO DO NOT HAVE IP COV PERIODIC ORAL EVALUATION LIMITED ORAL EVALUATION - PROBLEM FOCUSED Oral evaluation, pt < 3yrs COMPREHENSIVE ORAL EVALUATION-NEW OR ESTABLISHED P DETAILED AND EXTENSIVE ORAL EVALUATION - PROBLEM F RE-EVALUATION-LIMITED, PROBLEM FOCUSED (ESTABLISHE RE-EVALUATION - POST-OPERATIVE OFFICE VISIT COMPREHENSIVE PERIODONTAL EVALUATION - NEW OR ESTA SCREENING OF A PATIENT ASSESSMENT OF A PATIENT INTRAORAL-COMPLETE SERIES (INCLUDING BITEWINGS) INTRAORAL-PERIAPICAL-FIRST FILM INTRAORAL-PERIAPICAL-EACH ADDITIONAL FILM INTRAORAL-0CCLUSAL FILM EXTRAORAL-FIRST FILM EXTRAORAL-EACH ADDITIONAL FILM BITEWING-SINGLE FILM BITEWINGS-TWO FILMS 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2008 1/1/2009 1/1/2009 6/3/2010 6/3/2010 6/3/2010 6/3/2010 6/3/2010 6/3/2010 1/1/2006 10/1/2014 10/1/2014 1/1/2015 1/1/2004 1/1/2004 10/1/2013 1/1/2015 1/1/2009 7/1/2009 1/1/2010 1/1/2010 10/1/2010 1/1/2011 4/1/2012 10/1/2012 1/1/2015 1/1/2008 1/1/2008 1/1/2008 7/1/2008 7/1/2009 1/1/2009 7/1/2009 7/1/2009 7/1/2009 7/1/2009 7/1/2009 1/1/2004 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2016 1/1/2016 1/1/2016 1/1/2014 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2006 1/1/2007 1/1/2010 1/1/2014 7/1/2014 7/1/2014 10/1/2014 1/1/2015 1/1/2016 3/23/2010 1/1/2009 1/1/2009 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2015 1/1/2004 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC D0273 D0274 D0277 D0290 D0310 D0320 D0321 D0322 D0330 D0340 D0350 D0351 D0363 D0364 D0365 D0366 D0367 D0368 D0369 D0370 D0371 D0380 D0381 D0382 D0383 D0384 D0385 D0386 D0391 D0393 D0394 D0395 D0415 D0416 D0417 D0418 D0421 D0425 D0431 D0460 D0470 D0472 D0473 D0474 D0475 D0476 D0477 D0478 D0479 D0480 D0481 D0482 D0483 D0484 D0485 D0486 D0502 D0601 D0602 D0603 D0999 D1110 D1120 D1206 D1208 D1310 D1320 D1330 D1351 D1352 D1353 D1510 D1515 D1520 D1525 D1550 D1555 D1999 D2140 D2150 D2160 D2161 D2330 D2331 D2332 D2335 D2390 D2391 D2392 D2393 D2394 Bitewings - three films BITEWINGS-FOUR FILMS VERTICAL BITEWINGS - 7 TO 8 FILMS POSTERIOR-ANTERIOR OR LATERAL SKULL AND FACIAL BON SIALOGRAPHY TEMPOROMANDIBULAR JOINT ARTHROGRAM, INCLUDING INJE OTHER TEMPOROMANDIBULAR JOINT FILMS, BY REPORT TOMOGRAPHIC SURVEY PANORAMIC FILM CEPHALOMETRIC FILM ORAL/FACIAL PHOTO IMAGE OBTAIN INTRA/EXTRAORLLY 3D PHOTOGRAPHIC IMAGE Cone beam, three dimensional CONE BEAM CT CAP&INTEPR LTD FD VIEW-<1 WHOLE JAW CONE BEAM CT CAP&INT FD VW 1 FULL DENT ARCH-MAND CONE BM CT CAP&INT FD VIEW 1 FULL DENT ARCH-MAX CONE BEAM CT CAPTURE & INTERP FD VIEW BOTH JAWS CONE BEAM CT CAP&INTEPR TMJ SERIES 2/> EXPOSURES MAXILLOFACIAL MRI CAPTURE AND INTERPRETATION MAXILLOFACIAL ULTRASOUND CAPTURE&INTERPRETATION SIALOENDOSCOPY CAPTURE AND INTERPRETATION CONE BEAM CT IMAG CAP W/LTD FD VIEW-<1 WHOLE JAW CONE BM CT IMAG CAP FD VW 1 FULL DENT ARCH-MAND CONE BEAM CT IMAG CAP FD VW 1 FULL DENT ARCH-MAX CONE BEAM CT IMAGE CAPTURE FIELD VIEW BOTH JAWS CONE BEAM CT IMAG CAP TMJ SERIES 2/> EXPOSURES MAXILLOFACIAL MRI IMAGE CAPTURE MAXILLOFACIAL ULTRASOUND IMAGE CAPTURE INTEPR DX IMAG PRACTITNER NOT ASSOC CAP IMAG RPT TREATMENT SIMULATION USING 3D IMAGE VOLUME DIGTL SUBTRACTION 2/> IMAGES/IMAG VOL SAME MODAL FUSION 2/MORE 3D IMAGES VOLUME 1/MORE MODALITIES COLLECTION OF MICROORGANISMS FOR CULTURE AND SENSI VIRAL CULTURE CLCT & PREP SALIVA SAMPLE FOR LAB DX TESTING ANALYSIS OF SALIVA SAMPLE GENETIC TEST FOR SUSCEPTIBILITY TO ORAL DISEASES CARIES SUSCEPTIBILITY TESTS ADJUNCTIVE PRE-DIAGNOSTIC TEST THAT AIDS IN DETECT PULP VITALITY TESTS DIAGNOSTIC CASTS ACCESSION OF TISSUE, GROSS EXAMINATION, PREPARATIO ACCESSION OF TISSUE, GROSS AND MICROSCOPIC EXAMINA ACCESSION OF TISSUE, GROSS AND MICROSCOPIC EXAMINA DECALCIFICATION PROCEDURE SPECIAL STAINS FOR MICROORGANISMS SPECIAL STAINS, NOT FOR MICROORGANISMS IMMUNOHISTOCHEMICAL STAINS TISSUE IN-SITU HYBRIDIZATION, INCLUDING INTERPRETA PROCESSING AND INTERPRETATION OF EXFOLIATIVE CYTOL ELECTRON MICROSCOPY - DIAGNOSTIC DIRECT IMMUNOFLUORESCENCE INDIRECT IMMUNOFLUORESCENCE CONSULTATION ON SLIDES PREPARED ELSEWHERE CONSULTATION, INCLUDING PREPARATION OF SLIDES FROM ACCESSION TRANSEPITHELIAL CYTOLOG SAMPL MIC EXAM OTHER ORAL PATHOLOGY PROCEDURES, BY REPORT CARIES RISK ASSESSMENT & DOC FINDING LOW RISK CARIES RISK ASSESSMENT & DOC FINDING MOD RISK CARIES RISK ASSESSMENT & DOC FINDING HIGH RISK UNSPECIFIED DIAGNOSTIC PROCEDURE, BY REPORT PROPHYLAXIS-ADULT PROPHYLAXIS; CHILD Topical fluoride varnish TOPICAL APPLICATION OF FLUORIDE NUTRITIONAL COUNSELING FOR THE CONTROL OF DENTAL D TOBACCO COUNSELING FOR THE CONTROL AND PREVENTION ORAL HYGIENE INSTRUCTION SEALANT - PER TOOTH PREV RSN REST MOD HIGH CARIES RISK PT-PERM TOOTH SEALANT REPAIR - PER TOOTH SPACE MAINTAINER; FIXED-UNILATERAL SPACE MAINTAINER; FIXED BILATERAL SPACE MAINTAINER-REMOVABLE UNILATERAL SPACE MAINTAINER-REMOVABLE BILATERAL RECEMENTATION OF SPACE MAINTAINER Remove fix space maintainer UNSPECIFIED PREVENTIVE PROCEDURE BY REPORT AMALGAM; ONE SURFACE, PRIMARY OR PERMANENT AMALGAM; TWO SURFACES, PRIMARY OR PERMANENT AMALGAM; THREE SURFACES, PRIMARY OR PERMANENT AMALGAM; FOUR OR MORE SURFACES, PRIMARY OR PERMANE RESIN-BASED COMPOSITE ONE SURFACE ANTERIOR RESIN-BASED COMPOSITE, TWO SURFACES, ANTERIOR RESIN-BASED COMPOSITE THREE SURFACES ANTERIOR RESIN-BASED COMPOSITE 4/> SURFACES INCISAL ANGLE RESIN-BASED COMPOSITE CROWN, ANTERIOR RESIN-BASED COMPOSITE - ONE SURFACE, POSTERIOR RESIN-BASED COMPOSITE - TWO SURFACES POSTERIOR RESIN-BASED COMPOSITE - 3 SURFACES POSTERIOR RESIN-BASED COMPOSITE-4 OR MORE SURFACES, POSTERIO 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2007 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2011 1/1/2015 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2014 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC D2410 D2420 D2430 D2510 D2520 D2530 D2542 D2543 D2544 D2610 D2620 D2630 D2642 D2643 D2644 D2650 D2651 D2652 D2662 D2663 D2664 D2710 D2712 D2720 D2721 D2722 D2740 D2750 D2751 D2752 D2780 D2781 D2782 D2783 D2790 D2791 D2792 D2794 D2799 D2910 D2915 D2920 D2921 D2929 D2930 D2931 D2932 D2933 D2934 D2940 D2941 D2949 D2950 D2951 D2952 D2953 D2954 D2955 D2957 D2960 D2961 D2962 D2970 D2971 D2975 D2980 D2981 D2982 D2983 D2990 D2999 D3110 D3120 D3220 D3221 D3222 D3230 D3240 D3310 D3320 D3330 D3331 D3332 D3333 D3346 D3347 D3348 D3351 D3352 D3353 D3354 GOLD FOIL-ONE SURFACE GOLD FOIL-TWO SURFACES GOLD FOIL-THREE SURFACES INLAY-METALLIC-ONE SURFACE INLAY-METALLIC-TWO SURFACES INLAY-METALLIC-THREE OR MORE SURFACES ONLAY-METALLIC-TWO SURFACES ONLAY - METALLIC - THREE SURFACES ONLAY - METALLIC - FOUR OR MORE SURFACES INLAY-PORCELAIN/CERAMIC-ONE SURFACE INLAY-PORCELAIN/CERAMIC-TWO SURFACES INLAY-PORCELAIN/CERAMIC-THREE OR MORE SURFACES ONLAY - PORCELAIN/CERAMIC - TWO SURFACES ONLAY - PORCELAIN/CERAMIC - THREE SURFACES ONLAY - PORCELAIN/CERAMIC - FOUR OR MORE SURFACES INLAY - RESIN-BASED COMPOSITE - ONE SURFACE INLAY - RESIN-BASED COMPOSITE - TWO SURFACES INLAY - RESIN-BASED COMPOSITE - THREE OR MORE SURF ONLAY - RESIN-BASED COMPOSITE - TWO SURFACES ONLAY - RESIN-BASED COMPOSITE - THREE SURFACES ONLAY - - RESIN-BASED COMPOSITE - FOUR OR MORE SUR CROWN - RESIN-BASED COMPOSITE (INDIRECT) CROWN - 3/4 RESIN-BASED COMPOSITE (INDIRECT) CROWN-RESIN WITH HIGH NOBLE METAL CROWN-RESIN WITH PREDOMINANTLY BASE METAL CROWN-RESIN WITH NOBLE METAL CROWN-PORCELAIN/CERAMIC SUBSTRATE CROWN-PORCELAIN FUSED TO HIGH NOBLE METAL CROWN-PROCELAIN FUSED TO PREDOMINANTLY BASE METAL CROWN-PORCELAIN FUSED TO NOBLE METAL CROWN - 3/4 CAST HIGH NOBLE METAL CROWN - 3/4 CAST PREDOMINANTLY BASE METAL CROWN - 3/4 CAST NOBLE METAL CROWN - 3/4 PORCELAIN/CERAMIC CROWN-FULL CAST HIGH NOBLE METAL CROWN-FULL CAST PREDOMINANTLY BASE METAL CROWN-FULL CAST NOBLE METAL CROWN-TITANIUM PROVISIONAL CROWN RECEMENT INLAY, ONLAY OR PARTIAL COVERAGE RESTORAT RECEMENT CAST OR PREFABRICATED POST AND CORE RECEMENT CROWN REATTACHMENT TOOTH FRAGMENT INCISAL EDGE/CUSP PREFAB PORCELAIN/CERAMIC CROWN - PRIMARY TOOTH PREFABRICATED STAINLESS STEEL CROWN; PRIMARY TOOTH PREFABRICATED STAINLESS STEEL CROWN; PERMANENT TOO PREFABRICATED RESIN CROWN PREFABRICATED STAINLESS STEEL CROWN W/ RESIN WINDO PREFABRICATED ESTHETIC COATED STAINLESS STEEL CROW PROTECTIVE RESTORATION INTERIM THERAPEUTIC RESTORATION-PRIMARY DENTITN RESTORATIVE FOUNDATION AN INDIRECT RESTORATION CORE BUILDUP INCLUDING ANY PINS WHEN REQUIRED PIN RETENTION-PER TOOTH, IN ADDITION TO RESTORATIO CAST POST AND CORE IN ADDITION TO CROWN EACH ADDITIONAL CAST POST - SAME TOOTH