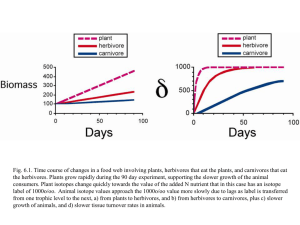
Induced Plant Responses to Herbivory
advertisement
ANNUAL
REVIEWS
Further
Quick links to online content
Annu. Rev. Ecol. Syst.
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
Copyright
© 1989
1989. 20:331-48
by Annual Reviews Inc. All rights reserved
INDUCED PLANT RESPONSES TO
HERBIVORY
Richard Karban
Department of Entomology, University of California, Davis, California 95616
Judith H. Myers
The Ecology Group and Departments of Plant Science and Zoology, University of
British Columbia, Vancouver, British Columbia, V6T lW5 Canada
PHENOMENA OF INDUCED PLANT RESPONSES
Changes in plants following damage or stress are called "induced responses."
In the broadest sense, these changes can increase the "resistance" of the plant
to further herbivore attack by reducing the preference for, or effect of,
herbivores on the damaged plant. It should not be assumed that these changes
which provide resistance evolved as a result of selection by herbivores. In
some cases the reponses may currently act as "induced defenses"; that is, they
are responses by the plant to herbivore injury or the invasion of microparasites
that decrease the negative fitness consequences of attacks on the plant. These
terms-"induced resistance" and "induced defense"-are used by different
people to mean a variety of different things. Workers in this field would
benefit by agreeing upon a set of definitions, and we offer a dichotomous key
of these terms (Table 1). Note that an induced response could conceivably
operate as a defense without decreasing herbivore preference or performance.
Instead, it may make the plant more tolerant to herbivory. Although "induced
defenses" are widely discussed, to our knowledge no one has shown an
induced response to be defensive, i.e. no one has explicitly measured the
influences of the change on the fitness of the plant.
Not all induced plant responses increase resistance by making plants less
suitable as hosts. On the contrary, an extensive literature describes increases
331
0066-4162/8911120-0331$02.00
332
KARBAN & MYERS
Table 1
A dichotomous key for induced responses
Does stress or injury change plant quality?
1
NO: No response
l' YES: INDUCED RESPONSE (proceed to 2)
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
Does the induced response decrease herbivore preference or performance?
2
NO: No effect or induced susceptibility
2' YES: INDUCED RESISTANCE (proceed to 3)
Does reduced herbivore preference/performance increase plant fitness?
3 NO: The plant is not defended by the response
3' YES: INDUCED DEFENSE
in plant quality following injury caused by drought (104), nutrient deficiency
(70), solar radiation (66), low temperature (46), high temperature (94), air
pollution (20), and previous damage caused by herbivory (107). Much of the
evidence for changes in resistance associated with induced responses comes
from bioassays of induced foliage under laboratory or artificial field con­
ditions (reviewed in 28, 84). While the proportion of cases in which induced
responses act as defenses against herbivores may be uncertain, we would like
to consider in this review the characteristics of changes that relate to their role
as defenses. What are the changes, why and how might they occur, and what
might be done to further understand their influence on plant-herbivore in­
teractions? Specifically, which changes are likely to act as effective defenses
and how might they work? Which herbivores are likely to be affected? Have
these responses evolved as defenses against herbivores? Under what con­
ditions might selection favor facultative induced defenses rather than pre­
formed constitutive defenses?
WHAT CHANGES FOLLOW DAMAGE?
Secondary Metabolites and Phytoalexins
Injuries to plant tissues cause a wide array of plant responses. The nature of
the response varies with plant type. One area of progress has been to recog­
nize that the way trees respond is associated with their growth pattern and
nutrient status (14). A cataloging of plant responses is beyond the scope of
this review, although a few representative examples are provided. Many
studies of induced responses have considered changes in tannins and phenols,
products of the shikimic acid pathway. Relative activity of the enzyme
phenylalanine ammonia lyase (PAL) can determine the production of pheno­
lics, including lignin (19). Induction of the phytohormone ethylene by tissue
damage may influence the production of PAL and therefore the concentration
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
INDUCED RESPONSES
333
of secondary metabolites (110) and leaf toughness (47). The exact role of
ethylene in this process remains controversial (72). Many agents of environ­
mental stress correlated to herbivory can also cause increases in secondary
metabolites (71). Patterns often vary depending on the history of the plant.
The balance between many primary and secondary metabolites influences the
response of plants to stress and also the effects that these plant responses will
have on herbivores. Herbivore damage often affects the concentrations of
available nitrogen and other important nutrients in foliage (7, 97). A major
problem facing workers in this area is determining which of the many
secondary plant chemicals and plant nutrients that change following damage
or stress are responsible for the overall effects on herbivores. The range of
induced changes is so great that it is impossible to investigate all these factors
and difficult to determine rationally which are worthy of study.
In some instances, herbivores elicit plants to synthesize phytoalexins (1,
68, 100). Phytoalexins are low molecular-weight, antimicrobial compounds
(63) usually present in plants at extremely low concentrations prior to infec­
tion. These can be synthesized de novo by plants following microbial infec­
tion, and effectiveness is determined by the speed and magnitude at which
they are produced and accumulated (62). Limited evidence suggests that
phytoalexins may be active against insects as well as plant pathogens (90, 95,
74, 32).
Physiological and Morphological Changes
The response of plants to herbivores can be more extensive than simply
modifications of secondary metabolite concentrations. For example, spider
mites cause widespread changes in the cytology, histology, and physiology of
their host plants, including modifications of photosynthetic and transpiration­
al rates, and they can inject substances that can act as plant growth regulators
(reviewed by 59).
Herbivores can influence the morphology of their food plants by causing
increases in the density of prickles, spines, and hairs (reviewed by 79), by
causing the return to juvenile growth form (11), or by affecting the phenology
of plant processes such as leaf abscission (106). Many herbivores are "spe­
cialists" on plant tissue of a particular physiological age, so that altering the
synchrony between plant and insect could act to make the plant appear more
resistant. All of these changes could have an influence on herbivores, or on
the extent of further herbivory.
DYNAMICS OF PLANT CHANGE FOLLOWING
HERBIVORE DAMAGE
Plants respond to herbivore damage over spatial scales ranging from single
leaves to whole trees and over temporal scales ranging from minutes to
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
334
KARBAN & MYERS
evolutionary time. Most of the studies that point to induced resistance,
assayed as a decrease in herbivore performance, have found that the response
was systemic at least to other parts of the damaged shoot. However, one study
measuring rapid increases in foliage phenols found that this chemical response
was not systemic in birch trees (99). The spatial extent of the induced
response may determine whether the response acts as a defense. A localized
response may encourage herbivores to feed elsewhere on the same plant;
damage to the plant will be spread but not reduced. Surprisingly, no study has
explicitly mapped the spatial extent of induced resistance in all parts of the
entire plant. Despite the exciting suggestion by Rhoades (86) and Baldwin &
Schultz (4) that plants may become more resistant in response to cues released
by damaged neighbors, subsequent experiments have been few and have not
supported the idea (80, 28).
