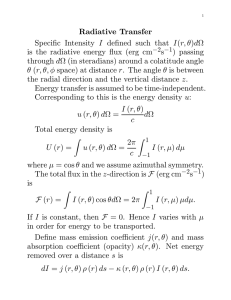
The Principle of Conservation of Energy In its most general form, the
advertisement

METR 201: Physical Processes Dr. Dave Dempsey The Principle of Conservation of Energy In its most general form, the Principle of Conservation of Energy says that energy is “conserved”—that is, it can’t be created or destroyed, so that the total amount of energy in any closed system (that is, a system that can’t exchange energy with anything outside the system) doesn’t change. However, energy exists in many forms, and it can change from one form to another. For example, when an object emits radiative energy (which most objects do, all the time), that energy must come at the expense of some other form of energy, and it in fact comes at the expense of an equivalent amount of heat energy in the emitting substance. (Heat is a form of internal energy associated with the random molecular motions of a substance). Similarly, when an object absorbs radiative energy, that energy is converted into an equivalent amount of heat in the object. As another example, when a substance changes phase (from gas, liquid, or solid to one of the other two phases), energy transforms from heat in the substance to an equivalent amount of latent heat (a form of chemical potential energy) in the substance, or vice versa, depending on the direction of the phase change. We can apply the principle of conservation of energy to write a version specific to the heat content of an object, creating a heat budget equation for the object. In it’s most general form, we can write the heat budget equation as: Rate of change of heat content of the object = Sum of rates at which object gains heat − by various mechanisms (sum of “sources” of heat) Sum of rates at which object loses heat by various mechanisms (sum of “sinks” of heat) If we apply this general form of the heat budget equation to patch of the earth’s surface (and ignore very small sources and sinks), we get: Rate of change of heat content of the object = Rate of absorption of solar radiative energy + ± Rate of transformation ± of latent heat to/from heat due to phase changes of water Rate of conduction of heat from/to air in contact with it ± Rate of conduction of heat from/to material below Rate of emission of radiative energy by the surface Rate of absorption of infrared radiation emitted downward by the atmosphere − Rate of absorption of solar radiative energy We can relate the rate of absorption of solar radiation by the surface [(Rsolabs)sfc], the first term on the right-­‐hand side above and a mechanism by which the surface gains heat, to: o the flux or intensity (rate per unit area) of solar radiative energy arriving at the top of the atmosphere on a surface directly facing the sun (that is, perpendicular to the sun’s rays), ( SArrAtm! ,where the symbol ! means “perpendicular to”); o the fraction of that radiation that reaches the surface without being scattered or reflected back to space or absorbed by the atmosphere (that is, transmitted by the atmosphere) (τatm, the transmissivity of the atmosphere); o a factor accounting for the degree of reduction in the intensity of the remaining solar radiation when it strikes the horizontal earth’s surface at an angle and spreads out across it (sin(θsun), where θsun is the angle between the sun’s rays and the horizontal surface, or sun angle); o the fraction of the solar radiation arriving at the surface that the surface absorbs (asfc, the absorptivity of the surface); and o the area of the surface (Asfc) Specifically: (Rsolabs)sfc = SArrAtm! ✕ τatm✕ sin(θsun) ✕ asfc ✕ Asfc (Note that the solar absorption flux at the earth’s surface, (Sabs)sfc = SArrAtm! ✕ τatm✕ sin(θsun) ✕ asfc., and we invoke the generalized relation between any rate and flux: flux = rate/area.) Three categories of things can happen to radiative energy arriving on a surface: (1) it can be scattered or reflected away (without changing form); (2) it can be transmitted (that is, pass through without changing form); or it can be absorbed (and converted into heat). That is, we can write: Flux, rate, or amount Flux, rate, or amount of radiative energy = scattered or reflected striking a surface back by the surface + Flux, rate, or amount transmitted through the surface + Flux, rate, or amount absorbed by the surface Dividing both sides of this equation by the left-­‐hand side (the total flux, rate, or amount of radiative energy striking the surface) gives us: 1 = α + τ + a where α is the albedo (reflectivity) of the surface, τ is the transmissivity of the surface, and a is the absorptivity of the surface, each of which is defined as a fraction of the total flux, rate, or amount of radiative energy arriving at the surface