PREFABRICATED POST AND CORE IN ADDITION TO CROWN POST REMOVAL (NOT IN CONJUCTION WITH ENDODONTIC TH EACH ADDITIONAL PREFABRICATED POST - SAME TOOTH LABIAL VENEER RESIN LAMINATE - CHAIRSIDE LABIAL VENEER (RESIN LAMINATE)-LABORATORY LABIAL VENEER (PORCELAIN LAMINATE)-LABORATORY TEMPORARY CROWN FRACTURED TOOTH ADDITIONAL PROCEDURES TO CONSTRUCT NEW CROWN UNDER COPING CROWN REPAIR, BY REPORT INLAY REPAIR NECESSITATED RESTORATIVE MATL FAIL ONLAY REPAIR NECESSITATED RESTORATIVE MATL FAIL VENEER REPAIR NECESSITATED RESTORATIVE MATL FAIL RESIN INFILTRATION INCIPIENT SMOOTH SURFACE LES UNSPECIFIED RESTORATIVE PROCEDURE, BY REPORT PULP CAP-DIRECT (EXCLUDING FINAL RESTORATION) PULP CAP-INDIRECT (EXCLUDING FINAL RESTORATION) THERAPEUTIC PULPOTOMY (EXCLUDING FINAL RESTORATION PULPAL DEBRIDEMENT, PRIMARY AND PERMANENT TEETH PART PULPOTOMY FOR APEXOGENEIS PERM TOOTH PULPAL THERAPY (RESORBABLE FILLING)-ANTERIOR, PRIM PULPAL THERAPY (RESORBABLE FILLING)-POSTERIOR, PRI ENDODONTIC THERAPY ANTERIOR (EXCLUDING FINAL RESTO ENDODONTIC THERAPY BICUSPID (EXCLUDING FINAL RESTO ENDODONTIC THPY(EXCLUDING FINAL RESTORATION) MOLAR TREATMENT OF ROOT CANAL OBSTRUCTION; NON-SURGICAL INCOMPLETE ENDODONTIC THERAPY; INOPERABLE, UNRESTO INTERNAL ROOT REPAIR OF PERFORATION DEFECTS RETREATMENT OF PREVIOUS ROOT CANAL THERAPY; ANTERI RETREATMENT OF PREVIOUS ROOT CANAL THERAPY; BICUSP RETREATMENT OF PREVIOUS ROOT CANAL THERAPY; MOLAR APEXIFICATION/RECALCIFICATION INITIAL VISIT APEXIFICATION/RECALCIFICATN INTERIM MED REPLACE APEXIFICATION/RECALCIFICATION-FINAL VISIT (INCLUDE PUPAL REGENERATION; NOT INCL FINAL RESTORATION 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2014 1/1/2013 1/1/2007 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2014 1/1/2014 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2011 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC D3355 D3356 D3357 D3410 D3421 D3425 D3426 D3427 D3428 D3429 D3430 D3431 D3432 D3450 D3460 D3470 D3910 D3920 D3950 D3999 D4210 D4211 D4212 D4230 D4231 D4240 D4241 D4245 D4249 D4260 D4261 D4263 D4264 D4265 D4266 D4267 D4268 D4270 D4273 D4274 D4275 D4276 D4277 D4278 D4283 D4285 D4320 D4321 D4341 D4342 D4355 D4381 D4910 D4920 D4921 D4999 D5110 D5120 D5130 D5140 D5211 D5212 D5213 D5214 D5225 D5226 D5281 D5410 D5411 D5421 D5422 D5510 D5520 D5610 D5620 D5630 D5640 D5650 D5660 D5670 D5671 D5710 D5711 D5720 D5721 D5730 D5731 D5740 D5741 D5750 D5751 PULPAL REGENERATION - INITIAL VISIT PULPAL REGENERATION - INTERIM MEDICATION REPLACE PULPAL REGENERATION - COMPLETION OF TREATMENT APICOECTOMY - ANTERIOR APICOECTOMY - BICUSPID FIRST ROOT APICOECTOMY - MOLAR FIRST ROOT APICOECTOMY EACH ADDITIONAL ROOT PERIRADICULAR SURGERY WITHOUT APICOECTOMY BONE GRAFT W/PERIRADICULAR SURG PER TOOTH 1 SITE BONE GRAFT PERIRADICULR SURG EA ADD CONTIG TOOTH RETROGRADE FILLING - PER ROOT BIOL MATL SOFT OSS TISS REGEN PERIRADICULAR SURG GUIDED TISS REGEN RESORB BARR PERIRADICULAR SURG ROOT AMPUTATION-PER ROOT ENDODONTIC ENDOSSEOUS IMPLANT INTENTIONAL REIMPLANTATION W/NECESSARY SPLINTING SURGICAL PROCEDURE FOR ISOLATION OF TOOTH WITH RUB HEMISECTION (INCLUDING ANY ROOT REMOVAL), NOT INCL Canal preparation & fitting of performed dowel or UNSPECIFIED ENDODONTIC PROCEDURE, BY REPORT Gingivectomy or gingivoplasty 4 or more continuous GINGIVECTOMY OR GINGIVOPLASTY - ONE TO THREE CONTI GING/GINGIVOPLASTY ALLW ACSS RESTORATV PRO-TOOTH Ana crown exp 4 or> per quad Ana crown exp 1-3 per quad GINGIVAL FLAP PROCEDURE, INCLUDING ROOT PLANING GINGIVAL FLAP PROCEDURE, INCLUDING ROOT PLANING APICALLY POSITIONED FLAP CLINICAL CROWN LENGTHENING-HARD TISSUE OSSEOUS SURGERY (INCLUDING FLAP ENTRY AND CLOSURE) OSSEOUS SURGERY(INCLUDING FLAP ENTRY & CLOSURE) BONE REPLACEMENT GRAFT - FIRST SITE IN QUADRANT BONE REPLACEMENT GRAFT - EACH ADDITIONAL SITE IN Q BIOLOGIC MATERIALS TO AID IN SOFT AND OSSEOUS TISS GUIDED TISSUE REGENERATION - RESORBABLE BARRIER, P GUIDED TISSUE REGENERATION - NONRESORBABLE BARRIER SURGICAL REVISION PROCEDURE, PER TOOTH PEDICLE SOFT TISSUE GRAFT PROCEDURE SUBEPITHELIAL CONNECTIVE TISSUE GRAFT PROCEDURES, DISTAL OR PROXIMAL WEDGE PROCEDURE (WHEN NOT PERFO SOFT TISSUE ALLOGRAFT COMBINED CONNECTIVE TISSUE AND DOUBLE PEDICLE GRAF FREE SFT TSS GFT PROC 1ST T/EDNTULS T PSTN GFT FREE SFT TSS GFT EA ADD CNTIG T/EDNT T SAME SITE AUTOGENOUS CONNECTIVE TISSUE GRAFT PROCEDURE NON-AUTOGENOUS CONNECTIVE TISSUE GRAFT PROCEDURE PROVISIONAL SPLINTING-INTRACORONAL PROVISIONAL SPLINTING-EXTRACORONAL Perio Scaling/Root Planing 4 or more teeth per qua PERIODONTAL SCALING AND ROOT PLANING - 1 TO 3 TEET FULL MOUTH DEBRIDEMENT TO ENABLE COMPREHENSIVE EVA LOCALIZED DELIVERY OF ANTIMICROBIAL AGENTS VIA A C PERIODONTAL MAINTENANCE UNSCHEDULED DRESSING CHANGE NOT TX DENTIST STAFF GINGIVAL IRRIGATION - PER QUADRANT UNSPECIFIED PERIODONTAL PROCEDURE, BY REPORT COMPLETE DENTURE - MAXILLARY COMPLETE DENTURE - MANDIBULAR IMMEDIATE DENTURE - MAXILLARY IMMEDIATE DENTURE - MANDIBULAR MAXILLARY PARTIAL DENTURE - RESIN BASE MANDIBULAR PARTIAL DENTURE - RESIN BASE MAXILLARY PARTIAL DENTURE - CAST METAL FRAMEWORK W MANDIBULAR PARTIAL DENTURE - CAST METAL FRAMEWORK MAXILLARY PARTIAL DENTURE - FLEXIBLE BASE (INCLUDI MANDIBULAR PARTIAL DENTURE - FLEXIBLE BASE (INCLUD REMOVABLE UNILATERAL PARTIAL DENTURE-ONE PIECE CAS ADJUST COMPLETE DENTURE - MAXILLARY ADJUST COMPLETE DENTURE - MANDIBULAR ADJUST PARTIAL DENTURE - MAXILLARY ADJUST PARTIAL DENTURE - MANDIBULAR REPAIR BROKEN COMPLETE DENTURE BASE REPLACE MISSING OR BROKEN TEETH-COMPLETE DENTURE ( REPAIR RESIN DENTURE BASE REPAIR CAST FRAMEWORK REPAIR OR REPLACE BROKEN CLASP REPLACE BROKEN TEETH-PER TOOTH ADD TOOTH TO EXISTING PARTIAL DENTURE ADD CLASP TO EXISTING PARTIAL DENTURE REPLACE ALL TEETH AND ACRYLIC ON CAST METAL FRAMEW REPLACE ALL TEETH AND ACRYLIC ON CAST METAL FRAMEW REBASE COMPLETE MAXILLARY DENTURE REBASE COMPLETE MANDIBULAR DENTURE REBASE MAXILLARY PARTIAL DENTURE REBASE MANDIBULAR PARTIAL DENTURE RELINE COMPLETE MAXILLARY DENTURE (CHAIRSIDE) RELINE COMPLETE MANDIBULAR DENTURE CHAIRSIDE RELINE MAXILLARY PARTIAL DENTURE (CHAIRSIDE) RELINE MANDIBULAR PARTIAL DENTURE (CHAIRSIDE) RELINE COMPLETE MAXILLARY DENTURE (LABORATORY) RELINE COMPLETE MANDIBULAR DENTURE (LABORATORY) 1/1/2014 1/1/2014 1/1/2014 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2014 1/1/2014 1/1/2014 1/1/2007 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2013 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC D5760 D5761 D5810 D5811 D5820 D5821 D5850 D5851 D5860 D5861 D5862 D5863 D5864 D5865 D5866 D5867 D5875 D5899 D5911 D5912 D5913 D5914 D5915 D5916 D5919 D5922 D5923 D5924 D5925 D5926 D5927 D5928 D5929 D5931 D5932 D5933 D5934 D5935 D5936 D5937 D5951 D5952 D5953 D5954 D5955 D5958 D5959 D5960 D5982 D5983 D5984 D5985 D5986 D5987 D5988 D5991 D5992 D5993 D5994 D5999 D6010 D6011 D6012 D6013 D6040 D6050 D6051 D6052 D6055 D6056 D6057 D6058 D6059 D6060 D6061 D6062 D6063 D6064 D6065 D6066 D6067 D6068 D6069 D6070 D6071 D6072 D6073 D6074 D6075 D6076 D6077 RELINE MAXILLARY PARTIAL DENTURE (LABORATORY) RELINE MANDIBULAR PARTIAL DENTURE (LABORATORY) INTERIM COMPLETE DENTURE (MAXILLARY) INTERIM COMPLETE DENTURE (MANDIBULAR) INTERIM PARTIAL DENTURE (MAXILLARY) INTERIM PARTIAL DENTURE (MANDIBULAR) TISSUE CONDITIONING, MAXILLARY TISSUE CONDITIONING, MANDIBULAR OVERDENTURE-COMPLETE, BY REPORT OVERDENTURE-PARTIAL, BY REPORT PRECISION ATTACHMENT, BY REPORT OVERDENTURE - COMPLETE MAXILLARY OVERDENTURE - PARTIAL MAXILLARY OVERDENTURE - COMPLETE MANDIBULAR OVERDENTURE - PARTIAL MANDIBULAR REPLACEMENT OF REPLACEABLE PART OF SEMI-PRECISION MODIFICATION OF REMOVABLE PROSTHESIS FOLLOWING IMP UNSPECIFIED REMOVABLE PROSTHODONTIC PROCEDURE, BY FACIAL MOULAGE (SECTIONAL) FACIAL MOULAGE (COMPLETE) NASAL PROSTHESIS AURICULAR PROSTHESIS ORBITAL PROSTHESIS OCULAR PROSTHESIS FACIAL PROSTHESIS NASAL SEPTAL PROSTHESIS OCULAR PROSTHESIS, INTERIM CRANIAL PROSTHESIS FACIAL AUGMENTATION IMPLANT PROSTHESIS NASAL PROSTHESIS, REPLACEMENT AURICULAR PROSTHESIS, REPLACEMENT ORBITAL PROSTHESIS, REPLACEMENT FACIAL PROSTHESIS, REPLACEMENT OBTURATOR PROSTHESIS, SURGICAL OBTURATOR PROSTHESIS, DEFINITIVE OBTURATOR PROSTHESIS, MODIFICATION MANDIBULAR RESECTION PROSTHESIS WITH GUIDE FLANGE MANDIBULAR RESECTION PROSTHESIS WITHOUT GUIDE FLAN OBTURATOR/PROSTHESIS, INTERIM TRISMUS APPLIANCE NOT FOR TMD TREATMENT FEEDING AID SPEECH AID PROSTHESIS, PEDIATRIC SPEECH AID PROSTHESIS, ADULT PALATAL AUGMENTATION PROSTHESIS PALATAL LIFT PROSTHESIS, DEFINITIVE PALATAL LIFT PROSTHESIS, INTERIM