Some responses are known to occur within several hours after damage, as
in the case of proteinase inhibitors in damaged foliage of solanaceous plants
(reviewed in 108) or latex in damaged cucurbits (16). What component of
damage signals rapidly induced responses is generally not known. Damage to
tissues may release cellwall fragments that are translocated to other parts of
the plant where they activate genes that code for enzymes, such as proteinase
inhibitors (91). In this case the signal is transported systemically within
injured tomato plants but is directed primarily up the stem from older leaves to
younger ones (69). The proteinase inhibitors accumulate in vacuoles of
uninjured cells of injured plants and are deleterious to some caterpillars 00).
Induced resistance need not involve de novo synthesis; damage may bring
preformed enzymes and substrates into contact, causing the production of
active agents (21). Enzymatic activation of compartmentalized precursors is
responsible for many reactions, including the cyanogenic response of plants to
herbivores (21, 49). Damage to tissue may release ethylene that stimulates the
production of PAL and increases in phenolics (110, see also 72). Phenolics
are not transported from damaged to undamaged birch leaves; rather, they are
synthesized in undamaged leaves following increases in PAL activity (34).
The mechanisms of responses that occur over several years are also poorly
understood. Plant tissue that dcvelops in the growing season after marked
defoliation often shows increases in phenolics and fiber, declines in nutrient
concentrations, regrowth of juvenile tissue, and changes in plant morphology
(rcvicwed in 102, 79).
MECHANISMS: ACTIVE RESISTANCE OR PASSIVE
DETERIORATION?
While enzymatic activation of precursors and synthesis of phytoalexins and
proteinase inhibitors are clearly active processes, changes in plant chemistry
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
INDUCED RESPONSES
335
following defoliation may result from a passive rearrangement of resources
within the plant. The distinction is that active responses involve de novo
synthesis or energetically costly enzymatic processes, whereas passive re­
sponses involve only the consequences of tissue removal. Passive responses
have been described as nutrient stress by Tuomi and coworkers (98, 99), as
carbon-nutrient imbalance by Bryant and his associates (13, 14), and as
passive deterioration by Myers & Williams (80). According to this hypoth­
esis, a tree growing in an area with abundant soil nutrients (a fast growing
tree) loses proportionately more nitrogen and other nutrients and less carbon
during defoliation because it had proportionately more nitrogen in its leaves.
Subsequently, carbon may be replaced in the leaves at a faster rate than
nitrogen, and the surplus allocated to carbon-based allelochemicals (terpenes,
resins, tannins, and other phenolics) and fiber. These foliar changes are
expected to reduce the preference and performance of herbivores on trees that
were previously defoliated. On the other hand, trees growing in nutrient-poor
conditions or which store proportionately more carbon in their leaves (ever­
greens) may respond in the opposite way; defoliation may reduce the con­
centrations of carbon-based chemicals and increase the palatability of leaves
of these slow-growing trees in the next growing season (14, 15, 24, 98).
This model leads to several testable predictions (see also 98). (a) Nitrogen
fertilization of defoliated trees should negate the nutrient imbalance and
cancel the induced response; (b) carbon stress should result in a collapse of
carbon-based resistance; (c) if herbivory and plant crowding reduce the same
nutrients, then the effects of these two stresses should be qualitatively similar
(57). Experimental N fertilization of birch trees increased foliar nitrogen and
reduced phenolics, while root damage, which reduced nutrient uptake, re­
duced foliar nitrogen and increased phenolics (97). Larsson et al (64) found
similar patterns between carbon availability (light) and carbon-based pheno­
lics. Shading (reduced C) increased the palatability of willows to snowshoe
hares, presumably because of reduced carbon-based defenses (12). Clipped
and shaded willows produced regrowth shoots with lower concentrations of
carbon-based secondary compounds, that were more preferred than clipped
and unshaded trees.
The resource rearrangement model does not explain all observations,
however. Nitrogen fertilization of artificially defoliated birch trees did not
negate the induced resistance as assayed by autumnal moth caterpillars (39).
Crowding cotton plants reduced their suitability to spider mites; however,
crowding and herbivore damage did not act additively to reduce foliage
quality for mites or verticillium fungus (57). On the contrary, induced resis­
tance was only apparent when plants were not crowded, suggesting that
resources are required for the induced response to occur.
These tests of the passive model are not easy to interpret. For example,
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
336
KARBAN & MYERS
nitrogen fertilization and carbon stress could produce the result predicted by
the model for many reasons having nothing to do with the hypothesized
nutrient stress. Nitrogen fertilization could cause ratios of specific amino
acids to become unnaturally lopsided or levels of nitrogen to become higher
than optimal for herbivores (82). More convincing tests of the model would
cause changes in nutrient ratios by means other than herbivory (by plant
crowding or more careful fertilization treatments). The effects of these treat­
ments on both plant chemistry and plant quality for herbivores could be
measured.
Tissue removal by herbivores may alter the plant physiologically, making it
more resistant in the process. Pruning commonly causes shoots to exhibit
juvenile characters compared to unattacked shoots of similar plants. Juvenile
growth is often characterized by greater concentrations of secondary chemi­
cals or physical resistance (14, 79). Although these responses cause an
increase in less palatable tissue, they are probably examples of generally high
protection of the juvenile stage.
INFLUENCE OF INDUCED RESPONSES ON
HERBIVORES
Field studies on the effects of induced responses on herbivores have yielded
extremely variable results among plants within a population, and among
populations (reviewed in 26, 34). Much of this variation may be the result of
differences in species, age, genotype, history, and environmental factors (17,
26, 48). Despite this variability, we can make preliminary generalizations
about the timing and spatial extent of induced responses, and specificity of
their effects on herbivores.
Timing of Induced Responses
The rate at which induced changes occur and the rate at which they are relaxed
determines whether they affect particular herbivores. The critical distinction
between rapid or short-term responses versus long-term responses is neither
the rate at which the response occurs nor the rate of relaxation of the response.
Rather, these rates must be compared to the relevant events of attack and
resultant damage. Short-term responses occur during the attack such that the
attacking individuals experience the consequences of the changes they induce.
Long-term responses occur following the attack and have little effect on the
attacking individuals but can influence herbivores that attempt to use the plant
at later times. The effect of an induced response must be considered in terms
of the life history and mobility of particular herbivores. The same plant
response may affect only subsequent generations of short-lived herbivores
such as spider mites, or it may affect the attacker in the case of a longer lived
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
INDUCED RESPONSES
337
caterpillar. Less mobile herbivores, such as leaf miners, gall formers, and
bark beetles are more likely to be affected by localized responses than are
herbivores that constantly move.