in the first place. For an opaque surface on the surface of the earth, for example (that is, a surface that doesn’t transmit radiative energy, so τsfc = 0), then 1 = αsfc + asfc ! asfc = 1 – αsfc, and we can express the absorptivity in terms of the albedo very simply. Hence, for an opaque surface at the surface of the earth we can write the absorption rate of solar radiation by that surface as: (Rsolabs)sfc = SArrAtm! ✕ τatm✕ sin(θsun) ✕ (1 – αsfc)✕ Asfc Rate of Conduction Conduction is the direct transfer of heat from one object to another that in direct contact with it and at a different temperature, via collisions between the molecules of each substance in random motion. When the faster-­‐moving molecules of the warmer object collide with the slower-­‐moving molecules of the cooler object, the faster molecules slow down and the slower molecules speed up. Because temperature is a measure of the average energy of random molecular motions in a material, this means that the cooler object warms and the warmer object, which loses exactly as much heat as the cooler object gains, cools. The rate at which heat conducts from one object to another is proportional to the temperature difference between them—the bigger the temperature difference between them, the faster heat will conduct from the warmer object to the colder one. Not all objects conduct heat equally well, though. Air, for example, is a poor conductor of heat, and so heat does not conduct through air well. Rock, sand, soil, ice, and liquid water are not great heat conductors, either, but are somewhat better than air. In the atmosphere, we typically ignore heat conduction except between the earth’s surface and the atmosphere, where temperature differences can become large enough to overcome the poor conductivity of the materials involved. Rate of emission of radiative energy For this term (the last term in the conservation equation for heat, representing a sink of heat for an object), we start with the Stefan-­‐ Boltzmann Law, which states that the intensity of emission of radiative energy (that is, the radiative energy emission flux) of a blackbody ( E(T ) ) is proportional to the absolute temperature of the object (T), raised to the fourth power: E(T ) = ! T 4 A blackbody is an object that absorbs all radiative energy that strikes it (that is, it reflects and transmits no radiation), and emits radiative energy with the theoretically maximum intensity (flux) possible. (The sun acts nearly as a blackbody, for example, and the earth acts as a blackbody for infrared radiation, and while the earth is not a blackbody for solar radiation [clouds reflect visible light well, for example], the earth isn’t hot enough to emit solar radiation so the Stefan-­‐Boltzmann relation still applies to it well. Not all objects act as blackbodies, however, so the Stefan-­‐Boltzmann relation sometimes has to be modified for them, and even when modified it doesn’t apply well to some objects.) Note that the radiative emission flux, E(T ) , is written to emphasize that it is a function of—that is, it depends upon or varies with—the absolute temperature, T. The Greek symbol ! (lower case “sigma”) represents the constant of proportionality between E(T ) and T 4 . In MKS units it has the value of 5.67×10-­‐8(Watts/m2)/K4. E(T ) is a flux, not a rate, so to determine the total rate at which the object loses heat, we have to multiply this by the total surface area of the object, Asfc: Rate of blackbody radiative emission = Asfc ! E(T ) By substituting from the Stefan-­‐Boltzmann relation, we relate this to the object’s absolute temperature: Rate of blackbody radiative emission = Asfc ! ! T 4 This tells us that the warmer an object is, the faster it loses heat by radiative emission. Relating rate of change of heat content to rate of change of temperature of an object: Heat is a form of energy, the total (kinetic) energy of random molecular motions in an object. Temperature is a measure of the average kinetic energy of random molecular motions in a material. Heat and temperature aren’t the same, but they are related. We can relate the rates at which an object’s heat content and temperature change: " T (t2 ) ! T (t1 ) % H (t2 ) ! H (t1 ) = cH m $ '& t2 ! t1 t2 ! t1 # where H (t ) represents the heat content of an object (written to emphasize that is a function of—that is, it depends upon or varies with—time, t); m is the mass of the object; cH is the specific heat of the object; and T (t ) is the temperature of the object (also written to emphasize that it is a function of time). Using common shorthand notational convention for the differences in this relation (the ! , or “difference”), we can also write the relation as: !H (t ) !T (t ) = cH m !t !t