PALATAL LIFT PROSTHESIS, MODIFICATION SPEECH AID PROSTHESIS, MODIFICATION SURGICAL STENT RADIATION CARRIER RADIATION SHIELD RADIATION CONE LOCATOR FLUORIDE GEL CARRIER COMMISSURE SPLINT SURGICAL SPLINT VESICULOBULLOUS DISEASE MEDICAMENT CARRIER ADJUST MAXILLOFACIAL PROSTH APPLIANCE BY REPORT MAINT CLEAN MAXILLOFACIAL PROSTH OTH THN REQ ADJ PERIODONTAL MED CARR PERIPH SEAL LAB PROCESSED UNSPECIFIED MAXILLOFACIAL PROSTHESIS, BY REPORT SURGICAL PLACEMENT OF IMPLANT BODY: ENDOSTEAL IMP SECOND STAGE IMPLANT SURGERY Endosteal implant SURGICAL PLACEMENT OF MINI IMPLANT SURGICAL PLACEMENT: EPOSTEAL IMPLANT SURGICAL PLACEMENT: TRANSOSTEAL IMPLANT INTERIM ABUTMENT SEMI-PRECISION ATTACHMENT ABUTMENT CONNECTING BAR IMPLANT OR ABUTMENT SUPPORTED PREFABRICATED ABUTMENT - INCLUDES PLACEMENT CUSTOM ABUTMENT - INCLUDES PLACEMENT ABUTMENT SUPPORTED PORCELAIN/CERAMIC CROWN ABUTMENT SUPPORTED PORCELAIN FUSED TO METAL CROWN ABUTMENT SUPPORTED PORCELAIN FUSED TO METAL CROWN ABUTMENT SUPPORTED PORCELAIN FUSED TO METAL CROWN ABUTMENT SUPPORTED CAST METAL CROWN (HIGH NOBLE ME ABUTMENT SUPPORTED CAST METAL CROWN (PREDOMINANTLY ABUTMENT SUPPORTED CAST METAL CROWN (NOBLE METAL) IMPLANT SUPPORTED PORCELAIN/CERAMIC CROWN IMPLANT SUPPORTED PORCELAIN FUSED TO METAL CROWN ( IMPLANT SUPPORTED METAL CROWN (TITANIUM, TITANIUM ABUTMENT SUPPORTED RETAINER FOR PORCELAIN/CERAMIC ABUTMENT SUPPORTED RETAINER FOR PORCELAIN FUSED TO ABUTMENT SUPPORTED RETAINER FOR PORCELAIN FUSED TO ABUTMENT SUPPORTED RETAINER FOR PORCELAIN FUSED TO ABUTMENT SUPPORTED RETAINER FOR CAST METAL FPD (H ABUTMENT SUPPORTED RETAINER FOR CAST METAL FPD (P ABUTMENT SUPPORTED RETAINER FOR CAST METAL FPD (N IMPLANT SUPPORTED RETAINER FOR CERAMIC FPD IMPLANT SUPPORTED RETAINER FOR PORCELAIN FUSED TO IMPLANT SUPPORTED RETAINER FOR CAST METAL FPD (TIT 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2014 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2011 1/1/2011 1/1/2014 1/1/2004 1/1/2004 1/1/2014 1/1/2007 1/1/2014 1/1/2004 1/1/2004 1/1/2013 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC D6080 D6090 D6091 D6092 D6093 D6094 D6095 D6100 D6101 D6102 D6103 D6104 D6110 D6111 D6112 D6113 D6114 D6115 D6116 D6117 D6190 D6194 D6199 D6205 D6210 D6211 D6212 D6214 D6240 D6241 D6242 D6245 D6250 D6251 D6252 D6253 D6545 D6548 D6549 D6600 D6601 D6602 D6603 D6604 D6605 D6606 D6607 D6608 D6609 D6610 D6611 D6612 D6613 D6614 D6615 D6624 D6634 D6710 D6720 D6721 D6722 D6740 D6750 D6751 D6752 D6780 D6781 D6782 D6783 D6790 D6791 D6792 D6793 D6794 D6920 D6930 D6940 D6950 D6980 D6985 D6999 D7111 D7140 D7210 D7220 D7230 D7240 D7241 D7250 D7251 D7260 IMPL MAINT PROC REMV REINSRT CLEAN PROSTH & ABUT REPAIR IMPLANTSUPPORTED PROSTHESIS BY REPORT Repl semi/precision attach Recement supp crown Recement supp part denture ABUTMENT SUPPORTED CROWN - (TITANIUM) REPAIR IMPLANT ABUTMENT, BY REPORT IMPLANT REMOVAL, BY REPORT DEBR PERIIMPL DFCT CLN EXPSD IMPL FLP ENTRY CLO DEBR&OSS CNTR PERIIMPL DFCT;SURF&FLAP ENTRY&CLOS BN GFT PERIIMPL DFCT-NO FLP/BR MB/BIOL MATL BONE GRAFT AT TIME OF IMPLANT PLACEMENT IMPL/ABUT SUPP REMV DENTURE EDENTULOUS ARCH-MAX IMPL/ABUT SUPP REMV DENTURE EDENTULOUS ARCH-MND IMPL/ABUT SUPP REMV DENTURE PART EDENT ARCH-MAX IMPL/ABUT SUPP REMV DENTURE PART EDENT ARCH-MAND IMPL/ABUT SUPP FIXED DENTURE EDENTULOUS ARCH-MAX IMPL/ABUT SUPP FIXD DENTURE EDENTULOUS ARCH-MAND IMPL/ABUT SUPP FIXED DENTURE PART EDENT ARCH-MAX IMPL/ABUT SUPP FIXD DENTURE PART EDENT ARCH-MAND RADIOGRAPHIC/SURGICAL IMPLANT INDEX, BY REPORT ABUTMENT SUPPORTED RETAINER CROWN FOR FPD - (TITAN UNSPECIFIED IMPLANT PROCEDURE, BY REPORT PONTIC - INDIRECT RESIN BASED COMPOSITE PONTIC-CAST HIGH NOBLE METAL PONTIC-CAST PREDOMINANTLY BASE METAL PONTIC-CAST NOBLE METAL PONTIC - TITANIUM PONTIC-PORCELAIN FUSED TO HIGH NOBLE METAL PONTIC-PORCELAIN FUSED TO PREDOMINANTLY BASE METAL PONTIC-PORCELAIN FUSED TO NOBLE METAL PONTIC - PORCELAIN/CERAMIC PONTIC-RESIN WITH HIGH NOBLE METAL PONTIC-RESIN WITH PREDOMINANTLY BASE METAL PONTIC-RESIN WITH NOBLE METAL PROVISIONAL PONTIC RETAINER-CAST METAL FOR RESIN BONDED FIXED PROSTHE RETAINER - PORCELAIN/CERAMIC FOR RESIN BONDED FIXE RESIN RETAINER-FOR RESIN BONDED FIXED PROSTHESIS INLAY-PORCELAIN/CERAMIC, TWO SURFACES INLAY - PORCELAIN/CERAMIC, THREE OR MORE SURFACES INLAY - CAST HIGH NOBLE METAL, TWO SURFACES INLAY - CAST HIGH NOBLE METAL, THREE OR MORE SURFA INLAY - CAST PREDOMINANTLY BASE METAL, TWO SURFACE INLAY - CAST PREDOMINANTLY BASE METAL, THREE OR MO INLAY - CAST NOBLE METAL, TWO SURFACES INLAY - CAST NOBLE METAL, THREE OR MORE SURFACES ONLAY - PORCELAIN/CERAMIC, TWO SURFACES ONLAY - PORCELAIN/CERAMIC, THREE OR MORE SURFACES ONLAY - CAST HIGH NOBLE METAL, TWO SURFACES ONLAY - CAST HIGH NOBLE METAL, THREE OR MORE SURFA ONLAY - CAST PREDOMINANTLY BASE METAL, TWO SURFACE ONLAY - CAST PREDOMINANTLY BASE METAL, THREE OR MO ONLAY - CAST NOBLE METAL, TWO SURFACES ONLAY - CAST NOBLE METAL, THREE OR MORE SURFACES INLAY - TITANIUM ONLAY - TITANIUM CROWN - INDIRECT RESIN BASED COMPOSITE CROWN-RESIN WITH HIGH NOBLE METAL CROWN-RESIN WITH PREDOMINANTLY BASE METAL CROWN-RESIN WITH NOBLE METAL CROWN - PORCELAIN/CERAMIC CROWN-PORCELAIN FUSED TO HIGH NOBLE METAL CROWN-PORCELAIN FUSED TO PREDOMINANTLY BASE METAL CROWN-PORCELAIN FUSED TO NOBLE METAL CROWN-3/4 CAST HIGH NOBLE METAL CROWN - 3/4 CAST PREDOMINANTLY BASED METAL CROWN - 3/4 CAST NOBLE METAL CROWN - 3/4 PORCELAIN/CERAMIC CROWN-FULL CAST HIGH NOBLE METAL CROWN-FULL CAST PREDOMINANTLY BASE METAL CROWN-FULL CAST NOBLE METAL PROVISIONAL RETAINER CROWN CROWN - TITANIUM CONNECTOR BAR RECEMENT FIXED PARTIAL DENTURE STRESS BREAKER PRECISION ATTACHMENT FIXED PARTIAL DENTURE REPAIR BY REPORT PEDIATRIC PARTIAL DENTURE, FIXED UNSPECIFIED FIXED PROSTHODONTIC PROCEDURE, BY REPO EXTRACTION, CORONAL REMNANTS - DECIDUOUS TOOTH EXTRACTION, ERUPTED TOOTH OR EXPOSED ROOT (ELEVATI SURG REMOVAL ERUPTED TOOTH REMV BONE ELEV FLAP REMOVAL OF IMPACTED TOOTH-SOFT TISSUE REMOVAL OF IMPACTED TOOTH-PARTIALLY BONY REMOVAL OF IMPACTED TOOTH-COMPLETELY BONY OF IMPACTED TOOTH; COMPLETELY BONY WITH UNUSUAL S SURGICAL REMOVAL OF RESIDUAL TOOTH ROOTS (CUTTING CORONECTOMY - INTENTIONAL PARTIAL TOOTH REMOVAL OROANTRAL FISTULA CLOSURE 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2013 1/1/2013 1/1/2013 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2011 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC D7261 D7270 D7272 D7280 D7282 D7283 D7285 D7286 D7287 D7288 D7290 D7291 D7292 D7293 D7294 D7295 D7310 D7311 D7320 D7321 D7340 D7350 D7410 D7411 D7412 D7413 D7414 D7415 D7440 D7441 D7450 D7451 D7460 D7461 D7465 D7471 D7472 D7473 D7485 D7490 D7510 D7511 D7520 D7521 D7530 D7540 D7550 D7560 D7610 D7620 D7630 D7640 D7650 D7660 D7670 D7671 D7680 D7710 D7720 D7730 D7740 D7750 D7760 D7770 D7771 D7780 D7810 D7820 D7830 D7840 D7850 D7852 D7854 D7856 D7858 D7860 D7865 D7870 D7871 D7872 D7873 D7874 D7875 D7876 D7877 D7880 D7899 D7910 D7911 D7912 D7920 PRIMARY CLOSURE OF A SINUS PERFORATION TOOTH REIMPLANTATION AND/OR STABILIZATION OF ACCID TOOTH TRANSPLANTATION (INCLUDES REIMPLANTATION FRO SURGICAL ACCESS OF AN UNERUPTED TOOTH MOBILIZATION OF ERUPTED OR MALPOSITIONED TOOTH TO PLACEMENT OF DEVICE TO FACILITATE ERUPTION OF IMPA BIOPSY OF ORAL TISSUE; HARD (BONE, TOOTH) BIOPSY OF ORAL TISSUE; SOFT EXFOLIATIVE CYTOLOGICAL SAMPLE COLLECTION BRUSH BIOPSY - TRANSEPITHELIAL SAMPLE COLLECTION SURGICAL REPOSITIONING OF TEETH TRANSSEPTAL FIBEROTOMY/SUPRA CRESTAL FIBEROTOMY, B Screw retained plate Temp anchorage dev w flap Temp anchorage dev w/o flap HARVEST BONE FOR USE AUTOGENOUS GRAFTING PROC ALVEOLOPLASTY IN CONJUNCTION WITH EXTRACTIONS - PE ALVEOLOPLASTY IN CONJUNCTION WITH EXTRACTIONS - ON ALVEOLOPLASTY NOT IN CONJUNCTION WITH EXTRACTIONS ALVEOLOPLASTY NOT IN CONJUNCTION WITH EXTRACTIONS VESTIBULOPLASTY-RIDGE EXTENSION (SECOND EPITHELIAL VESTIBULOPLASTY-RIDGE EXTENSION (INCLUDING SOFT TI EXCISION OF BENIGN LESION UP TO 1.25 CM EXCISION OF BENIGN LESION GREATER THAN 1.25 CM EXCISION OF BENIGN LESION, COMPLICATED EXCISION OF MALIGNANT LESION UP TO 1.25 CM EXCISION OF MALIGNANT LESION GREATER THAN 1.25 CM EXCISION OF MALIGNANT LESION, COMPLICATED EXCISION OF MALIGNANT TUMOR-LESION DIAMETER UP TO EXCISION OF MALIGNANT TUMOR-LESION DIAMETER GREATE REMOVAL OF BENIGN ODONTOGENIC CYST OR TUMOR-LESION REMOVAL OF BENIGN ODONTOGENIC CYST OR TUMOR-LESION REMOVAL OF BENIGN NONODONTOGENIC CYST OR TUMOR-LES REMOVAL OF BENIGN NONODONTOGENIC CYST OR TUMOR-LES DESTRUCTION OF LESION(S) BY