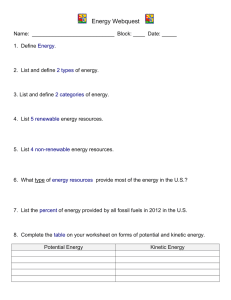
Performance Benchmark P.12.C.2 Students know energy forms can be converted. E/S capacity
advertisement

Performance Benchmark P.12.C.2 Students know energy forms can be converted. E/S Energy is the capacity to produce physical changes. The word capacity is important because change may or may not be actually occurring (i.e., energy can be thought of as being “stored” in some situations). Physical change is often equated with “work,” a term in physics defined as the product of force and distance. But change mechanisms associated with energy include not only work, but also heat and electromagnetic radiation. Figure 1. A schematic of the many connections within energy (from http://hyperphysics.phyastr.gsu.edu/hbase/enecon.html). Energy is a scalar quantity, which means energy values do not have a directional component. The SI unit for energy is the Joule (J), where 1 J = 1 kg × 1 m/s2× 1 m = 1 kg∙m2/s2. To learn more about energy units and conversions, go to http://www.physics.uci.edu/~silverma/units.html Energy is broken down into two types: potential and kinetic. Potential energy can be thought of as “stored” energy of an object because of its position. For example, the position of an object within a gravitational field gives it potential energy. On Earth, the higher an object is above the ground, the greater its gravitational potential energy. Another example is potential energy stored in a spring. In this case, an elastic object is deformed and will have potential energy until it moves back to a neutral (i.e., non- deformed) state. The greater the compression or elongation of the spring (in other words, the greater the deformation) the greater its potential energy. To learn more about potential energy, go to http://hyperphysics.phy-astr.gsu.edu/hbase/pegrav.html#pe Kinetic energy is associated with moving objects. If an object’s velocity increases, its kinetic energy will rapidly increase. Also, the more massive a moving object is, the 1 greater its kinetic energy. The equation that describes kinetic energy is KE mv 2 , 2 where m is mass of object in kilograms and v is the velocity of the object in meters/second. A detailed discussion of kinetic energy is found at http://www.physicsclassroom.com/Class/energy/U5L1c.html. In a system, the total energy is the sum of the potential and kinetic energies. Within the system, potential energy may be transformed into kinetic energy and vice versa. If the system is closed (no energy can enter or leave), the total energy will remain the same even though energy may be transformed from one kind to another within the system. This principle is called the Law of Conservation of Energy. The Law of Conservation of Energy also applies to open systems, where energy may enter and leave the system boundaries. In an open system, the net change in total energy is equivalent to the amount of energy added or removed from outside the boundaries, regardless of energy form. In other words, if net energy is added to a system, then the total energy is equal to the original amount of potential and kinetic energies plus the new amount of potential and kinetic energies added. On the other hand, if net energy is removed from a system, then the total energy is equal to the original amount of potential and kinetic energies minus the new amount of potential and kinetic energies subtracted. Simply stated, the Law of Conservation says that energy can neither be created from nothing nor destroyed completely, but it can be converted from one form into another. To learn more about the Law of Conservation of Energy, go to http://www.eia.doe.gov/kids/energyfacts/science/formsofenergy.html. Figure 2. In a pile driver, energy is transferred from potential energy (PE) to kinetic energy (KE) to do work on the post (i.e., a force moving the post downward). (from http://www.physicsclassroom.com/Class/energy/U5L1d.html) At its most fundamental level, energy is some combination of potential and kinetic. Energy can be more commonly described in terms of (1) chemical energy, (2) radiant energy, (3) electrical energy, and (4) thermal energy. These can be thought of as more complex combinations of potential and kinetic energy, and sometimes it is easier to talk about energy transformations in these terms. For example, the chemical energy in food, which is a combination of the potential and kinetic energy of molecules within the food, can be converted to kinetic energy of muscle motion in animals causing them to move. Figure 3. Common examples of energy transformations. (from http://www.eia.doe.gov/kids/ener gyfacts/science/formsofenergy.ht ml) It is common to refer to heat as energy, but heat is actually a process where energy is transferred from a high temperature object to a lower temperature object. Therefore, an object does not possess heat. The appropriate term for