The distinction between responses that influence the attacking organisms
and those that influence only later challengers to the plant is important
because, in theory, the consequences of these two effects should be quite
different. Induced resistance effective against the organisms causing the
response is more likely to reduce the local population of this herbivore species
(37). Induced resistance activated only after the attacker has left works as a
negative factor with a time delay and is much less likely to have a stabilizing
effect (37, 73). However, increased instability caused by a delay in the
induced response could still be accompanied by a reduction in mean herbivore
density. Using simple models of induced resistance involving mobile non­
selective herbivores with continuous generations, Edelstein-Keshet &
Rausher (22) argued that increasing the rate at which plants respond or
decreasing the rate of decay of the response make it more likely that induced
resistance will affect an herbivore population.
Both common sense and mathematical theory suggest that the rates of
induction and relaxation will influence the consequences on herbivores.
Nonetheless, we know relatively little about these rates because the appropri­
ate experiments are difficult, involving several treatments that must be sub­
sampled at several different time intervals. Most of the studies that followed
the time course of the induced response have found that the organism that
causes the damage also suffers the consequences [caterpillars on birch trees
(9, 41, 109), beetles on cucurbits (16, 96), spider mites on cotton plants (55),
caterpillars on tomato plants (10, 23), mites on avocado trees (75), beetles
and fungi on pines (83), aphids on cottonwoods (106), cicada eggs in cherry
trees (50), and caterpillars on oaks (89)]. However, three studies which
showed evidence of induced resistance found that the response was delayed so
that it had less chance of affecting the individuals (not the species) that caused
the induction [mammals on acacias (111), hares on birches (13), and caterpil­
lars on larches (6)]. The extent to which these individual herbivores are
territorial or otherwise feed on the same individual plants in successive years
determines the likelihood that they will suffer the consequences of their
previous feeding. Some induced effects can accumulate if the stress continues
for several years. For instance, performance of gypsy moth caterpillars on
black oak trees decreased as the number of years that the trees had been
defoliated increased from 0 to 3 (101). Several studies have found that
induced resistance increased as the level of injury to the plant increased [mites
on citrus (44), mites on cotton (Figure 4 in 53), caterpillars on birch (Figure 1
in 40)]. These results suggest that induced resistance should probably be
thought of as a graded response rather than as an on/off process.
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
338
KARBAN & MYERS
Many ecologists became interested initially in induced responses because
they provided a potential mechanism to explain multiyear population cycles
of forest insects. The hypothesis presented by Haukioja & Hakala (38) and
Benz (8)-that plant quality decreases after defoliation and then increases
gradually after a lag of several years-provides a delayed density-dependent
mechanism that could potentially drive population cycles of herbivores (11,
36, 87).
To explain regional synchrony of population fluctuations of forest Lepidop­
tera, we must test whether host trees respond in a consistent manner to insect
attack. This basic premise does not seem to be supported: Induced responses
of trees have been found to vary among species, among populations, among
years, and across environmental gradients (81a). On the other hand, changes
in the fecundity and survival of fluctuating populations of forest Lepidoptera
often show consistent patterns through the cycle, even when caterpillars feed
on different species of host plant, in different areas, and following different
histories of attack (77, 78).
Although the variation in response of trees to herbivore damage seems to
make inducible changes in food quality an unlikely explanation for the cyclic
population dynamics of forest Lepidoptera, we list in Table 2 further pre­
dictions of the hypothesis that can be tested. Observations on cyclic pop­
ulations of tent caterpillars and other forest Lepidoptera do not support
these predictions (77, 78). The importance of inducible changes in food
plant quality to population dynamics of nonoutbreak species has not been
studied.
Table 2
Testable predictions arising from the hypothesis that population cycles of forest
Lepidoptera are driven by deterioration in food plant quality following feeding damage from
increasing numbers of herbivores. Species and populations of host trees must respond in
a
consistent manner to herbivore damage for the fluctuations of different populations of insects to
remain in synchrony within a regio n .
1. Fecundity and survival of herbivores will be related to the history of attack on trees.
2. If the response of trees i s d ensit y dependent, fecundity and survival of herbivores will decline
with increasing density (level of attack) and deterioration in food quality.
3. Decreasing fecundity and survival of herbivores following damage to host plants will be
translated into a decline in the population density.
4. Cropping of herbivore density to reduce damage will prolong the outbreak phase of the
population.
5. Introduction of herbivores to suitable foodplants in sites with no previous herbivore damage
will lead to an outbreak out of synchrony with natural populations.
INDUCED RESPONSES
339
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
The Specificity of Induced Resistance
Most vertebrate immune responses are highly specific. We can ask two
questions regarding the specificity of induced resistance against herbivores
and other plant parasites: (a) Are plant responses triggered specifically by
particular parasites or injuries? and (b) do plant responses have activity only
against specific challengers?
Many studies have found that artificial damage causes responses in plants
that affect herbivores. However, these tell us little about whether the re­
sponses caused by artificial damage are physiologically the same and similar
in strength to those caused by herbivores. Studies that include at least three
treatments (plants damaged by herbivores, plants damaged artificially, and
undamaged controls) are more informative. Several such studies found that
artificial damage caused effects similar to those resulting from actual herbiv­
ory (30, 51, 81). However, several studies found that the effects of injury
inflicted by herbivores and by artificial means were different in extent (42,
39,25, 2) or in quality (33, 81). In a particularly elegant experiment, Hartley
& Lawton (34) found that insect feeding stimulated increased concentrations
of PAL and phenolics more than cutting the leaves with scissors. Fungi or
some component of insect saliva may stimulate the response since cutting
with scissors and applying caterpillar regurgitate produced a response similar
to that of insect damage. When designing experiments of induced responses,
investigators should not assume that artificial damage will produce results
similar to actual herbivory, unless this hypothesis is experimentally tested.
Induced responses in plants can influence a variety of different herbivores.
The inducer and the affected species may belong to very different feeding
guilds and be taxonomically unrelated. For instance, cotton seedlings dam­
aged by spider mites become more resistant to the symptoms of a fungal
disease (56). Similarly, seedlings that had been infected by the fungus became
less suitable hosts for spider mites. Many studies have found "cross­
resistance" between different herbivore species [many different herbivores on
cotton (58, 52, 54), insects on larch (5), caterpillars on lupines (31), insects
on oaks (103, 25, 45)]. However, several studies found that different species
reacted idiosyncratically to induced plant changes. For example, birch leaves
damaged by leaf mining were avoided by four species of caterpillars, whereas
leaves damaged by chewing caterpillars were avoided by one caterpillar
species but were equally preferred by another two species; leaves damaged
artificially were preferred by two caterpillar species and were preferred
equally by another two species (33). Unlike the antibody-antigen model of
immune responses in vertebrates, induced resistance in plants against herbi­
vores is characterized by low specificity. Interestingly, plant pathologists
have reached the same conclusions about the lack of specificity of induced
resistance against pathogenic organisms (61, 62).