PHYSICAL OR CHEMICAL M REMOVAL OF LATERAL EXOSTOSIS (MAXILLA OR MANDIBLE) REMOVAL OF TORUS PALATINUS REMOVAL OF TORUS MANDIBULARIS SURGICAL REDUCTION OF OSSEOUS TUBEROSITY RADICAL RESECTION OF MAXILLA OR MANDIBLE INCISION AND DRAINAGE OF ABSCESS-INTRAORAL SOFT TI INCISION AND DRAINAGE OF ABSCESS - INTRAORAL SOFT INCISION AND DRAINAGE OF ABSCESS-EXTRAORAL SOFT TI INCISION AND DRAINAGE OF ABSCESS - EXTRAORAL SOFT REMOVAL OF FOREIGN BODY FROM MUCOSA, SKIN, OR SUBC REMOVAL OF REACTION-PRODUCING FOREIGN BODIES-MUSCU PARTIAL OSTECTOMY/SEQUESTRECTOMY FOR REMOVAL OF NO MAXILLARY SINUSOTOMY FOR REMOVAL OF TOOTH FRAGMENT MAXILLA-OPEN REDUCTION (TEETH IMMOBILIZED IF PRESE MAXILLA-CLOSED REDUCTION (TEETH IMMOBILIZED IF PRE MANDIBLE-OPEN REDUCTION (TEETH IMMOBILIZED IF PRES MANDIBLE-CLOSED REDUCTION (TEETH IMMOBILIZED IF PR MALAR AND/OR ZYGOMATIC ARCH-OPEN REDUCTION MALAR AND/OR ZYGOMATIC ARCH-CLOSED REDUCTION ALVEOLUS - CLOSED REDUCTION, MAY INCLUDE STABILIZA ALVEOLUS - OPEN REDUCTION, MAY INCLUDE STABILIZATI FACIAL BONES-COMPLICATED REDUCTION WITH FIXATION A MAXILLA-OPEN REDUCTION MAXILLA-CLOSED REDUCTION MANDIBLE-OPEN REDUCTION MANDIBLE-CLOSED REDUCTION MALAR AND/OR ZYGOMATIC ARCH-OPEN REDUCTION MALAR AND/OR ZYGOMATIC ARCH-CLOSED REDUCTION ALVEOLUS - OPEN REDUCTION STABILIZATION OF TEETH ALVEOLUS, CLOSED REDUCTION STABILIZATION OF TEETH FACIAL BONES-COMPLICATED REDUCTION WITH FIXATION A OPEN REDUCTION OF DISLOCATION CLOSED REDUCTION OF DISLOCATION MANIPULATION UNDER ANESTHESIA CONDYLECTOMY SURGICAL DISCECTOMY; WITH/WITHOUT IMPLANT DISC REPAIR SYNOVECTOMY MYOTOMY JOINT RECONSTRUCTION ARTHROTOMY ARTHROPLASTY ARTHROCENTESIS NON-ARTHROSCOPIC LYSIS AND LAVAGE ARTHROSCOPY-DIAGNOSIS, WITH OR WITHOUT BIOPSY ARTHROSCOPY-SURGICAL: LAVAGE AND LYSIS OF ADHESION ARTHROSCOPY-SURGICAL: DISC REPOSITIONING AND STABI ARTHROSCOPY-SURGICAL: SYNOVECTOMY ARTHROSCOPY-SURGICAL: DISCECTOMY ARTHROSCOPY-SURGICAL: DEBRIDEMENT OCCLUSAL ORTHOTIC DEVICE BY REPORT UNSPECIFIED TMD THERAPY, BY REPORT SUTURE OF RECENT SMALL WOUNDS UP TO 5 CM COMPLICATED SUTURE-UP TO 5 CM COMPLICATED SUTURE-GREATER THAN 5 CM SKIN GRAFT (IDENTIFY DEFECT COVERED, LOCATION, AND 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2007 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC D7921 D7940 D7941 D7943 D7944 D7945 D7946 D7947 D7948 D7949 D7950 D7951 D7952 D7953 D7955 D7960 D7963 D7970 D7971 D7972 D7980 D7981 D7982 D7983 D7990 D7991 D7995 D7996 D7997 D7998 D7999 D8010 D8020 D8030 D8040 D8050 D8060 D8070 D8080 D8090 D8210 D8220 D8660 D8670 D8680 D8690 D8691 D8692 D8693 D8694 D8999 D9110 D9120 D9210 D9211 D9212 D9215 D9219 D9220 D9221 D9223 D9230 D9241 D9242 D9243 D9248 D9310 D9410 D9420 D9430 D9440 D9450 D9610 D9612 D9630 D9910 D9911 D9920 D9930 D9931 D9940 D9941 D9942 D9950 D9951 D9952 D9970 D9971 D9972 D9973 D9974 COLLECTION & APPLIC AUTO BLOOD CONCENTRATE PROD OSTEOPLASTY-FOR ORTHOGNATHIC DEFORMITIES OSTEOTOMY - MANDIBULAR RAMI OSTEOTOMY - MANDIBULAR RAMI WITH BONE GRAFT; INCLU OSTEOTOMY-SEGMENTED OR SUBAPICAL-PER SEXTANT OR QU OSTEOTOMY-BODY OF MANDIBLE LEFORT I (MAXILLA-TOTAL) LEFORT I (MAXILLA-SEGMENTED) LEFORT II OR LEFORT III (OSTEOPLASTY OF FACIAL BON LEFORT II OR LEFORT III-WITH BONE GRAFT OSSEOUS, OSTEOPERIOSTEAL, OR CARTILAGE GRAFT OF TH Sinus aug w bone/bone sup SINUS AUGMENTATION VIA A VERTICAL APPROACH BONE REPLACEMENT GRAFT FOR RIDGE PRESERVATION - PE REPAIR OF MAXILLOFACIAL SOFT AND/OR HARD TISSUE DE FRENULECTOMY SEP PROC NOT INCIDENTL ANOTHER PROC FRENULOPLASTY EXCISION OF HYPERPLASTIC TISSUE-PER ARCH EXCISION OF PERICORONAL GINGIVA SURGICAL REDUCTION OF FIBROUS TUBEROSITY SIALOLITHOTOMY EXCISION OF SALIVARY GLAND, BY REPORT SIALODOCHOPLASTY CLOSURE OF SALIVARY FISTULA EMERGENCY TRACHEOTOMY CORONOIDECTOMY SYNTHETIC GRAFT-MANDIBLE OR FACIAL BONES, BY REPOR IMPLANT-MANDIBLE FOR AUGMENTATION PURPOSES (EXCLUD APPLIANCE REMOVAL (NOT BY DENTIST WHO PLACED APPLI Intraoral place of fix dev UNSPECIFIED ORAL SURGERY PROCEDURE, BY REPORT LIMITED ORTHODONTIC TREATMENT OF THE PRIMARY DENTI LIMITED ORTHODONTIC TREATMENT OF THE TRANSITIONAL LIMITED ORTHODONTIC TREATMENT OF THE ADOLESCENT DE LIMITED ORTHODONTIC TREATMENT OF THE ADULT DENTITI INTERCEPTIVE ORTHODONTIC TREATMENT OF THE PRIMARY INTERCEPTIVE ORTHODONTIC TREATMENT OF THE TRANSITI COMPREHENSIVE ORTHODONTIC TREATMENT OF THE TRANSIT COMPREHENSIVE ORTHODONTIC TREATMENT OF THE ADOLESC COMPREHENSIVE ORTHODONTIC TREATMENT OF THE ADULT D REMOVABLE APPLIANCE THERAPY FIXED APPLIANCE THERAPY PREORTHODONTIC TREATMENT VISIT PERIODIC ORTHODONTIC TREATMENT VISIT (AS PART OF C ORTHODONTIC RETENTION (REMOVAL OF APPLIANCES, CONS ORTHODONTIC TREATMENT (ALTERNATIVE BILLING TO A CO REPAIR OF ORTHODONTIC APPLIANCE REPLACEMENT OF LOST OR BROKEN RETAINER REBONDING OR RECEMENTING OF FIXED RETAINER REPAIR OF FIXED RETAINERS INCLUDES REATTACHMENT UNSPECIFIED ORTHODONTIC PROCEDURE, BY REPORT PALLIATIVE (EMERGENCY) TREATMENT OF DENTAL PAIN-MI Fix partial denture section LOCAL ANESTHESIA N0T IN CONJUNCTION WITH OPERATIV REGIONAL BLOCK ANESTHESIA TRIGEMINAL DIVISION BLOCK ANESTHESIA LOCAL ANESTHESIA CONJUCTION OPERATIVE/SURG PROC EVALUATION FOR DEEP SEDATION/GENERAL ANESTHESIA DEEP SEDATION/GENERAL ANESTHESIA; FIRST 30 MINUTES DEEP SEDATION/GENERAL ANESTHESIA-EACH ADDITIONAL 1 DEEP SEDATION/GENERAL ANESTHESIA - EA 15 MIN INHALATION OF NITROUS OXIDE/ANXIOLYSIS ANALGESIA INTRAVENOUS CONSCIOUS SEDATION/ANALGESIA; FIRST 30 INTRAVENOUS CONSCIOUS SEDATION/ANALGESIA - EACH AD INTRAVENOUS MOD SEDATION/ANALGESIA - EA 15 MIN NON-INTRAVENOUS CONSCIOUS SEDATION CONSULTATION (DIAGNOSTIC SERVICE PROVIDED BY DENTI HOUSE/EXTENDED CARE FACILITY CALL HOSPITAL OR AMBULATORY SURGICAL CENTER CALL OFFICE VISIT FOR OBSERVATION (DURING REGULARLY SCH OFFICE VISIT-AFTER REGULARLY SCHEDULED HOURS CASE PRESENTATION, DETAILED AND EXTENSIVE TREATMEN THERAPEUTIC PARENTERAL DRUG, SINGLE ADMINISTRATION Thera par drugs 2 or > admin OTHER DRUGS AND/OR MEDICAMENTS, BY REPORT APPLICATION OF DESENSITIZING MEDICAMENT APPLICATION OF DESENSITIZING RESIN FOR CERVICAL AN BEHAVIOR MANAGEMENT, BY REPORT TREATMENT OF COMPLICATIONS (POSTSURGICAL) - UNUSUA CLEANING AND INSPECTION OF A REMOVABLE APPLIANCE OCCLUSAL GUARD BY REPORT FABRICATION OF ATHLETIC MOUTHGUARD REPAIR AND/OR RELINE OF OCCLUSAL GUARD OCCLUSION ANALYSIS-MOUNTED CASE OCCLUSAL ADJUSTMENT-LIMITED OCCLUSAL ADJUSTMENT-COMPLETE ENAMEL MICROABRASION ODONTOPLASTY 1 - 2 TEETH; INCLUDES REMOVAL OF ENAM EXTERNAL BLEACHING - PER ARCH EXTERNAL BLEACHING - PER TOOTH INTERNAL BLEACHING - PER TOOTH 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2014 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2007 1/1/2004 1/1/2016 1/1/2004 1/1/2007 1/1/2004 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC D9975 D9985 D9986 D9987 D9999 E0100 E0105 E0110 E0111 E0112 E0113 E0114 E0116 E0117 E0118 E0130 E0135 E0140 E0141 E0143 E0144 E0147 E0148 E0149 E0153 E0154 E0155 E0156 E0157 E0158 E0159 E0160 E0161 E0162 E0163 E0165 E0167 E0168 E0170 E0171 E0175 E0181 E0182 E0184 E0185 E0186 E0187 E0188 E0189 E0190 E0191 E0193 E0194 E0196 E0197 E0198 E0199 E0200 E0202 E0203 E0205 E0210 E0215 E0217 E0218 E0221 E0225 E0231 E0232 E0235 E0236 E0239 E0240 E0241 E0242 E0243 E0244 E0245 E0246 E0247 E0248 E0249 E0250 E0251 E0255 E0256 E0260 E0261 E0265 E0266 E0270 EXT BLEACH HOM APPLIC-ARCH; MATL FAB CSTM TRAYS SALES TAX MISSED APPOINTMENT CANCELLED APPOINTMENT UNSPECIFIED ADJUNCTIVE PROCEDURE, BY REPORT CANE, INCLUDES CANES OF ALL MATERIALS, ADJUSTABLE CANE, QUAD OR THREE PRONG, INCLUDES CANES OF ALL M CRUTCHES, FOREARM, INCLUDES CRUTCHES OF VARIOUS MA CRUTCH FOREARM, INCLUDES CRUTCHES OF VARIOUS MATER CRUTCHES UNDERARM, WOOD, ADJUSTABLE OR FIXED, PAIR CRUTCH UNDERARM, WOOD, ADJUSTABLE OR FIXED, EACH, CRUTCHES UNDERARM, OTHER THAN WOOD, ADJUSTABLE OR CRUTCH UNDERARM, OTHER THAN WOOD, ADJUSTABLE OR FI CRUTCH, UNDERARM, ARTICULATING, SPRING ASSISTED, E CRUTCH SUBSTITUTE, LOWER LEG PLATFORM, WITH OR WIT WALKER, RIGID (PICKUP), ADJUSTABLE OR FIXED HEIGHT WALKER, FOLDING (PICKUP), ADJUSTABLE OR FIXED HEIG WALKER, WITH TRUNK SUPPORT, ADJUSTABLE OR FIXED HE WALKER, RIGID, WHEELED, ADJUSTABLE OR FIXED HEIGHT WALKER, FOLDING, WHEELED, ADJUSTABLE OR FIXED HEIG WALKER, ENCLOSED, FOUR SIDED FRAMED, RIGID OR FOLD WALKER, HEAVY DUTY, MULTIPLE BRAKING SYSTEM, VARIA WALKER, HEAVY DUTY, WITHOUT WHEELS, RIGID OR FOLDI WALKER, HEAVY DUTY, WHEELED, RIGID OR FOLDING, ANY PLATFORM ATTACHMENT, FOREARM CRUTCH, EACH PLATFORM ATTACHMENT, WALKER, EACH WHEEL ATTACHMENT, RIGID