A 5: D
advertisement

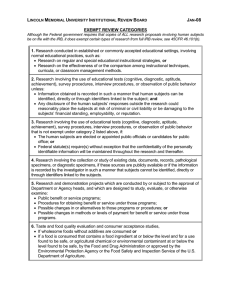
APPENDIX 5: DEFINITIONS Action Research – Research conducted for the purpose of informing or improving professional practices of the research practitioner. The research question is limited to the specific practices of the action researcher. The research findings are of interest and directly applicable to the practitioner. Cooperative Research—Research that is conducted by two institutions covered by 34 CFR. For CCSD, this will typically be the District and a University, but may also be any other entity covered by the regulations. Exempt Research—Research that, according to ED, does not need to meet the requirements of the human subjects regulations established by ED. According to 34 CFR 97.101 (b), “Research activities in which the only involvement of human subjects will be in one or more of the following categories are exempt: (1) Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (a) research on regular and special education instructional strategies, or (b) research on the effectives of or the comparison among instructional techniques, curricula, or classroom management methods. (2) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior, unless: i. Information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects; and ii. Any disclosure of the human subjects’ responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation. (3) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behavior that is not exempt under paragraph (b)(2) of this section, if: i. The human subjects are elected or appointed public officials or candidates for public office; or ii. Federal statute(s) require(s) without exception that the confidentiality of the personally identifiable information will be maintained throughout the research and thereafter. (4) Research, involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. (5) Research and demonstration projects which are conducted by or subject to the approval of department or agency heads, and which are designed to study, evaluate, or otherwise examine: i. Public benefit or service programs; ii. Procedures for obtaining benefits or services under those programs; iii. Possible changes in or alternatives to those programs or procedures; or iv. Possible changes in methods or levels of payment for benefits or services under those programs. (6) Taste and food quality evaluation and consumer acceptance studies, i. If wholesome foods without additives are consumed or ii. If a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the Food and Drug Administration or approved by the Environmental Protection Agency or the Food Safety and Inspection Service of the U.S. Department of Agriculture. Human Subject—Ed defines human subject as “…a living individual about whom an investigator (whether professional or student) conducting research obtains data through intervention or interaction with the individual or obtains identifiable private information.” (http://www.ed.gov/policy/fund/reg/humansub/part97-2.html#97.102) Institutional Review Board (IRB)—An internal, organizational mechanism charged with reviewing all proposed research to be conducted in the educational organization. ED states that the function of the IRB is to “…approve, request modification in, or disapprove research activities [ 34 CFR 97.109 (a) ] and to conduct continuing reviews of the research activities at intervals appropriate to the degree of risk, but not less than once a year [ 34 CFR 97.109 (e) ].” (http://www.ed.gov/policy/fund/reg/humansub/part97-2.html#97.102) Research—For the purposes of process being addressed herein, the definition set forth in the regulations ED governing the protection of human subjects is used. That is, research is “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.” [34 CFR 97.102 (d)] According to ED, “if an activity follows a deliberate plan whose purpose is to develop or contribute to generalizable knowledge, such as an exploratory study or the collection of data to test a hypothesis, it is research.” (http://www.ed.gov/policy/fund/reg/humansub/part972.html#97.102) The regulations do not distinguish between research and evaluation. Rather, they treat both as research. The Review Process will do the same. Research Review Committee—The body established and managed by CCSD Department of Research to conduct the reviews prescribed in the Review Process. The committee shall serve as the screening committee to determine which requests must comply with the Regulations and which are exempt. Sponsored Research—Proposed research work that a District administrator of a department or division considers important for that unit or for the District and that he/she wants to have performed within the District. Unsponsored Research—Proposed research work that has not been deemed of special interest by a CCSD division or department administrator. APPENDIX 6: REFERENCES Mills, G.E. (2003). Action Research: A Guide for the Teacher Researcher. Upper Saddle River, NJ: Pearson Education, Inc. United States Department of Education. Information About the Protection of Human Subjects in Research Sponsored by the Department of Education—Overview. Retrieved November 24, 2003 from http://www.ed.gov/policy/fund/guid/humansub/overview/html United States Department of Education. Code of Federal Regulations Title 34 Department of Education PART 97 - Protection of Human Subjects. Retrieved November 24, 2003 from http://www.ed.gov/policy/fund/reg/humansub/97.101. United States Department of Education. Excerpts from 34 CFR 350 and 34 CFR 356, Retrieved November 24, 2003 from http://www.ed.gov/policy/fund/reg/humansub/34cfr350.html). United States Department of Health and Human Services: National Institutes of Health. Human Participant Protections Education for Research Teams. Retrieved December 8, 2003 from http://cme.cancer.gov/c01/.