AP Chemistry Electron Configuration/Quantum Number Review Problems
advertisement

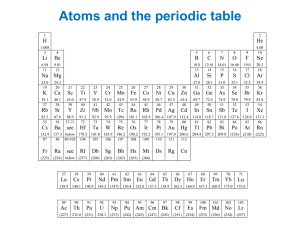
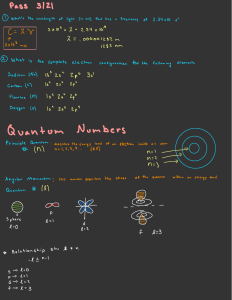
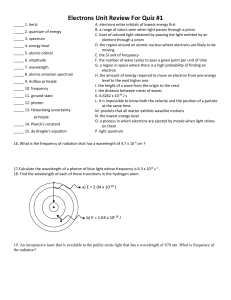
AP Chemistry Electron Configuration/Quantum Number Review Problems 1. For the last electron to enter Osmium (Z = 76). . . a. Write the unabridged electron configuration b. Determine the quantum numbers for that last electron c. How many filled energy levels are there? d. How many energy levels contain electrons? e. How many filled orbitals are there? f. How many electrons are not paired up? 2. What is the maximum number of electrons in an atom that can have these quantum numbers? a. n = 4; l = 3 b. n = 4; ml = -2