PICK-UP WALKER, PER PAIR SEAT ATTACHMENT, WALKER CRUTCH ATTACHMENT, WALKER, EACH LEG EXTENSIONS FOR WALKER, PER SET OF FOUR (4) BRAKE ATTACHMENT FOR WHEELED WALKER, REPLACEMENT, SITZ TYPE BATH OR EQUIPMENT, PORTABLE, USED WITH O SITZ TYPE BATH OR EQUIPMENT, PORTABLE, USED WITH O SITZ BATH CHAIR COMMODE CHAIR, STATIONARY, WITH FIXED ARMS COMMODE CHAIR, STATIONARY, WITH DETACHABLE ARMS PAIL OR PAN FOR USE WITH COMMODE CHAIR COMMODE CHAIR, EXTRA WIDE AND/OR HEAVY DUTY, STATI Commode chair with integrated seat lift mechanism Commode chair with integrated seat lift mechanism FOOT REST, FOR USE WITH COMMODE CHAIR, EACH PRESSURE PAD, ALTERNATING WITH PUMP, HEAVY DUTY PUMP FOR ALTERNATING PRESSURE PAD DRY PRESSURE MATTRESS GEL OR GEL-LIKE PRESSURE PAD FOR MATTRESS, STANDAR AIR PRESSURE MATTRESS WATER PRESSURE MATTRESS SYNTHETIC SHEEPSKIN PAD LAMBSWOOL SHEEPSKIN PAD, ANY SIZE POSITIONING CUSHION/PILLOW/WEDGE, ANY SHAPE OR SIZ HEEL OR ELBOW PROTECTOR, EACH POWERED AIR FLOTATION BED (LOW AIR LOSS THERAPY) AIR FLUIDIZED BED GEL PRESSURE MATTRESS AIR PRESSURE PAD FOR MATTRESS, STANDARD MATTRESS L WATER PRESSURE PAD FOR MATTRESS, STANDARD MATTRESS DRY PRESSURE PAD FOR MATTRESS, STANDARD MATTRESS L HEAT LAMP, WITHOUT STAND (TABLE MODEL), INCLUDES B PHOTOTHERAPY (BILIRUBIN) LIGHT WITH PHOTOMETER THERAPEUTIC LIGHTBOX, MINIMUM 10,000 LUX, TABLE TO HEAT LAMP, WITH STAND, INCLUDES BULB, OR INFRARED ELECTRIC HEAT PAD, STANDARD ELECTRIC HEAT PAD, MOIST WATER CIRCULATING HEAT PAD WITH PUMP WATER CIRCULATING COLD PAD WITH PUMP INFRARED HEATING PAD SYSTEM HYDROCOLLATOR UNIT, INCLUDES PADS NON-CONTACT WOUND WARMING DEVICE (TEMPERATURE CONT WARMING CARD FOR USE WITH THE NON CONTACT WOUND WA PARAFFIN BATH UNIT, PORTABLE (SEE MEDICAL SUPPLY C PUMP FOR WATER CIRCULATING PAD HYDROCOLLATOR UNIT, PORTABLE BATH/SHOWER CHAIR, WITH OR WITHOUT WHEELS, ANY SIZ BATH TUB WALL RAIL, EACH BATH TUB RAIL, FLOOR BASE TOILET RAIL, EACH RAISED TOILET SEAT TUB STOOL OR BENCH TRANSFER TUB RAIL ATTACHMENT TRANSFER BENCH FOR TUB OR TOILET WITH OR WITHOUT C TRANSFER BENCH, HEAVY DUTY, FOR TUB OR TOILET WITH PAD FOR WATER CIRCULATING HEAT UNIT HOSPITAL BED, FIXED HEIGHT, WITH ANY TYPE SIDE RAI HOSPITAL BED, FIXED HEIGHT, WITH ANY TYPE SIDE RAI HOSPITAL BED, VARIABLE HEIGHT, HI-LO, WITH ANY TYP HOSPITAL BED, VARIABLE HEIGHT, HI-LO, WITH ANY TYP HOSPITAL BED, SEMI-ELECTRIC (HEAD AND FOOT ADJUSTM HOSPITAL BED, SEMI-ELECTRIC (HEAD AND FOOT ADJUSTM HOSPITAL BED, TOTAL ELECTRIC (HEAD, FOOT AND HEIGH HOSPITAL BED, TOTAL ELECTRIC (HEAD, FOOT AND HEIGH HOSPITAL BED, INSTITUTIONAL TYPE INCLUDES: OSCILLA 1/1/2013 1/1/2004 1/1/2015 1/1/2015 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC E0271 E0272 E0273 E0274 E0275 E0276 E0277 E0280 E0290 E0291 E0292 E0293 E0294 E0295 E0296 E0297 E0300 E0301 E0302 E0303 E0304 E0305 E0310 E0315 E0316 E0325 E0326 E0328 E0329 E0350 E0352 E0370 E0371 E0372 E0373 E0424 E0425 E0430 E0431 E0433 E0434 E0435 E0439 E0440 E0441 E0442 E0443 E0444 E0445 E0446 E0450 E0455 E0457 E0459 E0460 E0461 E0462 E0463 E0464 E0465 E0466 E0470 E0471 E0472 E0480 E0481 E0482 E0483 E0484 E0485 E0486 E0487 E0500 E0550 E0555 E0560 E0561 E0562 E0565 E0570 E0572 E0574 E0575 E0580 E0585 E0600 E0601 E0602 E0603 E0604 E0605 MATTRESS, INNERSPRING MATTRESS, FOAM RUBBER BED BOARD OVER-BED TABLE BED PAN, STANDARD, METAL OR PLASTIC BED PAN, FRACTURE, METAL OR PLASTIC POWERED PRESSURE-REDUCING AIR MATTRESS BED CRADLE, ANY TYPE HOSPITAL BED, FIXED HEIGHT, WITHOUT SIDE RAILS, WI HOSPITAL BED, FIXED HEIGHT, WITHOUT SIDE RAILS, WI HOSPITAL BED, VARIABLE HEIGHT, HI-LO, WITHOUT SIDE HOSPITAL BED, VARIABLE HEIGHT, HI-LO, WITHOUT SIDE HOSPITAL BED, SEMI-ELECTRIC (HEAD AND FOOT ADJUSTM HOSPITAL BED, SEMI-ELECTRIC (HEAD AND FOOT ADJUSTM HOSPITAL BED, TOTAL ELECTRIC (HEAD, FOOT AND HEIGH HOSPITAL BED, TOTAL ELECTRIC (HEAD, FOOT AND HEIGH PEDIATRIC CRIB, HOSPITAL GRADE, FULLY ENCLOSED HOSPITAL BED, HEAVY DUTY, EXTRA WIDE, WITH WEIGHT HOSPITAL BED, EXTRA HEAVY DUTY, EXTRA WIDE, WITH W HOSPITAL BED, HEAVY DUTY, EXTRA WIDE, WITH WEIGHT HOSPITAL BED, EXTRA HEAVY DUTY, EXTRA WIDE, WITH W BED SIDE RAILS, HALF LENGTH BED SIDE RAILS, FULL LENGTH BED ACCESSORY: BOARD, TABLE, OR SUPPORT DEVICE, AN SAFETY ENCLOSURE FRAME/CANOPY FOR USE WITH HOSPITA URINAL; MALE, JUG-TYPE, ANY MATERIAL URINAL; FEMALE, JUG-TYPE, ANY MATERIAL HOSPITAL BED PEDIATRIC MANUAL INCLUDES MATTRESS HOSPITAL BED PEDIATRIC ELECTRIC INCLUDE MATTRESS CONTROL UNIT FOR ELECTRONIC BOWEL IRRIGATION/EVACU DISPOSABLE PACK (WATER RESERVOIR BAG, SPECULUM, VA AIR PRESSURE ELEVATOR FOR HEEL NONPOWERED ADVANCED PRESSURE REDUCING OVERLAY FOR POWERED AIR OVERLAY FOR MATTRESS, STANDARD MATTRES NONPOWERED ADVANCED PRESSURE REDUCING MATTRESS STATIONARY COMPRESSED GASEOUS OXYGEN SYSTEM, RENTA STATIONARY COMPRESSED GAS SYSTEM, PURCHASE; INCLUD PORTABLE GASEOUS OXYGEN SYSTEM, PURCHASE; INCLUDES PORTABLE GASEOUS OXYGEN SYSTEM, RENTAL; INCLUDES P PORTABL LIQUID OXYGEN SYS RENTAL; HOME LIQUEFIER PORTABLE LIQUID OXYGEN SYSTEM, RENTAL; INCLUDES PO PORTABLE LIQUID OXYGEN SYSTEM, PURCHASE; INCLUDES STATIONARY LIQUID OXYGEN SYSTEM, RENTAL; INCLUDES STATIONARY LIQUID OXYGEN SYSTEM, PURCHASE; INCLUDE OXYGEN CONTENTS, GASEOUS (FOR USE WITH OWNED GASEO OXYGEN CONTENTS, LIQUID (FOR USE WITH OWNED LIQUID PORTABLE OXYGEN CONTENTS, GASEOUS (FOR USE ONLY WI PORTABLE OXYGEN CONTENTS, LIQUID (FOR USE ONLY WIT OXIMETER DEVICE FOR MEASURING BLOOD OXYGEN LEVELS TOPICAL OXYGEN DELIVERY SYSTEM NOS INCL SUPPLIES VOLUME CONTROL VENTILATOR, WITHOUT PRESSURE SUPPOR OXYGEN TENT, EXCLUDING CROUP OR PEDIATRIC TENTS CHEST SHELL (CUIRASS) CHEST WRAP NEGATIVE PRESSURE VENTILATOR; PORTABLE OR STATION VOLUME CONTROL VENTILATOR, WITHOUT PRESSURE SUPPOR ROCKING BED WITH OR WITHOUT SIDE RAILS PRESSURE SUPPORT VENTILATOR WITH VOLUME CONTROL MO PRESSURE SUPPORT VENTILATOR WITH VOLUME CONTROL MO HOME VENTILATOR, ANY TYPE, USED WITH INVASIVE INTERFACE, (E.G., TRACHEOSTOMY TUBE HOME VENTILATOR, ANY TYPE, USED WITH NON-INVASIVE INTERFACE, (E.G., MASK, CHEST SH RESPIRATORY ASSIST DEVICE, BI-LEVEL PRESSURE CAPAB RESPIRATORY ASSIST DEVICE, BI-LEVEL PRESSURE CAPAB RESPIRATORY ASSIST DEVICE, BI-LEVEL PRESSURE CAPAB PERCUSSOR, ELECTRIC OR PNEUMATIC, HOME MODEL INTRAPULMONARY PERCUSSIVE VENTILATION SYSTEM AND R COUGH STIMULATING DEVICE, ALTERNATING POSITIVE AND HIGH FREQUENCY CHEST WALL OSCILLATION AIR-PULSE GE OSCILLATORY POSITIVE EXPIRATORY PRESSURE DEVICE, N ORAL DEVICE/APPLIANCE PREFAB, USED TO REDUCE UPPER ORAL DEVICE/APPLIANCE CUSTOM USED TO REDUCE UPPER SPIROMETER ELECTRONIC INCLUDES ALL ACCESSORIES IPPB MACHINE, ALL TYPES, WITH BUILT-IN NEBULIZATI HUMIDIFIER, DURABLE FOR EXTENSIVE SUPPLEMENTAL HUM HUMIDIFIER, DURABLE, GLASS OR AUTOCLAVABLE PLASTIC HUMIDIFIER, DURABLE FOR SUPPLEMENTAL HUMIDIFICATIO HUMIDIFIER, NON-HEATED, USED WITH POSITIVE AIRWAY HUMIDIFIER, HEATED, USED WITH POSITIVE AIRWAY PRES COMPRESSOR, AIR POWER SOURCE FOR EQUIPMENT WHICH I NEBULIZER, WITH COMPRESSOR AEROSOL COMPRESSOR, ADJUSTABLE PRESSURE, LIGHT DUT ULTRASONIC/ELECTRONIC AEROSOL GENERATOR WITH SMALL NEBULIZER, ULTRASONIC, LARGE VOLUME NEBULIZER, DURABLE, GLASS OR AUTOCLAVABLE PLASTIC, NEBULIZER, WITH COMPRESSOR AND HEATER RESPIRATORY SUCTION PUMP, HOME MODEL, PORTABLE OR CONTINUOUS POSITIVE AIRWAY PRESSURE DEVICE BREAST PUMP, MANUAL, ANY TYPE BREAST PUMP, ELECTRIC (AC AND/OR DC), ANY TYPE BREAST PUMP, HEAVY DUTY, HOSPITAL GRADE, PISTON OP VAPORIZER, ROOM TYPE 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N Y N N N N N N N N N N N N Y Y N N N N N N N N N N N Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y N N Y Y N N N N N N N N N N N N N N N N N N N N N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC E0606 E0607 E0610 E0615 E0616 E0617 E0618 E0619 E0620 E0621 E0625 E0627 E0628 E0629 E0630 E0635 E0636 E0637 E0638 E0639 E0640 E0641 E0642 E0650 E0651 E0652 E0655 E0656 E0657 E0660 E0665 E0666 E0667 E0668 E0669 E0670 E0671 E0672 E0673 E0675 E0676 E0691 E0692 E0693 E0694 E0700 E0705 E0710 E0720 E0730 E0731 E0740 E0744 E0745 E0746 E0747 E0748 E0749 E0755 E0760 E0761 E0762 E0764 E0765 E0766 E0769 E0770 E0776 E0779 E0780 E0781 E0782 E0783 E0784 E0785 E0786 E0791 E0830 E0840 E0849 E0850 E0855 E0856 E0860 E0870 E0880 E0890 E0900 E0910 E0911 E0912 POSTURAL DRAINAGE BOARD HOME BLOOD GLUCOSE MONITOR PACEMAKER MONITOR, SELF-CONTAINED, (CHECKS BATTERY PACEMAKER MONITOR, SELF CONTAINED, CHECKS BATTERY IMPLANTABLE CARDIAC EVENT RECORDER WITH MEMORY, AC EXTERNAL DEFIBRILLATOR WITH INTEGRATED ELECTROCARD APNEA