the microscopic energy is thermal energy. Thermal energy is a combination of the potential and kinetic energies associated with microscopic particles within a material. Electromagnetic radiation, more commonly called light, is also commonly referred to as an energy form. But, just as with heat, light is a process that transfers energy. Besides being a wave, light also has a particle nature, where each particle of light is called a photon. The total energy transferred via a photon is directly dependent on the light’s frequency. For example, high frequency light, such as x-rays have greater photon energies than low frequency light such as radio. The total energy transferred by an individual photon is calculated using Einstein’s photoelectric effect equation. Details about the photoelectric effect can be found at http://galileo.phys.virginia.edu/classes/252/photoelectric_effect.html. Performance Benchmark P.12.C.2 Students know energy forms can be converted. E/S Common misconceptions associate with this benchmark: 1. Students incorrectly believe that energy can be made, used, and lost. The Law of Conservation of Energy states that energy is not created or destroyed only transferred from potential to kinetic (or vice versa). Students should understand that just because they cannot see energy transfer (into and from some forms) does not mean that the energy has been destroyed. A discussion about the Second Law of Thermodynamics should aid in the explanation of energy transformation. The Second Law of Thermodynamics concerns entropy and its increasing amount in the universe and exploring this topic should deepen student understanding about how energy has been transferred via heat to a more disorganized kinetic energies of random molecular motions. To learn more about the Second Law of Thermodynamics, go to http://www.grc.nasa.gov/WWW/K-12/airplane/thermo2.html. 2. Students incorrectly use the terms “energy” and “force” interchangeably In Star Wars Episode IV, Obi Wan Kenobi tells Luke Skywalker that “The Force is an energy field created by all living things.” This statement typifies confusion about the terms energy and force. An appreciable part of this confusion resides in misconceptions about the relationship between force and motion. Many students incorrectly believe that a moving object must have an “impetus” force causing it to stay in motion. Students incorrectly believe that this impetus force is applied to an object by a collision with another object (i.e., hitting a baseball with a bat) and that this “impetus force” then resides within the object even after it has lost contact with the original impactor, causing it to continue moving until this impetus is somehow dissipated. To learn more about the impetus misconception, go to http://modeling.asu.edu/R&E/forceConceptionTaxon92.doc Before Galileo, the prevailing scientific thought upheld the belief of force impetus, where some incorrectly believed that this force depended on the speed and mass of the object. Note how closely the incorrect idea of force impetus relates to correct understandings about kinetic energy. Starting with Galileo and Newton, scientists now know that an object will remain in constant motion (either at rest or traveling in the same direction with the same speed) unless acted upon by a net force. Therefore, force is not required for motion, but only to change an object’s motion (direction and/or speed). Forces act upon objects, but are not an inherent quality within the object. A detailed discussion of Newton’s First Law of Motion is found at http://www.physicsclassroom.com/Class/newtlaws/U2L1a.html On the other hand, energy is an inherent quality of an object. If the object is moving, it has kinetic energy. Also, within the object there exists internal kinetic energy associated with molecular motions of the object’s material. The object would also have potential energy due to position within a gravitational, electrical, magnetic, and/or other type of force field. An overview about the forms of energy can be read at http://www.eia.doe.gov/kids/energyfacts/science/formsofenergy.html 3. Students incorrectly believe that energy is a substance, such as gasoline fuel. Many students often view energy as a type of material or substance that acts similarly to that of matter. This may stem from common use of fuels, such as gasoline, and the misconception that the fluid itself is energy. The bonds that hold the hydrocarbon together to form gasoline are stored chemical potential energy that is released with a minimal amount of activation energy. Gasoline, or any fuel, can be viewed as a “storage container” for energy. Energy is not a substance, but a quantitative way to measure how a system is changing or potentially can change. To learn more about the energy as a substance misconception and other energy misconceptions, go to http://www.uwsp.edu/cnr/WCEE/keep/Mod1/Whatis/energyforms.htm. Performance Benchmark P.12.C.2 Students know energy forms can be converted. E/S Sample Test Questions 1. Energy is created as the result of which activity? a. Burning gasoline in an internal combustion engine. b. Damming a river for hydroelectric power. c. Rolling a marble down an incline plane. d. Energy cannot be created from nothingness. 