340
KARBAN & MYERS
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
WHY INDUCED RESPONSES RATHER THAN
CONSTITUTIVE ONES?
Some fraction of the induced responses elicited by damage result in greater
resistance to herbivores. If these changes increase the resistance of plants to
their herbivores, why are they inducible rather than constitutive? The problem
becomes more perplexing for those cases in which induced responses are
general reactions to many stresses and have activity against many different
herbivores and parasites. The problem applies only to active responses since
passive deterioration can only be inducible, by definition. We consider four
hypotheses.
Phytotoxic Responses and Packaging Problems
If the products induced by damage are toxic to herbivores and plant diseases,
they may also be toxic to the plants themselves, and self-toxicity may increase
if the effect is maintained for an extended time. For example, some phyto­
alexins are toxic to plants at concentrations that inhibit microorganisms (62).
Repeated applications of fungus-derived elicitors of these phytoalexins to the
foliage of beans caused severe necrosis and stunted growth. This autotoxicity
is avoided by a system in which the phytoalexins are only produced when
needed. Many plant products that are released following herbivory are locally
toxic to the plant. However, precursors may be stored safely in vacuoles so
that enzymes and substrates are mixed only after the vacuoles are ruptured by
feeding damage (reviewed in 21).
Plants Are Induced Much of the Time
For some plants, the induced state might be the most common one. For
example. tomato plants must be carefully protected in the greenhouse to
prevent the induction of high levels of proteinase inhibitors. Plants in the field
are likely to be in the induced state most of the time following stimulation
from wind (R. M. Broadway. personal cummunication). This argument
probably does not apply to those examples of induced resistance in which an
effect on herbivores has been demonstrated in the field. This is not really an
explanation for why a particular response should be inducible but rather an
observation that the distinction between induced and constitutive traits may be
largely semantic. in some cases.
The Induced Response Creates a Changing Target
Most studies measure induced responses by looking at only a restricted group
of chemicals or by doing a bioassay. Even so. results often vary considerably
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
INDUCED RESPONSES
341
among parts of plants, and among different plants within a population. The
responses to damage of different plants and different parts within an in­
dividual plant may be quite idiosyncratic. The plant may not simply be in an
"induced state." Rather, induction likely involves differential changes occur­
ring in different types of organs of a plant, and in different organs of the same
type (leaves on a tree). Induced responses include many traits that affect
herbivores, all of which can change, rather than the turning on of a single
"defensive chemical." Each of these traits in each plant part may respond with
its own rate of induction and relaxation. A changing, heterogeneous target
may allow for a more rapid response and may retard or prevent the adaptation
of herbivores or diseases to the plant defense (105). A changing plant
phenotype may allow the plant to respond more rapidly to herbivores and
other parasites than it could if it relied on constitutive defenses that changed
only in evolutionary time. Constitutive defenses have no lag time at all, but
also no ability to change when herbivores circumvent them. Induced re­
sponses may allow the plant to respond to unpredictable environmental
variability (65). Phenotypic plasticity in resistance is expected to be more
effective than genetic adaptation in response to selective factors, such as
herbivores and plant pathogens, that may vary during the life span of an
individual plant. This hypothesis predicts that induced resistance will be most
common for plants that experience unpredictable selective pressures from
herbivores sporodically in relation to the generation time of the plant. This
argument would be strengthened if we knew that phenotypic plasticity in
resistance to herbivores was heritable, as plasticity of some other plant traits
appears to be (92).
Induced Defenses Are Less Costly
Much of the recent theory concerning the evolution of plant defenses has
centered around the notion that defenses are costly (18, 27, 76, 85, 88).
Accordingly, plants should allocate resources to defenses only when and
where such allocation will result in increased fitness.
This leads to several testable predictions: (a) Herbivory should reduce plant
fitness and induced plants should have greater fitness than noninduced plants
when herbivores are present. (b) Plants without induced defenses should have
higher fitness in environments without herbivores. (c) Plants that are well
defended by constitutive defenses against a particular herbivore should not
allocate resources to induced defenses against that same herbivore. If con­
stitutive defenses are effective, induced defenses, which are presumably
costly, would be redundant. In other words, these two should be negatively
correlated.
These predictions have not been tested adequately. Cotton plants that were
induced at the cotyledon stage supported smaller populations of spider mites
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
342
KARBAN & MYERS
during the remainder of that field season (52). However, growth and yield of
these induced plants did not differ from plants that were not induced, contrary
to prediction (a). Either spider mites did not reduce these aspects of plant
fitness or else the reduction in fitness to control plants caused by greater
herbivory was offset by the costs of inducing resistance. Since cotton has
undergone intense selection as an agricultural crop this may not be an
appropriate model system. In native tobacco plants, both constitutive levels of
alkaloids and increases in alkaloid titers induced by damage were negatively
correlated with seed output, suggesting a cost to this presumed defense (3).
The best examples of estimates of costs of induced resistance come from
small invertebrates in fresh water and marine environments. Some of these
organisms respond to predators through morphological modifications such as
the production of helmets in daphnia (43), heavier shells in barnacles (67),
and spines in rotifers (29) and bryozoans (35). Induced resistance for rotifers
did not reduce survival, fecundity, or population growth, but for barnacles,
daphnia, and bryozoans, these induced morphological changes reduced
growth and/or fecundity. When predators are not present, unarmored in­
dividuals have the fitness advantage.
CONCLUSIONS
The initial observations of changes in chemical composition of plants follow­
ing stress or damage seemed obvious examples of plant adaptations against
herbivores. If, in a bioassay, the quality of foliage was reduced (as indicated
by poorer survival and fecundity of the herbivore), then an impact on the
future density of the herbivore seemed an obvious conclusion. Many studies
have now found that induction causes changes in performance of bioassay
herbivores. However, all stages in the interactions between plants and herbi­
vores have been found to vary; insects vary in their choice of damaged and
undamaged foliage and in their growth and survival on damaged and un­
damaged tissue. Some plants respond to damage, some do not; some improve
as hosts following damage, others deteriorate. After a decade of work, there
are few generalities concerning the effects of induced plant responses on
population dynamics.
The hypothesis outlined by Haukioja (36) and Rhoades (87), in which
changes in food p�ilnt chemistry were proposed as the driving mechanism
behind large-scale cyclic fluctuations in folivorous insects, has met with
equivocal support. In some instances, variation among populations of trees is
too great to provide the consistent impact on the insects sufficient for wide­
spread cyclic declines. More work is needed to examine the effects of induced
host changes on populations of herbivores in natural and agricultural systems.