MONITOR, WITHOUT RECORDING FEATURE APNEA MONITOR, WITH RECORDING FEATURE SKIN PIERCING DEVICE FOR COLLECTION OF CAPILLARY B SLING OR SEAT, PATIENT LIFT, CANVAS OR NYLON PATIENT LIFT, BATHROOM OR TOILET, NOT OTHERWISE CL SEAT LIFT MECHANISM INCORPORATED INTO A COMBINATIO SEPARATE SEAT LIFT MECHANISM FOR USE WITH PATIENT SEPARATE SEAT LIFT MECHANISM FOR USE WITH PATIENT PATIENT LIFT, HYDRAULIC, WITH SEAT OR SLING PATIENT LIFT, ELECTRIC WITH SEAT OR SLING MULTIPOSITIONAL PATIENT SUPPORT SYSTEM, WITH INTEG COMB SIT STAND FRAME/TABLE SYS SEATLIFT FEATURE STANDING FRAME/TABLE SYS ONE POSITION ANY SZ PATIENT LIFT, MOVEABLE FROM ROOM TO ROOM WITH DISA PATIENT LIFT, FIXED SYSTEM, INCLUDES ALL COMPONENT STANDING FRAME/TABLESYSTEM, MULTI-POSITION (E.G. THREE-WAY STANDER), ANY SIZE INC STANDING FRAME/TABLE SYSTEM, MOBILE (DYNAMIC STANDER), ANY SIZE INCLUDING PEDIAT PNEUMATIC COMPRESSOR, NON-SEGMENTAL HOME MODEL PNEUMATIC COMPRESSOR, SEGMENTAL HOME MODEL WITHOUT PNEUMATIC COMPRESSOR, SEGMENTAL HOME MODEL WITH CA NON-SEGMENTAL PNEUMATIC APPLIANCE FOR USE WITH PNE SEG PNEUMAT APPLIANCE USE W/PNEUMAT COMPRS TRUNK SEG PNEUMAT APPLIANCE USE W/PNEUMAT COMPRS CHEST NON-SEGMENTAL PNEUMATIC APPLIANCE FOR USE WITH PNE NON-SEGMENTAL PNEUMATIC APPLIANCE FOR USE WITH PNE NON-SEGMENTAL PNEUMATIC APPLIANCE FOR USE WITH PNE SEGMENTAL PNEUMATIC APPLIANCE FOR USE WITH PNEUMAT SEGMENTAL PNEUMATIC APPLIANCE FOR USE WITH PNEUMAT SEGMENTAL PNEUMATIC APPLIANCE FOR USE WITH PNEUMAT SEG PNEU APPLINC PNEU COMPRS IN 2 FULL LEGS TRNK SEGMENTAL GRADIENT PRESSURE PNEUMATIC APPLIANCE, F SEGMENTAL GRADIENT PRESSURE PNEUMATIC APPLIANCE, F SEGMENTAL GRADIENT PRESSURE PNEUMATIC APPLIANCE, H PNEUMATIC COMPRESSION DEVICE, HIGH PRESSURE, RAPID Inter limb compress dev NOS UV LIGHT TX SYS BULB/LAMP TIMER; TX 2 SQ FT/LESS ULTRAVIOLET LIGHT THERAPY SYSTEM PANEL, INCLUDES B ULTRAVIOLET LIGHT THERAPY SYSTEM PANEL, INCLUDES B ULTRAVIOLET MULTIDIRECTIONAL LIGHT THERAPY SYSTEM SAFETY EQUIPMENT (E.G., BELT, HARNESS OR VEST) TRANSFER BOARD OR DEVICE, ANY TYPE, EACH RESTRAINTS, ANY TYPE (BODY, CHEST, WRIST OR ANKLE) TENS, TWO LEAD, LOCALIZED STIMULATION TRANSCUTANEOUS ELECTRICAL NERVE STIMULATION DEVICE FORM FITTING CONDUCTIVE GARMENT FOR DELIVERY OF TE INCONTINENCE TREATMENT SYSTEM, PELVIC FLOOR STIMUL NEUROMUSCULAR STIMULATOR FOR SCOLIOSIS NEUROMUSCULAR STIMULATOR, ELECTRONIC SHOCK UNIT ELECTROMYOGRAPHY (EMG), BIOFEEDBACK DEVICE OSTEOGENESIS STIMULATOR, ELECTRICAL, NON-INVASIVE, OSTEOGENESIS STIMULATOR, ELECTRICAL, NON-INVASIVE, OSTEOGENESIS STIMULATOR, ELECTRICAL, SURGICALLY IM ELECTRONIC SALIVARY REFLEX STIMULATOR (INTRA-ORAL/ OSTOGENESIS STIMULATOR, LOW INTENSITY ULTRASOUND, NON-THERMAL PULSED HIGH FREQUENCY RADIOWAVES, HIGH Transcutaneous electrical joint stimulation device Functional neuromuscular stimulator, transcutaneou FDA APPROVED NERVE STIMULATOR, WITH REPLACEABLE BA ELEC STIM DVC U CANCER TX INCL ALL ACC ANY TYPE ELECTRICAL STIMULATION OR ELECTROMAGNETIC WOUND TR FES TRANSQ STIM NERV&/MUSC GRP CMPL SYS NOS IV POLE AMBULATORY INFUSION PUMP, MECHANICAL, REUSABLE, FO AMBULATORY INFUSION PUMP, MECHANICAL, REUSABLE, FO AMBULATORY INFUSION PUMP, SINGLE OR MULTIPLE CHANN INFUSION PUMP, IMPLANTABLE, NON-PROGRAMMABLE (INCL INFUSION PUMP SYSTEM, IMPLANTABLE, PROGRAMMABLE (I EXTERNAL AMBULATORY INFUSION PUMP, INSULIN IMPLANTABLE INTRASPINAL (EPIDURAL/INTRATHECAL) CAT IMPLANTABLE PROGRAMMABLE INFUSION PUMP, REPLACEMEN PARENTERAL INFUSION PUMP, STATIONARY, SINGLE OR MU AMBULATORY TRACTION DEVICE, ALL TYPES, EACH TRACTION FRAME, ATTACHED TO HEADBOARD, CERVICAL TR TRACTION EQUIPMENT, CERVICAL, FREE-STANDING STAND/ TRACTION STAND, FREE STANDING, CERVICAL TRACTION CERVICAL TRACTION EQUIPMENT NOT REQUIRING ADDITION CERVICAL TRACTION DEVICE INFLATABLE AIR BLADDER TRACTION EQUIPMENT, OVERDOOR, CERVICAL TRACTION FRAME, ATTACHED TO FOOTBOARD, EXTREMITY T TRACTION STAND, FREE STANDING, EXTREMITY TRACTION, TRACTION FRAME, ATTACHED TO FOOTBOARD, PELVIC TRAC TRACTION STAND, FREE STANDING, PELVIC TRACTION, (E TRAPEZE BARS, A/K/A PATIENT HELPER, ATTACHED TO BE Trapeze bar, heavy duty, for patient weight capaci Trapeze bar, heavy duty, for patient weight capaci 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2014 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2013 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2004 1/1/2014 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N Y N Y N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N N Y N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N Y HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC E0920 E0930 E0935 E0936 E0940 E0941 E0942 E0944 E0945 E0946 E0947 E0948 E0950 E0951 E0952 E0955 E0956 E0957 E0958 E0959 E0960 E0961 E0966 E0967 E0968 E0969 E0970 E0971 E0973 E0974 E0978 E0980 E0981 E0982 E0983 E0984 E0985 E0986 E0988 E0990 E0992 E0994 E0995 E1002 E1003 E1004 E1005 E1006 E1007 E1008 E1009 E1010 E1011 E1012 E1014 E1015 E1016 E1017 E1018 E1020 E1028 E1029 E1030 E1031 E1035 E1036 E1037 E1038 E1039 E1050 E1060 E1070 E1083 E1084 E1085 E1086 E1087 E1088 E1089 E1090 E1092 E1093 E1100 E1110 E1130 E1140 E1150 E1160 E1161 E1170 E1171 FRACTURE FRAME, ATTACHED TO BED, INCLUDES WEIGHTS FRACTURE FRAME, FREE STANDING, INCLUDES WEIGHTS PASSIVE MOTION EXERCISE DEVICE CPM device, other than knee TRAPEZE BAR, FREE STANDING, COMPLETE WITH GRAB BAR GRAVITY ASSISTED TRACTION DEVICE, ANY TYPE CERVICAL HEAD HARNESS/HALTER PELVIC BELT/HARNESS/BOOT EXTREMITY BELT/HARNESS FRACTURE, FRAME, DUAL WITH CROSS BARS, ATTACHED TO FRACTURE FRAME, ATTACHMENTS FOR COMPLEX PELVIC TRA FRACTURE FRAME, ATTACHMENTS FOR COMPLEX CERVICAL T WHEELCHAIR ACCESSORY, TRAY, EACH HEEL LOOP/HOLDER, ANY TYPE, WITH OR WITHOUT ANKLE TOE LOOP/HOLDER, ANY TYPE, EACH WHEELCHAIR ACCESSORY, HEADREST, CUSHIONED, ANY TYP WHEELCHAIR ACCESSORY, LATERAL TRUNK OR HIP SUPPORT WHEELCHAIR ACCESSORY, MEDIAL THIGH SUPPORT, ANY TY MANUAL WHEELCHAIR ACCESSORY, ONE-ARM DRIVE ATTACHM MANUAL WHEELCHAIR ACCESSORY, ADAPTER FOR AMPUTEE, WHEELCHAIR ACCESSORY, SHOULDER HARNESS/STRAPS OR C MANUAL WHEELCHAIR ACCESSORY, WHEEL LOCK BRAKE EXTE MANUAL WHEELCHAIR ACCESSORY, HEADREST EXTENSION, E MANUAL WHEELCHAIR ACCESSORY, HAND RIM WITH PROJECT COMMODE SEAT, WHEELCHAIR NARROWING DEVICE, WHEELCHAIR NO.2 FOOTPLATES, EXCEPT FOR ELEVATING LEG REST Anti-tipping device, wheelchair WHEELCHAIR ACCESSORY, ADJUSTABLE HEIGHT, DETACHABL MANUAL WHEELCHAIR ACCESSORY, ANTI-ROLLBACK DEVICE, WHEELCHAIR ACCESSORY, POSITIONING BELT/SAFETY BEL SAFETY VEST, WHEELCHAIR WHEELCHAIR ACCESSORY, SEAT UPHOLSTERY, REPLACEMENT WHEELCHAIR ACCESSORY, BACK UPHOLSTERY, REPLACEMENT MANUAL WHEELCHAIR ACCESSORY, POWER ADD-ON TO CONVE MANUAL WHEELCHAIR ACCESSORY, POWER ADD-ON TO CONVE WHEELCHAIR ACCESSORY, SEAT LIFT MECHANISM MNL WHEELCHAIR ACSS PUSH-RIM ACT PWR ASSIST SYS MANUAL WC ACCESSORY LEVR-ACTIVATD WHL DRIVE PAIR WHEELCHAIR ACCESSORY, ELEVATING LEG REST, COMPLETE MANUAL WHEELCHAIR ACCESSORY, SOLID SEAT INSERT ARM REST, EACH WHEELCHAIR ACCESSORY, CALF REST/PAD, EACH WHEELCHAIR ACCESSORY, POWER SEATING SYSTEM, TILT O WHEELCHAIR ACCESSORY, POWER SEATING SYSTEM, RECLIN WHEELCHAIR ACCESSORY, POWER SEATING SYSTEM, RECLIN WHEELCHAIR ACCESSORY, POWER SEATNG SYSTEM, RECLINE WHEELCHAIR ACCESSORY, POWER SEATING SYSTEM, COMBIN WHEELCHAIR ACCESSORY, POWER SEATING SYSTEM, COMBIN WHEELCHAIR ACCESSORY, POWER SEATING SYSTEM, COMBIN WHEELCHAIR ACCESSORY, ADDITION TO POWER SEATING SY WHEELCHAIR ACCESSORY, ADDITION TO POWER SEATING SY MODIFICATION TO PEDIATRIC SIZE WHEELCHAIR, WIDTH A WC ACCSS PWR SEAT SYS CNTR MNT PWR ELEV LEG EA RECLINING BACK, ADDITION TO PEDIATRIC SIZE WHEELCH SHOCK ABSORBER FOR MANUAL WHEELCHAIR, EACH SHOCK ABSORBER FOR POWER WHEELCHAIR, EACH HEAVY DUTY SHOCK ABSORBER FOR HEAVY DUTY OR EXTRA HEAVY DUTY SHOCK ABSORBER FOR HEAVY DUTY OR EXTRA RESIDUAL LIMB SUPPORT SYSTEM FOR WHEELCHAIR WHEELCHAIR ACCESSORY, MANUAL SWINGAWAY, RETRACTABL WHEELCHAIR ACCESSORY, VENTILATOR TRAY, FIXED WHEELCHAIR ACCESSORY, VENTILATOR TRAY, GIMBALED ROLLABOUT CHAIR, ANY AND ALL TYPES WITH CASTORS 5" MULTI-POSITIONAL PATIENT TRANSFER SYSTEM, WITH INT MULTI-PSTN PT TRNSF SYS EXTRA WIDE PT >300 LBS TRANSPORT CHAIR, PEDIATRIC SIZE TRANSPORT CHAIR, ADULT SIZE, PATIENT WEIGHT CAPACI TRANSPORT CHAIR, ADULT SIZE, HEAVY DUTY, PATIENT W FULLY-RECLINING WHEELCHAIR, FIXED FULL LENGTH ARMS FULLY-RECLINING WHEELCHAIR, DETACHABLE ARMS, DESK FULLY-RECLINING WHEELCHAIR, DETACHABLE ARMS (DESK HEMI-WHEELCHAIR, FIXED FULL LENGTH ARMS, SWING AWA HEMI-WHEELCHAIR, DETACHABLE