Study on the Effects of Discharge Rates on the Capacity Fade of
advertisement

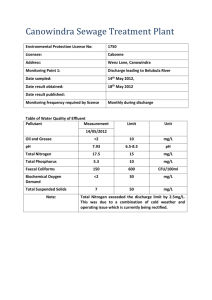
Gang Ning, Bala S. Haran, Branko N. Popov Department of Chemical Engineering University of South Carolina, Columbia, SC Introduction: Lithium-ion rechargeable batteries with LiCoO2 cathode and carbon anodes are rapidly replacing other battery systems due to their high energy and power densities. While the discharge properties and safety issues with these batteries have been studied in details, not much attention has been placed on the capacity fade due to cycling, especially under different discharge rates. This capacity fade is caused by various mechanisms, which depend on not only the electrode materials but also on the charge and discharge protocols [1, 2, 3, 4]. Commercial Li-ion cells lose capacity continuously. This capacity fade is accompanied by an increase in the internal impedance of the battery upon cycling. Therefore, it is critical to clarify the contribution of each of the components in the cell to the internal impedance increase of the battery at different discharge rates. Objectives of this study were to determine the mechanism of the capacity fade of Sony lithium-ion batteries during continuous cycling under different discharge rates as well as to determine if the battery follows the same mechanism of capacity fade under different discharge rates. The Half-cell analysis provides an accurate way of determining the capacity fade resulting from LiCoO2 and carbon respectively. Using EIS, the change of impedance at the positive and negative electrodes was estimated at different cycle numbers. Data for high discharge rates will also be used as baseline for the future capacity fade analysis of hybrid system. Experimental: All experimental studies were done on Sony Sony 18650 commercial cells. Constant current (CC) and constant voltage (CV) protocol was utilized in charging the batteries. The cell was charged at a constant current of 1 A until the potential reached 4.2 V. Subsequently, the voltage was held constant at 4.2 V until the charge current decayed to 50 mA. Discharge was carried out at different rates of 1C, 2C and 3C within the voltage window from 4.2 to 2.5 V. Discharge capacity of cells was determined by C/2 discharge rate. Cycling studies were carried out using Arbin Battery Test (BT-2000) System. Potentiostat/Galvanostat Model 273A were used for the electrochemical characterization of Sony 18650 cells. The impedance studies were carried out on cells that were previously kept at open circuit for 3 hours in order to stabilize the open cell voltage. The cell voltage changed less than 1 mV during the experiments. EIS measurements were done on the Sony cells as well as on individual T-Cells initially and after 300 cycles. Impedance studies were done on the cells at both charged and discharged states. The frequency range for the whole battery in the EIS test is from 0.01 Hz to 10000 Hz while for the LiCoO2 T-cell is from 0.01 to 100000 Hz and for the carbon T-cell is from 0.001 Hz to 100000 Hz. The amplitude of the AC signal is 10 mV. In order to identify the contribution of the positive and negative electrodes to the total cell impedance, studies were done on the individual electrodes. Sony 18650 batteries were stripped off at fully discharged state in a glove box filled with ultra pure argon (National Gas and Welders). Both the positive (LiCoO2) and negative (carbon) electrodes were carfully removed from the cell. Pellets with a diameter of 1.2 cm were punched from the removed electrodes. To ensure the best contact between pellet and current collector of the T-cell (made of stainless steel), the material on one side of the pellet was scraped to the extent that the original fresh copper or aluminum (collectors in the commercial battery) would appear. Dimethyl carbonate (DMC) is used to clean the surface of the pellets. Electrochemical characterization of these individual pellets was done in Tcell using a three-electrode setup. Li/Li+ was used as both reference electrode and counter electrode. The working electrode was the well-prepared pellet. Electrolyte used was 1 M LiPF6 in a 1:1 mixture of ethylene carbonate (EC), and dimethyl carbonate (DMC). Results and Discussions: Fig 1 shows the discharge capacity change among initially, after 300 cycles under 1C discharge rate, after 300 cycles under 2C discharge rate, and after 300 cycles under 3C discharge rate. It is clear that due to the ohmic resistance increase in the internal battery that the flat plat form voltage region is shortened. Specific analyses of individual contribution to the resistance increase and capacity fade are currently in progress. Acknowledgment: Financial support provided by National Reconnaissance Office for Hybrid Advanced Power Sources # NRO-00-C-1034 is acknowledged gratefully. References 1. B. Johnson and R.E. White. J. Power Sources 70 (1998), p. 48. 2. D. Linden, Editor, Handbook of Batteries (2nd edn. ed.), McGraw-Hill, New York (1995), pp. 36.44 -36.48. 3. P. Arora, R.E. White and M. Doyle. J. Electrochem. Soc. 145 (1998), p. 3647. 4. D. Zhang, B.N.Popov. J. Power Sources 91 (2000), p. 122. 4.1 3.9 3.7 Voltage (V) Study on the Effects of Discharge Rates on the Capacity Fade of Lithium-ion Battery Initial Discharge 2C Discharge 3.5 1C Discharge 3.3 3.1 3C Discharge 2.9 2.7 2.5 0.1 0.3 0.5 0.7 0.9 1.1 1.3 Discharge Capacity (Ah) Fig1: Discharge capacity vs. voltage, initially and after 300 cycled under different discharge rates