The variation that may have surprised ecologists searching for simple
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
INDUCED RESPONSES
343
answers and general patterns will perhaps come as little surprise to plant
physiologists. Recognizing that fast- and slow-growing trees will respond to
defoliation in different ways and that loss of buds in the winter or spring will
cause different patterns of foliage quality has greatly helped interpretations of
conflicting findings. However, still controversial is whether chemical changes
following damage can be wholly attributed to passive changes by damaged
plants, or if active defensive processes must be invoked. The role of micro­
parasites, fungi, bacteria, or viruses in eliciting active responses in damaged
plants following contamination by herbivores will be an exciting area for
future research and one that may help answer questions about the mechanisms
of induction. The controversy between active and passive responses of plants
to herbivore damage will almost certainly be resolved by the realization that a
combination of mechanisms are involved. We must find out what is happen­
ing, where, why, and how often.
If, as ecologists, we wish to understand induced changes we should be
prepared to devote ourselves to long-term and multidimensional studies. If we
aim to understand the chemical mechanisms of induced resistance, we should
consider all of the chemicals within a plant with potential activity against
herbivores, rather than specializing on a particular subset that are easy to work
with or are thought to bc important. Certainly, we should seek experimental
evidence that allows us to vary only one constituent, using artificial diets and
isogenic lines, when available. This careful experimentation must be con­
ducted for all of the plausible mechanisms. At the same time we should keep
in mind that the effects we observe in these highly artificial experiments may
be very different from effects experienced by herbivores dealing with the
chemicals in plants, where interactions and synergisms arc likely to be
important. We have now learned that many plants change in response to
herbivory and that no single mechanism will explain all of these diverse plant
responses.
At the other extreme, we must extend our bioassay results to field ex­
periments on natural populations of herbivores. Rather than asking whether
induced responses can be shown to affect the performance or behavior of
herbivores we should assess the relative importance of induced plant resis­
tance compared to other ecological factors that may also affect the population
dynamics of herbivores.
Induced responses should not be assumed to be defenses. Instead, we must
observe whether they defend plants by comparing fitness of induced and
uninduced plants in an environment that includes herbivores. Fitness will be
most easily measured on small, short-lived plants which show evidence of
induced responses following low levels of herbivore damage [e.g. cucurbits
(96), wild tobacco (3), crucifers (93)]. It should be kept in mind that results
with these systems may have little relevance to what is happening with trees.
Even after an induced response is shown to provide resistance against a
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
344
KARBAN & MYERS
particular herbivore and to defend the plant against that herbivore, we should
not conclude that it evolved in response to that herbivore. Plants are affected
by many different selective pressures; thus, limiting our consideration to a
single herbivore at one point in time is likely to be misleading.
The speed with which the study of induced changes in plant quality has
progressed from the "simple understanding" phase to the "chaos of variation"
phase and is now entering the "patterns of variation" phase is due both to
initially stimulating ideas and to efforts of a large number of researchers. In
the future we should concentrate our efforts toward (a) understanding the
mechanisms of induced responses, (b) understanding the consequences of
induced resistance on populations of herbivores, and (c) applying what we
learn about induced resistance and defense to protecting agricultural crops
(60, 55). Continued progress in each of these directions will be most rapid if
we can maintain a broad perspective and consider a wide variety of nonexclu­
sive hypotheses.
ACKNOWLEDGMENTS
This research has been supported by grants from NSF and USDA to R.
Karban and grants from NSERC to J. H. Myers. Joy Bergelson, Alison
Brody, John Bryant, Greg English-Loeb, Murray Isman, and Bill Morris
made useful comments on the manuscript.
Literature Cited
I. Akazawa, T. , Uritani, 1., Kubota, H.
1960. Isolation of ipomearamone and
two coumarin derivatives from sweet
potato roots injured by the weevil, Cylas
formicarius
elegantulus.
Biochem. Biophys. 88:150-56
Arch.
2. Baldwin,1. T. 1988. The alkaloidal re­
sponses of wild tobacco to real and sim­
ulated herbivory. Oecologia 77:378-381
3. Baldwin, 1. T. 1990. Damage-induced
alkaloids in wild tobacco. See Ref. 84.
In press
4. Baldwin, I. T. , Schultz, J. C. 1983.
Rapid changes in tree leaf chemistry in­
duced by damage: evidence for com­
munication between plants. Science 221:
227-79
5. Baltensweiler, W. 1985. On the extent
and the mechanisms of the outbreaks of
the larch bud moth (Zeiraphera diniana
Gn., Lepidoptera, Tortricidae) and its
impact on the subalpine larch-cembran
pine forest ecosystem. In Establishment
and Tending of Subalpine Forest: Re­
search and Management, ed. H. Turner,
W. Tranquillini, pp. 215-219 Proc. 3rd
IUFRO Workshop Birmensdorf, Swit­
zerland
6. Baltensweiler, W. , Benz, G. , Bovey,
P., Delucchi, V. 1977. Dynamics of
larch bud moth populations. Annu. Rev.
Entomol. 22:79-100
7. Benz, G. 1974. Negative Ruckkoppe­
lung durch Raum- und Nahrungskonkur­
renz sowie zyklische Veranderungen der
Nahrungsgrandlage als Regelprinzip in
der Populations-dynamik des Grauen
Larchenwicklers, Zeiraphera diniana
(Guenee) (Lepidoptera, Tortricidae). Z.
Angew. Entomol. 76:196--228
8. Benz, G. 1977. Insect induced resis­
tance as a means of self defence of
plants. In EUCA RPlAflOBC Working
Group Breeding for Resistance to In­
sects and Mites (Bulletin SROP 1977),
pp. 155-59
9. Bergelson, J., Fowler, S. , Hartley, S.
1986. The effects of foliage damage on
casebearing moth larvae, Coleophora
serratella, feeding on birch. Ecol. En­
tomol. 11:241-250
10. Broadway, R. M., Duffey, S. S.,
Pearce, G. , Ryan, C. A. 1986. Plant
proteinase inhibitors: a defense against
herbivorous insects? Entomol. Exp.
Appl. 41:33--3 8
INDUCED RESPONSES
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
II. Bryant, J. P. 1981. Phytochemical de­
terrence of snowshoe hare browsing by
adventitious shoots of four Alaskan
trees. Science 213:889-90
12. Bryant, J. P. 1987. Feltleaf willow­
snowshoe hare interactions: plant car­
bon/nutrient balance and floodplain suc­
cession. Ecology 68:1319-27
13. Bryant, J. P., Chapin, F. S., Klein, D.
R. 1983. Carbon/nutrient balance of
boreal plants in relation to vertebrate
herbivory. Oikos 40:357-68
14. Bryant, J. P., Danell, K., Provenza, F.,
Reichardt, P., Clausen, T. 1990. Effects
of mammal browsing on the chemistry
of deciduous woody plants. See Ref. 84.