ARMS DESK OR FULL LENG HEMI-WHEELCHAIR, FIXED FULL LENGTH ARMS, SWING AWA HEMI-WHEELCHAIR DETACHABLE ARMS DESK OR FULL LENGT HIGH STRENGTH LIGHTWEIGHT WHEELCHAIR, FIXED FULL L HIGH STRENGTH LIGHTWEIGHT WHEELCHAIR, DETACHABLE A HIGH STRENGTH LIGHTWEIGHT WHEELCHAIR, FIXED LENGTH HIGH STRENGTH LIGHTWEIGHT WHEELCHAIR, DETACHABLE A WIDE HEAVY DUTY WHEEL CHAIR, DETACHABLE ARMS (DESK WIDE HEAVY DUTY WHEELCHAIR, DETACHABLE ARMS DESK O SEMI-RECLINING WHEELCHAIR, FIXED FULL LENGTH ARMS, SEMI-RECLINING WHEELCHAIR, DETACHABLE ARMS (DESK O STANDARD WHEELCHAIR, FIXED FULL LENGTH ARMS, FIXED WHEELCHAIR, DETACHABLE ARMS, DESK OR FULL LENGTH, WHEELCHAIR, DETACHABLE ARMS, DESK OR FULL LENGTH S WHEELCHAIR, FIXED FULL LENGTH ARMS, SWING AWAY DET MANUAL ADULT SIZE WHEELCHAIR, INCLUDES TILT IN SPA AMPUTEE WHEELCHAIR, FIXED FULL LENGTH ARMS, SWING AMPUTEE WHEELCHAIR, FIXED FULL LENGTH ARMS, WITHOU 1/1/2004 1/1/2004 1/1/2004 1/1/2007 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2016 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2010 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y N Y Y N N N N Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N Y N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC E1172 E1180 E1190 E1195 E1200 E1220 E1221 E1222 E1223 E1224 E1225 E1226 E1227 E1228 E1229 E1230 E1231 E1232 E1233 E1234 E1235 E1236 E1237 E1238 E1239 E1240 E1250 E1260 E1270 E1280 E1285 E1290 E1295 E1296 E1297 E1298 E1300 E1310 E1352 E1353 E1354 E1355 E1356 E1357 E1358 E1372 E1390 E1391 E1392 E1399 E1405 E1406 E1500 E1510 E1520 E1530 E1540 E1550 E1560 E1570 E1575 E1580 E1590 E1592 E1594 E1600 E1610 E1615 E1620 E1625 E1630 E1632 E1634 E1635 E1636 E1637 E1639 E1699 E1700 E1701 E1702 E1800 E1801 E1802 E1805 E1806 E1810 E1811 E1812 E1815 E1816 AMPUTEE WHEELCHAIR, DETACHABLE ARMS (DESK OR FULL AMPUTEE WHEELCHAIR, DETACHABLE ARMS (DESK OR FULL AMPUTEE WHEELCHAIR, DETACHABLE ARMS (DESK OR FULL HEAVY DUTY WHEELCHAIR, FIXED FULL LENGTH ARMS, SWI AMPUTEE WHEELCHAIR, FIXED FULL LENGTH ARMS, SWING WHEELCHAIR; SPECIALLY SIZED OR CONSTRUCTED, (INDIC WHEELCHAIR WITH FIXED ARM, FOOTRESTS WHEELCHAIR WITH FIXED ARM, ELEVATING LEGRESTS WHEELCHAIR WITH DETACHABLE ARMS, FOOTRESTS WHEELCHAIR WITH DETACHABLE ARMS, ELEVATING LEGREST WHEELCHAIR ACCESSORY, MANUAL SEMI-RECLINING BACK, WHEELCHAIR ACCESSORY, MANUAL FULLY RECLINING BACK, SPECIAL HEIGHT ARMS FOR WHEELCHAIR SPECIAL BACK HEIGHT FOR WHEELCHAIR WHEELCHAIR, PEDIATRIC SIZE, NOT OTHERWISE SPECIFIE POWER OPERATED VEHICLE (THREE OR FOUR WHEEL NONHIG WHEELCHAIR, PEDIATRIC SIZE, TILT-IN-SPACE, RIGID, WHEELCHAIR, PEDIATRIC SIZE, TILT-IN-SPACE, FOLDING WHEELCHAIR, PEDIATRIC SIZE, TILT-IN-SPACE, RIGID, WHEELCHAIR, PEDIATRIC SIZE, TILT-IN-SPACE, FOLDING WHEELCHAIR, PEDIATRIC SIZE, RIGID, ADJUSTABLE, WIT WHEELCHAIR, PEDIATRIC SIZE, FOLDING, ADJUSTABLE, W WHEELCHAIR, PEDIATRIC SIZE, RIGID, ADJUSTABLE, WIT WHEELCHAIR, PEDIATRIC SIZE, FOLDING, ADJUSTABLE, W POWER WHEELCHAIR, PEDIATRIC SIZE, NOT OTHERWISE SP LIGHTWEIGHT WHEELCHAIR, DETACHABLE ARMS, (DESK OR LIGHTWEIGHT WHEELCHAIR, FIXED FULL LENGTH ARMS, SW LIGHTWEIGHT WHEELCHAIR, DETACHABLE ARMS (DESK OR F LIGHTWEIGHT WHEELCHAIR, FIXED FULL LENGTH ARMS, SW HEAVY DUTY WHEELCHAIR, DETACHABLE ARMS (DESK OR FU HEAVY DUTY WHEELCHAIR, FIXED FULL LENGTH ARMS, SWI HEAVY DUTY WHEELCHAIR, DETACHABLE ARMS (DESK OR FU HEAVY DUTY WHEELCHAIR, FIXED FULL LENGTH ARMS, ELE SPECIAL WHEELCHAIR SEAT HEIGHT FROM FLOOR SPECIAL WHEELCHAIR SEAT DEPTH, BY UPHOLSTERY SPECIAL WHEELCHAIR SEAT DEPTH AND/OR WIDTH, BY CON WHIRLPOOL, PORTABLE (OVERTUB TYPE) WHIRLPOOL, NON-PORTABLE (BUILT-IN TYPE) OXYGEN ACC FLOW REG CPBL POS INSPIRATORY PRESS REGULATOR O2 ACCESS WHEELED CART PRTBLE CYL/CONC REPL EA STAND/RACK O2 ACCESS BTTRY PACK/CRTRDGE PRTBLE CONC REPL EA O2 ACCESS BATTRY CHARGER PRTBLE CONC REPL EA O2 ACCESS DC POWER ADAPTER PRTBLE CONC REPL EA IMMERSION EXTERNAL HEATER FOR NEBULIZER OXYGEN CONCENTRATOR, SINGLE DELIVERY PORT, CAPABLE OXYGEN CONCENTRATOR, DUAL DELIVERY PORT, CAPABLE O Portable oxygen concentrator, rental DURABLE MEDICAL EQUIPMENT, MISCELLANEOUS OXYGEN AND WATER VAPOR ENRICHING SYSTEM WITH HEATE OXYGEN AND WATER VAPOR ENRICHING SYSTEM WITHOUT HE CENTRIFUGE, FOR DIALYSIS KIDNEY, DIALYSATE DELIVERY SYST. KIDNEY MACHINE, P HEPARIN INFUSION PUMP FOR HEMODIALYSIS AIR BUBBLE DETECTOR FOR HEMODIALYSIS, EACH, REPLAC PRESSURE ALARM FOR HEMODIALYSIS, EACH, REPLACEMENT BATH CONDUCTIVITY METER FOR HEMODIALYSIS, EACH BLOOD LEAK DETECTOR FOR HEMODIALYSIS, EACH, REPLAC ADJUSTABLE CHAIR, FOR ESRD PATIENTS TRANSDUCER PROTECTORS/FLUID BARRIERS, FOR HEMODIAL UNIPUNCTURE CONTROL SYSTEM FOR HEMODIALYSIS HEMODIALYSIS MACHINE AUTOMATIC INTERMITTENT PERITIONEAL DIALYSIS SYSTEM CYCLER DIALYSIS MACHINE FOR PERITONEAL DIALYSIS DELIVERY AND/OR INSTALLATION CHARGES FOR HEMODIALY REVERSE OSMOSIS WATER PURIFICATION SYSTEM, FOR HEM DEIONIZER WATER PURIFICATION SYSTEM, FOR HEMODIALY BLOOD PUMP FOR HEMODIALYSIS, REPLACEMENT WATER SOFTENING SYSTEM, FOR HEMODIALYSIS RECIPROCATING PERITONEAL DIALYSIS SYSTEM WEARABLE ARTIFICIAL KIDNEY, EACH PERITONEAL DIALYSIS CLAMPS, EACH COMPACT (PORTABLE) TRAVEL HEMODIALYZER SYSTEM SORBENT CARTRIDGES, FOR HEMODIALYSIS, PER 10 HEMOSTATS, EACH SCALE, EACH DIALYSIS EQUIPMENT, NOT OTHERWISE SPECIFIED JAW MOTION REHABILITATION SYSTEM REPLACEMENT CUSHIONS FOR JAW MOTION REHABILITATION REPLACEMENT MEASURING SCALES FOR JAW MOTION REHABI DYNAMIC ADJUSTABLE ELBOW EXTENSION/FLEXION DEVICE, BI-DIRECTIONAL STATIC PROGRESSIVE STRETCH ELBOW DE DYNAMIC ADJUSTABLE FOREARM PRONATION/SUPINATION DE DYNAMIC ADJUSTABLE WRIST EXTENSION / FLEXION DEVIC BI-DIRECTIONAL STATIC PROGRESSIVE STRETCH WRIST DE DYNAMIC ADJUSTABLE KNEE EXTENSION / FLEXION DEVICE BI-DIRECTIONAL STATIC PROGRESSIVE STRETCH KNEE DEV DYNAMIC KNEE, EXTENSION/FLEXION DEVICE W/ACTIVE RE DYNAMIC ADJUSTABLE ANKLE EXTENSION/FLEXION DEVICE, BI-DIRECTIONAL STATIC PROGRESSIVE STRETCH ANKLE DE 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2014 1/1/2004 1/1/2009 1/1/2004 1/1/2009 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 N N N N N N N N N N N N N N N Y N Y Y Y Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y Y Y Y N Y Y N Y N N Y N OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 OUTPT ASC 13X AND 83X & POS 22 OR 24 HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC E1818 E1820 E1821 E1825 E1830 E1831 E1840 E1841 E1902 E2000 E2100 E2101 E2120 E2201 E2202 E2203 E2204 E2205 E2206 E2207 E2208 E2209 E2210 E2211 E2212 E2213 E2214 E2215 E2216 E2217 E2218 E2219 E2220 E2221 E2222 E2224 E2225 E2226 E2227 E2228 E2230 E2231 E2291 E2292 E2293 E2294 E2295 E2300 E2301 E2310 E2311 E2312 E2313 E2321 E2322 E2323 E2324 E2325 E2326 E2327 E2328 E2329 E2330 E2331 E2340 E2341 E2342 E2343 E2351 E2358 E2359 E2360 E2361 E2362 E2363 E2364 E2365 E2366 E2367 E2368 E2369 E2370 E2371 E2372 E2373 E2374 E2375 E2376 E2377 E2378 E2381 BI-DIRECTIONAL STATIC PROGRESSIVE STRETCH FOREARM REPLACEMENT SOFT INTERFACE MATERIAL, DYNAMIC ADJUS REPLACEMENT SOFT INTERFACE MATERIAL/CUFFS FOR BI-D DYNAMIC ADJUSTABLE FINGER EXTENSION/FLEXION DEVICE DYNAMIC ADJUSTABLE TOE EXTENSION/FLEXION DEVICE, I STATIC PROGRESSIVE STRETCH TOE DEVICE DYNAMIC ADJUSTABLE SHOULDER FLEXION / ABDUCTION / MULTI-DIRECTIONAL STATIC PROGRESSIVE STRETCH SHOUL COMMUNICATION BOARD, NON-ELECTRONIC AUGMENTATIVE O GASTRIC SUCTION PUMP, HOME MODEL, PORTABLE OR STAT BLOOD GLUCOSE MONITOR WITH INTEGRATED VOICE SYNTHE BLOOD GLUCOSE MONITOR WITH INTEGRATED LANCING/BLOO PULSE GENERATOR SYSTEM FOR TYMPANIC TREATMENT OF I MANUAL WHEELCHAIR ACCESSORY, NONSTANDARD SEAT FRAM MANUAL WHEELCHAIR ACCESSORY, NONSTANDARD SEAT FRAM MANUAL WHEELCHAIR ACCESSORY, NONSTANDARD SEAT FRAM MANUAL WHEELCHAIR ACCESSORY, NONSTANDARD SEAT FRAM MANUAL WHEELCHAIR ACCESSORY, HANDRIM WITHOUT PROJE MANUAL WHEELCHAIR ACCESSORY, WHEEL LOCK ASSEMBLY, Wheelchair accessory, crutch and cane holder, each Wheelchair accessory, cylinder tank carrier, each Wheelchair accessory, arm trough, each Wheelchair accessory, bearings, any type replaceme Manual wheelchair accessory, pneumatic propulsion Manual wheelchair accessory, tube for pneumatic pr Manual wheelchair accessory, insert for pneumatic Manual wheelchair accessory, pneumatic caster tire Manual wheelchair accessory, tube for pneumatic ca MANUAL WHEELCHAIR ACCESSORY,FOAM FILLED PROPULSION MANUAL WHEELCHAIR ACCESSORY, FOAM FILLED CASTER TI MANUAL WHEELCHAIR ACCESSORY,FOAM PROPULSION TIRE,A Manual wheelchair accessory, foam caster tire, any Manual