2. Using the figure below, which of the following statements is correct? Figure 4. Energy of a skier-mountain system. (from http://www.physicsclassroom.com/Class/energy/U5L2bc.html) a. b. c. d. The total energy in the system is 100,000 J. The total energy in the system is 50,000 J. The total energy in the system is 0 J. The total energy at the end is half as much as it was in the beginning. 3. Potential energy is the “stored” energy an object has because of its a. Speed b. Size c. Position d. Density 4. In a closed system, a. Energy can be transformed with a net gain or loss of energy. b. Energy cannot be transformed and gradually flows out of the system. c. Energy can be transformed without any net gain or loss of energy. d. Energy can be transformed and gradually flows out of the system. 5. Kinetic energy is a. The energy stored in an object. b. The thermal energy within an object. c. The useful energy emitted by an object. d. The energy of an object’s movement. 6. A ball falls from a height of 20 meters. As the ball is free falling towards the ground (and ignoring air resistance) a. The ball does not have any potential energy and is gaining kinetic energy with the total energy increasing. b. The ball’s potential energy increases and its kinetc energy decreases with the total energy remaining the same. c. The ball’s potential energy decreases more rapidly than the kinetic energy with the total energy decreasing. d. The ball is losing potential energy and gaining kinetic energy with the total energy remaining the same. 7. From rest, a stone is dropped straight downward from a tower. Before being dropped, the stone has a gravitational potential energy of 1000 J. What is the kinetic energy of the stone when it has fallen half-way to the ground? a. 250 J b. 500 J c. 1000 J d. 2000 J 8. What is energy? a. The capacity to produce a physical change. b. The force needed to produce a physical change. c. The position of an object above a reference state. d. The power of an object above a reference state. Performance Benchmark P.12.C.2 Students know energy forms can be converted. E/S Answers to Sample Test Questions 1. 2. 3. 4. 5. 6. 7. 8. (d) (b) (c) (c) (d) (d) (b) (a) Performance Benchmark P.12.C.2 Students know energy forms can be converted. E/S Intervention Strategies and Resources The following list of intervention strategies and resources will facilitate student understanding of this benchmark. 1. Energy Modeling Unit Arizona State University runs a physics modeling program, which research has shown that students experiencing the unit undergo conceptual change for better student understanding. The complete energy unit comes with teacher notes and resources that thoroughly explain mechanical energy, and discuss heat and radiation. The modeling strategy forces students to problem solve and formulate a mathematical model of behavior that syncs with current scientific knowledge. To view the unit on energy, go to: http://modeling.asu.edu/Modelingpub/Mechanics_curriculum/7-Energy/ . Please note that this link is an ftp site appearing as a folder containing several PDF files for download. 2. Renewable Energy Lessons and Resources The New York State Energy Research and Development Authority has a program with schools in the state called School Power…Naturally, where solar panels are provided to schools for use in generating the schools’ electrical power. In association with the program, several curricular materials have been developed, including lessons that cover the nature of energy and conservation of energy. Teacher materials, instructions, and links to resource can be found at http://www.powernaturally.org/Programs/SchoolPowerNaturally/InTheClassroom / 3. Energy Tutorial The Physicsclassroom.com offers a comprehensive tutorial that explains the concepts of kinetic energy and potential energy. This site is useful for educators as a quick review of content and as a resource for students needing further clarification. The concepts are described well with many examples and the site has review questions for students to test their understanding. To view the tutorial, go to http://www.physicsclassroom.com/Class/energy/energtoc.html. 4. Energy Lesson Plans PhysicsFront.org is a Web site for Physics Educators that has a collection of ready to use lesson plans. The lesson plans are complete with labs and activities and further resources. Lesson plans and activities for energy can be found at http://www.thephysicsfront.org/static/unit.cfm?sb=5