In press
15. Bryant, J. P., Heitkonig, 1., Kuropat,
P., Owen-Smith, N. 1989. Effects of
severe defoliation upon the long-term re­
sistance to insect attack and chemistry of
leaves of six South African savannah
woody species. Manuscript
16. Carroll, C. R., Hoffman, C. A. 1980.
Chemical feeding deterrent mobilized in
response to
insect
herbivory
and
Epilachna
by
counteradaptation
tredecimnotata. Science 209:414-16
17. Coleman, 1. S., Jones, C. G. 1990. A
phytocentric perspective of phytochemi­
cal induction by herbivores. See Ref.
84, In press
18. Coley, P. D., Bryant, J. P., Chapin, F.
S. 1985. Resource availability and plant
antiherbivore defence. Science 230:89599
19. DiCosmo, P., Towers, G. H. N. 1984.
Stress and secondary metabolism in cul­
tured plant cells. In Recent Advances in
Phytochemistry, ed. B. N. Timmer­
mann, C. Steelink, F. Loewus, 18:97175. New York: Plenum
20. Dohman, G. P., McNeill, S., Bell, J. N.
B. 1984. Air pollution increases Aphis
fabae pest potential. Nature 307:52-53
21. Duffey, S. S., Felton, G. W. 1989. Role
of plant enzymes in resistance to insects.
In Enzymes in A griculture, ed. J. R.
Whittaker, D. E. Sonnet, pp. 289-313.
Amer. Chern. Soc.
22. Edelstein-Keshet, L., Rausher, M.
1989. The effects of inducible plant de­
fenses on herbivore populations. I. Mo­
bile herbivores in continuous time. Am.
Nat. In press
23. Edwards, P. J., Wratten, S. D., Cox, H.
1985. Wound-induced changes in the
acceptability of tomato to larvae of Spo­
doptera littoralis: a laboratory bioassay.
Ecol. Entomol. 10:155-58
24. Ericsson, A., Hellquist, c., Langstrom,
B., Larsson, S., Tenow, o. 1985.
Effects on growth of simulated and in-
345
duced shoot pruning by Tomicus
piniperda as related to carbohydrate and
nitrogent dynamics in Scots pine. 1.
Appl. Ecol. 22:105-24
25. Faeth, S. H. 1986. Indirect interactions
between
temporally-separated herbi­
vores mediated by the host plant. Ecolo­
gy 67:479-94
26. Faeth, S. H. 1990. Inducible responses
and interactions among oak folivores.
See Ref. 84. In press
27. Feeny, P. P. 1976. Plant apparency and
chemical defense. Recent Adv. Phy­
tochem. 10:1-40
28. Fowler, S. Y., Lawton, J. H. 1985.
Rapidly induced defenses and talking
trees: the devil's advocate position. Am.
Nat. 126:181-95
29. Gilbert, J. J. 1980. Further observations
on developmental polymorphism and its
evolution in the rotifer Brachionus caly­
ciflorus. Freshwater BioI. 10:281-94
30. Green, T. R., Ryan, C. A. 1972
Wound-induced proteinase inhibitor in
plant leaves: a possible defense mech­
anism against insects. Science 175:77677
31. Harrison, S., Karban, R. 1986. Effects
of an early-season folivorous moth on
the success of a later-season species,
mediated by a change in the quality of
the shared host, Lupinus arboreus Sims.
Oecologia 69:354-59
32. Hart, S. Y., Kogan, M., Paxton, J. D.
1983. Effect of soybean phytoalexins on
the herbivorous insects Mexican bean
beetle and soybean looper. 1. Chem.
Eml. 9:657-72
33. Hartley, S. E., Lawton, J. H. 1987.
Effects of different types of damage on
the chemistry of birch foliage, and the
responses of birch feeding insects.
Oecologia 74:432-37
34. Hartley, S. E., Lawton, J. H. 1990. A
biochemical basis and significance of
rapidly induced changes in birch. See
Ref. 84. In press
35. Harvell, C. D. 1986. The ecology and
evolution of inducible defenses in a
marine bryozoan: cues, costs, and con­
sequences. Am. Nat. 128:810-23
36. Haukioja, E. 1980. On the role of plant
defenses in the fluctuation of herbivore
populations. Oikos 35:202-13
37. Haukioja, E. 1982. Inducible defences
of white birch to a geometrid defoliator,
Epirrita autumnata. Proc. 5th Int.
Symp. Insect-Plant Relationships, pp.
199-203. Pudoc, Wageningen
38. Haukioja, E., Hakala, T. 1975. Herbi­
vore cycles and periodic outbreaks.
Formulation of a general hypothesis.
Rep. Kevo Subarctic Res. Stat. 12:1-9
346
KARBAN & MYERS
39. Haukioja, E., Neuvonen, S. 1985. In­
duced long-term resistance of birch
foliage against defoliators: defensive or
incidental? Ecology 66:1303-8
40. Haukioja, E., Neuvonen, S. 1987. In­
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
sect population dynamics and induction
of plant resistance: the testing of hypoth­
eses. In Insect Outbreaks, ed. P. Barbo­
sa, J. C. Schultz. pp. 411-432. New
York: Academic
41. Haukioja, E., Niem ela, P. 1977. Re­
tarded growth of a geometrid larva after
mechanical damage to leaves of its host
tree. Ann. Zool. Fennici 14: 48-52
42. Haukioja, E., Suomela, J. , Neuvonen,
S. 1985. Long-term inducible resistance
in birch foliage: triggering cues and
efficacy on a defoliator. Oecologia
65:363-69
43. Havel, J. E., Dodson, S. I. 1987. Re­
productive costs of Chaoborus-induced
polymorphism in Daphnia pulex. Hy­
drobiologia 150:273-81
44. Henderson, C. F., Holloway, J. K.
1942. Influence of leaf age and feeding
injury on the citrus red mite. J. Econ.
Ent. 35:683-86
45. Hunter, M. D. 1987. Opposing effects
of spring defoliation on late season oak
c aterpillars. Ecol. Entomol. 12:373-82
46. Isaak, A., Sorensen, E. L . , Painter, R.
H. 1965. Stability of resistance to pear
aphid and spotted alfalfa aphid in several
alfalfa clones under various temperature
regimes. J. Econ. Entomol. 58:140-43
47. Jaffe, M. J., Te lew ski , F. W. 1984.
Thigmomorphogenesis:
callose
and
ethylene in the h ardening of mechanical­
ly stressed plants. See Ref. 19, 18:79-95
48. Jones, C. G. , Coleman, J. S. 1989.
Plant stress and insect herbivory: toward
an inte grate d p erspect ive. In Integrated
Responses of Plants to Stress, ed. H. A.