wheelchair accessory, solid (rubber/plastic Manual wheelchair accessory, solid (rubber/plastic Manual wheelchair accessory, solid (rubber/plastic Manual wheelchair accessory, propulsion wheel excl Manual wheelchair accessory, caster wheel excludes Manual wheelchair accessory, caster fork, any size MANUAL WC ACCESS GEAR REDUCTION DRIVE WHEEL EACH MNL WC ACCESS WHEEL BRAKING SYS&LOCK COMPLETE EA MANUAL WHEELCHAIR ACCESSORY MANUAL STANDING SYS MNL WC ACCESS SOLID SEAT SUPP BASE INCL HARDWARE BACK, PLANAR, FOR PEDIATRIC SIZE WHEELCHAIR INCLUD SEAT, PLANAR, FOR PEDIATRIC SIZE WHEELCHAIR INCLUD BACK, CONTOURED, FOR PEDIATRIC SIZE WHEELCHAIR INC SEAT, CONTOURED, FOR PEDIATRIC SIZE WHEELCHAIR INC MNL WC ACCESS PED SIZE WC DYNAMIC SEATING FRAME WHEELCHAIR ACC PWR SEAT ELEVATION SYS ANY TYPE WHEELCHAIR ACCESSORY POWER STANDING SYS ANY TYPE POWER WHEELCHAIR ACCESSORY, ELECTRONIC CONNECTION POWER WHEELCHAIR ACCESSORY, ELECTRONIC CONNECTION POWER WC ACCESS HAND OR CHIN CONTROL INTERFACE POWER WC ACCESS HARNESS UPGRADE EXP CONTROLLR EA POWER WHEELCHAIR ACCESSORY, HAND CONTROL INTERFACE POWER WHEELCHAIR ACCESSORY, HAND CONTROL INTERFACE POWER WHEELCHAIR ACCESSORY, SPECIALTY JOYSTICK HAN POWER WHEELCHAIR ACCESSORY, CHIN CUP FOR CHIN CONT POWER WHEELCHAIR ACCESSORY, SIP AND PUFF INTERFACE POWER WHEELCHAIR ACCESSORY, BREATH TUBE KIT FOR SI POWER WHEELCHAIR ACCESSORY, HEAD CONTROL INTERFACE POWER WHEELCHAIR ACCESSORY, HEAD CONTROL OR EXTREM POWER WHEELCHAIR ACCESSORY, HEAD CONTROL INTERFACE POWER WHEELCHAIR ACCESSORY, HEAD CONTROL INTERFACE POWER WHEELCHAIR ACCESSORY, ATTENDANT CONTROL, PRO POWER WHEELCHAIR ACCESSORY, NONSTANDARD SEAT FRAME POWER WHEELCHAIR ACCESSORY, NONSTANDARD SEAT FRAME POWER WHEELCHAIR ACCESSORY, NONSTANDARD SEAT FRAME POWER WHEELCHAIR ACCESSORY, NONSTANDARD SEAT FRAME POWER WHEELCHAIR ACCESSORY, ELECTRONIC INTERFACE T PWR WC ACCESS GRP 34 NONSEALED LEAD ACID BATT EA PWR WC ACCESSORY GRP 34 SEALED LEAD ACID BATT EA POWER WHEELCHAIR ACCESSORY, 22 NF NON-SEALED LEAD POWER WHEELCHAIR ACCESSORY, 22NF SEALED LEAD ACID POWER WHEELCHAIR ACCESSORY, GROUP 24 NON-SEALED LE POWER WHEELCHAIR ACCESSORY, GROUP 24 SEALED LEAD A POWER WHEELCHAIR ACCESSORY, U-1 NON-SEALED LEAD AC POWER WHEELCHAIR ACCESSORY, U-1 SEALED LEAD ACID B POWER WHEELCHAIR ACCESSORY, BATTERY CHARGER, SINGL POWER WHEELCHAIR ACCESSORY, BATTERY CHARGER, DUAL POWER WHEELCHAIR COMPONENT, MOTOR, REPLACEMENT ONL POWER WHEELCHAIR COMPONENT, GEAR BOX, REPLACEMENT POWER WHEELCHAIR COMPONENT, MOTOR AND GEAR BOX COM Power wheelchair accessory, group 27 sealed lead a POWER WHEELCHAIR ACCESSORY,GROUP 27 NON-SEALED LEA Hand/chin ctrl spec joystick Hand/chin ctrl std joystick Non-expandable controller Expandable controller, repl Expandable controller, initl POWER WHEELCHAIR COMPONENT ACTUATOR REPLACE ONLY Pneum drive wheel tire 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2006 1/1/2008 1/1/2008 1/1/2009 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2009 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2008 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2006 1/1/2006 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2013 1/1/2007 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 Y N N Y N N Y Y N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N Y N N N N N N N N N N N Y Y N Y Y N N Y N Y Y Y Y N N N N N N N N N N N N N N N N N N N N N N N N Y N N N HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC HCPC E2382 E2383 E2384 E2385 E2386 E2387 E2388 E2389 E2390 E2391 E2392 E2394 E2395 E2396 E2397 E2402 E2500 E2502 E2504 E2506 E2508 E2510 E2511 E2512 E2599 E2601 E2602 E2603 E2604 E2605 E2606 E2607 E2608 E2609 E2610 E2611 E2612 E2613 E2614 E2615 E2616 E2617 E2619 E2620 E2621 E2622 E2623 E2624 E2625 E2626 E2627 E2628 E2629 E2630 E2631 E2632 E2633 E8000 E8001 E8002 G0008 G0009 G0010 G0027 G0101 G0102 G0103 G0104 G0105 G0106 G0108 G0109 G0117 G0118 G0120 G0121 G0122 G0123 G0124 G0127 G0128 G0129 G0130 G0141 G0143 G0144 G0145 G0147 G0148 G0151 G0152 Tube, pneum wheel drive tire Insert, pneum wheel drive Pneumatic caster tire Tube, pneumatic caster tire Foam filled drive wheel tire Foam filled caster tire Foam drive wheel tire Foam caster tire Solid drive wheel tire Solid caster tire Solid caster tire, integrate Drive wheel excludes tire Caster wheel excludes tire Caster fork POWER WHLCHAIR ACCESSORY LITHIUM-BASED BATTRY EA NEGATIVE PRESSURE WOUND THERAPY ELECTRICAL PUMP, S SPEECH GENERATING DEVICE, DIGITIZED SPEECH, USING SPEECH GENERATING DEVICE, DIGITIZED SPEECH, USING SPEECH GENERATING DEVICE, DIGITIZED SPEECH, USING SPEECH GENERATING DEVICE, DIGITIZED SPEECH, USING SPEECH GENERATING DEVICE, SYNTHESIZED SPEECH, REQU SPEECH GENERATING DEVICE, SYNTHESIZED SPEECH, PERM SPEECH GENERATING SOFTWARE PROGRAM, FOR PERSONAL C ACCESSORY FOR SPEECH GENERATING DEVICE, MOUNTING S ACCESSORY FOR SPEECH GENERATING DEVICE, NOT OTHERW GENERAL USE WHEELCHAIR SEAT CUSHION, WIDTH LESS TH GENERAL USE WHEELCHAIR SEAT CUSHION, WIDTH 22 INCH SKIN PROTECTION WHEELCHAIR SEAT CUSHION, WIDTH LES SKIN PROTECTION WHEELCHAIR SEAT CUSHION, WIDTH 22 POSITIONING WHEELCHAIR SEAT CUSHION, WIDTH LESS TH POSITIONING WHEELCHAIR SEAT CUSHION, WIDTH 22 INCH SKIN PROTECTION AND POSITIONING WHEELCHAIR SEAT CU SKIN PROTECTION AND POSITIONING WHEELCHAIR SEAT CU CUSTOM FABRICATED WHEELCHAIR SEAT CUSHION, ANY SIZ WHEELCHAIR SEAT CUSHION, POWERED GENERAL USE WHEELCHAIR BACK CUSHION, WIDTH LESS TH GENERAL USE WHEELCHAIR BACK CUSHION, WIDTH 22 INCH POSITIONING WHEELCHAIR BACK CUSHION, POSTERIOR, WI POSITIONING WHEELCHAIR BACK CUSHION, POSTERIOR, WI POSITIONING WHEELCHAIR BACK CUSHION, POSTERIOR-LAT POSITIONING WHEELCHAIR BACK CUSHION, POSTERIOR-LAT CUSTOM FABRICATED WHEELCHAIR BACK CUSHION, ANY SIZ REPLACEMENT COVER FOR WHEELCHAIR SEAT CUSHION OR B POSITIONING WHEELCHAIR BACK CUSHION, PLANAR BACK W POSITIONING WHEELCHAIR BACK CUSHION, PLANAR BACK W SKIN PROTECT WC SEAT CUSH WIDTH <22 IN ANY DEPTH SKIN PROTCT WC SEAT CUSH WIDTH 22 IN/> ANY DEPTH SKIN PROTECT & POSITIONING WC CUSH WIDTH < 22 IN SKIN PROTECT & POSITIONING WC CUSH WIDTH 22 IN/> WC ACCESS SHLDR ELB MOBIL ARM SUPP WC ADJUSTBLE WC ACCESS SHLDR ELB M ARM SUPP ADJUSTBL RANCHO WC ACCESS SHLDR ELB MOBIL ARM SUPP WC RECLINING WC ACCESS SHLDR ELB M ARM SUPP FRICTION ARM SUPP WC ACCESS SHLDR ELB MOBIL MONOSUSP ARM HAND SUPP WC ACCESS ADD MOBILE ARM SUPPORT ELEV PROX ARM WC ACCESS ADD MOBIL ARM SUPP OFFSET/LAT RCKR ARM WC ACCESS ADD MOBILE ARM SUPPORT SUPINATOR GAIT TRAINER, PEDIATRIC SIZE, POSTERIOR SUPPORT, I GAIT TRAINER, PEDIATRIC SIZE, UPRIGHT SUPPORT, INC GAIT TRAINER, PEDIATRIC SIZE, ANTERIOR SUPPORT, IN ADMINISTRATION OF INFLUENZA VIRUS VACCINE ADMINISTRATION OF PNEUMOCOCCAL VACCINE ADMINISTRATION OF HEPATITIS B VACCINE SEMEN ANALYSIS; PRESENCE AND/OR MOTILITY OF SPERM CERVICAL OR VAGINAL CANCER SCREENING; PELVIC AND C PROSTATE CANCER SCREENING; DIGITAL RECTAL EXAMINAT PROSTATE CANCER SCREENING; PROSTATE SPECIFIC ANTIG COLORECTAL CANCER SCREENING; FLEXIBLE SIGMOIDOSCOP COLORECTAL CANCER SCREENING; COLONOSCOPY ON INDIVI COLORECTAL CANCER SCREENING; ALTERNATIVE TO G0104, DIABETES OUTPATIENT SELF-MANAGEMENT TRAINING SERVI DIABETES OUTPATIENT SELF-MANAGEMENT TRAINING SERVI GLAUCOMA SCREENING FOR HIGH RISK PATIENTS FURNISHE GLAUCOMA SCREENING FOR HIGH RISK PATIENT FURNISHED COLORECTAL CANCER SCREENING; ALTERNATIVE TO G0105, COLORECTAL CANCER SCREENING; COLONOSCOPY ON INDIVI COLORECTAL CANCER SCREENING; BARIUM ENEMA SCREENING CYTOPATHOLOGY, CERVICAL OR VAGINAL (ANY SCREENING CYTOPATHOLOGY, CERVICAL OR VAGINAL (ANY TRIMMING OF DYSTROPHIC NAILS, ANY NUMBER DIRECT (FACE-TO-FACE WITH PATIENT) SKILLED NURSING OCCUPATIONAL THERAPY REQUIRING THE SKILLS OF A QUA SINGLE ENERGY X-RAY ABSORPTIOMETRY (SEXA) BONE DEN SCREENING CYTOPATHOLOGY SMEARS, CERVICAL OR VAGINA SCREENING CYTOPATHOLOGY, CERVICAL OR VAGINAL (ANY SCREENING CYTOPATHOLOGY, CERVICAL OR VAGINAL (ANY SCREENING CYTOPATHOLOGY, CERVICAL OR VAGINAL (ANY SCREENING CYTOPATHOLOGY SMEARS, CERVICAL OR VAGIN SCREENING CYTOPATHOLOGY SMEARS, CERVICAL OR VAGINA SERVICE PHYS THERAP HOME HLTH/HOSPICE EA 15 MIN SERVICE OCCUP THERAP HOME HLTH/HOSPICE EA 15 MIN 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2007 1/1/2008 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2011 1/1/2011 1/1/2011 1/1/2011 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2012 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 1/1/2004 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/31/9999 12/3