Mooney, W. E. Winner, E. J. Pell. New
York: Academic. In press
49. Jones, D. A. 1972. Cyanogenic glyco­
sides and their function. In Phvtochemi­
cal Ecology, ed. J. B. Harborne. pp.
103-124. New York: Academic
50. Karban, R. 1983. Induced responses of
cherry trees to periodical cicada oviposi­
tion. Oecologia 59:226-31
51. Karban, R. 1985. Resistance against
spider mites in cotton induced by me­
chanical abrasion. Entomol. Exp. Appl.
37:137-41
52. Karban, R. 1986. Induced resistance
against spider mites in cotton: field veri ­
fication. Entomol. Exp. Appl. 42:23942
53. Karban, R. 1987. Environmental con­
ditions affecting the strength of induced
resistance against mites in cotton. Oeco­
logia 73:414-19
54. Karban, R. 1988. Resistance to beet
armyw orms . (Spodopter a exigua) in­
duced by exposure to spider mites. (Tet­
ranychus turkestani) in cotton. Am.
Midi. Nat. 119:77-82
55. Karban, R. 1990. Inducible resistance in
agricultural systems. See Ref. 84. In
press
56. Karban,
57.
58.
59.
60.
R.,
Adamchak,
R.,
Schna­
thorst, W. C. 1987. Induced resistance
and i nterspecific comp etition between
spider mites and a vascular wilt fungus.
Science 235:678-80
Karban, R., Bro dy , A. K., Schnathorst,
W. C. 1989. Crowding and a plant's
ability to defend itself again st herbivores
and diseases. Am. Nat. In press
Karhan, R., Carey, J. R. 1984. Induced
resistance of cotton see dlings to mites.
Science 225:53-54
Karban, R., English-Loeb, G. M. 1988.
Effects of herbivory and plant condition­
ing on the population dynamics of spider
mites. Exp. Appl. Acar. 4:225-46
Kogan, M., Paxton, J. 1983. Natural
inducers of plant resistance to insects. In
Plant Resistance to Insects, ed. P. A.
Hedin, pp. 153-71. Washington, DC:
61.
62.
63.
64.
65.
Am. Chern. Soc.
Kuc, J. 1981. Multiple mechanisms,
reaction rates, and induced resistance in
p lants. In Plant D isease Control, ed. R.
C. Staples, pp. 259-72. New York:
Wiley
Kuc, J. 1987. Plant immunization and
its applicability for disease control. In
Innovative Approaches to Plant D isease
Control, ed. I. Chet, pp. 255-74, New
York: John Wiley
Kuc, J., Rush , J. S. 1985. Phytoalexins.
Arch. Biochem. B iophys. 236:455-72
Larsson, S . , Wiren , A . , Lundgren, L.,
Ericsson, T. 1986. Effects of light and
nutrient stress on leaf phenolic chemistry
in Salix dasyclados and s usceptibility to
Galerucella lineola ( Co leoptera) . Oikos
47:205-10
Levins, R. 1968. Evolution in Changing
Environments: Some Theoretical Explo­
rations. Princeton, NJ: Princeton Univ.
Press
66. Lincoln, D. E., Mooney, H. A. 1984.
Herbivory on Diplacus aurantiacus
shrubs in sun and shade. Oecologia
64:173-76
67. Lively, C. M. 1986. Predator-induced
shell dimorphism in the acorn barnacle
Chthamalus anisopoma. Evolution 40:
232-42
68. Loper, G. M. 1968. Effect of aphid in-
INDUCED RESPONSES
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
69.
70.
71.
72.
73.
74.
75.
76.
77.
78.
79.
80.
81.
festation on the coumestrol content of
alfalfa varieties differing in aphid resis­
tance. Crop Sci . 8 : 1 04-6
Makus, D . , Zuroske, G . , Ryan, C. A .
1 990. The direction and rate o f transport
of the proteinase inhibiting factor out of
wounded tomato leaves. Plant Physiol.
65: 1 50 (Suppl . )
Mattson, W . J . , Addy, N . D . 1 975 .
Phytophagous insects as regulators of
forest primary production. Science 1 90:
5 1 5-22
Mattson, W. 1 . , Haack, R . A . 1 987. The
role of drought in outbreaks of plant­
eating insects. Biuscience 37: 1 1 0- 1 8
Mauch, F . , Hadwiger, L . A . , Boller, T.
1 984. Ethylene: symptom not signal for
the induction of chitinase and b- 1 ,3glucanase in pea pods by pathogens and
elicitors. Plant Physiol. 76:607- 1 1
May, R. H. 1 98 1 . Models for single
populations. In Theoretical Ecology:
Principles and Applications, ed . R . H .
May, pp. 5-29. Sunderland, Mass:
Sinauer. 2nd ed.
Mcintyre, J . L . , Dodds, J . A . , Hare, J .
D. 1 98 1 . Effects of localized infections
of Nicotiana tabacum by tobacco mosiac
virus on systemic resistance against di­
verse pathogens and an insect. Phyto­
pathology 7 1 :297-3 0 1
McMurtry, J . A. 1 970. Some factors of
foliage condition limiting population
growth of Oligonychus punicae (Acar­
ina: Tetranychidae) . Ann. Entomol. Soc.
Am. 63:406- 1 2
Mooney, H . A . , Gulmon, S . L. 1 982.
Constraints on leaf structure and func­
tion in reference to herbivory. Biosci­
ence 32: 198-206
Myers, J. H. 1 99 8 . The induced defense
hypothesis. Does it apply to the popula­
tion dynamics of insects? In Chemical
Mediation of Coevolution,
ed.
K.
Spencer, pp. 530-37. New York: Aca­
demic
Myers, J. H. 1 988. Can a general hy­
pothesis explain population cycles of
forest Lepidoptera? Adv. Ecol. Res. 1 8:
1 80-242
Myers, J. H . , Bazely, D. 1 990. Thoms,
spines, prickles and hairs: are they
stimulated by herbivory and do they
deter herbivores? See Ref. 84. I n
press
Myers, J. H . , Williams, K. S. 1984.
Does tent caterpillar attack reduce the
food quality of red alder foliage? Oeco­
{ogia 62:74-79
Neuvonen, S . , Haukioja, E. , Molarius,
A. 1 987. Delayed inducible resistance
against a leaf-chewing insect for four
347
deciduous tree species. Oecologia 74:
363-69
8 1 a . Neuvonen, S . , Haukioja, E. 1 990. The
effects of inducible resistance in host
foliage on birch feeding herbivores. See
Ref. 84. In press
82. Prestidge, R. A . , McNeill, S. 1 983. Au­
chenorrhyncha-host plant interactions:
leafhoppers and grasses. Ecol . Ent. 8:
33 1-339
83. Raffa, K. F . , Berryman, A . A . 1 987.
Interacting selective pressures in coni­
fer-bark beetle systems: a basis for recip­
rocal adaptations? Am. Nat. 1 29:23462
84. Raupp, M. J . , Tallamy, D. W . , cds.
1 990. Phytochemical Induction by Her­
bivores. New York: Wiley
85. Rhoades, D. F. 1 979. Evolution of plant
chemical defense against herbivores . In
Herbivores:
Their Interaction With
Secondary Plant Metabolites, ed . G. A .
Rosenthal , D . H . Janzen , pp. 4-54.
New York: Academic
86. Rhoades, D. F. 1 983. Responses of
alder and willow to attack by tent cater­
pillars and webworms: evidence for
pheromonal sensitivity of willows. See
Ref. 60, pp. 55-68
87. Rhoades, D. F. 1 983. Herbivore popula­
tion dynamics and plant chemistry. In
Variable Plants and Herbivores in Nat­
ural and Managed Systems, ed. R. F.
Denno, M. S . McClure, pp. 1 5 5-220.
New York: Academic
88. Rhoades, D. F . , Cates, R. G. 1 976.
Toward a general theory of plant anti­
herbivore chemistry. Recent Adv. Phy­
tochem . 1 0: 1 68-2 1 3
89. Roland, J . , Myers, J. H . 1 987. Im­
proved insect performance from host­
plant defoliation: winter moth on oak
and apple. Ecol. Entomol. 1 2:40914
90. Russell, G . B . , Sutherland, O . R . W . ,
Hutchins, R . F. N . , Christmas, P. E.
1 978. Vcstitol: a phytoalexin with insect
feeding-deterrent activity . 1. Chem .
Ecol. 4:57 1 -79
9 1 . Ryan, C. A. 1 983. Insect-induced chem­
ical signals regulating natural plant pro­
tection responses. In Variable Plants
and Herbivores in Natural and Managed
Systems. ed. R. F. Denno, M. S .
McClure, pp. 43-60. New York: Aca­
demic
92. Schlichting, C. D. 1 986. The evolution
of phenotypic plasticity in plants. Annu.
Rev. Eco!. Syst. 1 7:667-93
93. Shapiro, A. M . , DeVay, J. E. 1987.
Hypersensitivity reaction of Brassica
nigra L. (Cruciferae) kills eggs of Pieris
348
KARBAN & MYERS
butterflies
(Lepidoptera:
Pieridae).
Annu. Rev. Ecol. Syst. 1989.20:331-348. Downloaded from www.annualreviews.org
by ETH- Eidgenossische Technische Hochschule Zurich - BIBLIOTHEK on 03/29/11. For personal use only.
Oecologia 7 1 :63 1 -32
94. Sosa, O. 1 979. Hessian fly: resistance of
wheat as affected by temperature and
duration of exposure. Env. Entomol. 8:
280-8 1
95. Sutherland, O. R. W. , Russell, G. B. ,
Biggs, D. R . , Lane, G. A. 1 980. Insect
feeding deterrent activity of phytoalexin
isoflavonoids. Biochem . Syst. Ecol. 8:
73-75
96. Tallamy, D. W. 1 985. Squash beetle
feeding behavior: an adaptation against
induced cucurbit defenses. Ecology 66:
1 574-79
97. Tuomi, J., Niemela, P . , Haukioja, E.,
Siren, S . , Neuvonen, S . 1 984. Nutrient
stress: an explanation for plant anti­
herbivore responses to defoliation.
Oecologia 6 1 :208- 1 0
98. Tuomi, J., Niemela, P . , Chapin, F . S.,
Bryant, J. P . , Siren, S. 1 988. Defensive
responses of trees in relation to their
carbon/nutrient balance. In Mechanisms
of Woody Plant Defenses Against In­
sects: Search for Pattern, ed. W. J.
Mattson, J. Levieux , C. Bernard-Dagan,
pp. 57-72. New York: Springer-Verlag
99. Tuomi , J. , Niemela, P . , Rousi, M . ,
Siren, S . , Vuorisalo, T. 1 988. Induced
accumulation of foliage phenols in
mountain birch: brauch response to de­
foliation? Am. Nat. 1 32:602-8
1 00. Uritani, I . , Saito, T . , Honda, H. , Kim,
W. K. 1 975. Induction of furano­
terpenoids in sweet potato roots by the
larval components of the sweet potato
weevils. Agric. Bioi. Chem . 37: 1 857-62
1 0 1 . Valentine, H. T . , Wallner, W. E., War­
go, P. M. 1 983. Nutritional changes in
host foliage during and after defoliation,
and their relation to the weight of gypsy
moth pupae. Oecologia 57:298-302
1 02. Wagner, M. R. 1 988. Induced defenses
in ponderosa pine against defoliating in­
sects. See Ref. 98 , pp. 1 4 1-55
1 03. West, C . 1 985. Factors underlying the
late
seasonal
appearance
of
the
lepidopterous leaf-mining guild on oak.
Ecol Entomol. 1 0: 1 1 1-20
1 04. White, T. C. R. 1 969. An index to
measure weather-induced stress of trees
associated with outbreaks of psyllids in
Australia. Ecology 50: 905-9
\ 05. Whitham, T. G. 1 983. Host manipula­
tion of parasites: within-plant variation
as a defense against rapidly evolving
pests. In Variable Plants and Herbivores
in Natural and Managed Systems, ed. R .
F . Denno, M . S. McClure. p p . 1 5-4 1 .
New York: Academic
1 06. Williams, A. G. , Whitham, T. G. 1986.
Premature leaf abscission: and induced
plant defense against gall aphids . Ecolo­
gy 67: 1 6 1 9-- 27
1 07. Williams, K. S . , Myers, J. H. 1 984.
Previous herbivore attack of red alder
may improve food quality for fall web­
worm larvae. Oecologia 63: 1 6&-70
108. Wolfson, J. 1990. The effects and non­
effects of proteinase inhibitors on insect
herbivores. See Ref. 84. In press
1 09. Wratten, S. D . , Edwards, P. J . , Dunn,
I. 1 984. Wound-induced changes in
palatability of Betula pubescens and B.
pendula. Oecologia 6 1 :372-75
1 1 0. Yang, S. F . , Pratt, H. K. 1 978. The
physiology of ethylene in wounded plant
tissues. In Biochemistry of Wounded
Plant Tissue, ed. G. Kahl, pp. 595-622.
New York: De Gruyter
I l l . Young, T. P. 1 987. Increased thorn
length in Acacia drepanolobium-an in­
duced response to browsing. Oecologia
7 1 :43&-38