Lecture 6.0 Properties of Dielectrics
advertisement

Lecture 6.0
Properties of Dielectrics
Dielectric use in Silicon Chips
Capacitors
– On chip
– On Circuit Board
Insulators
– Transistor gate
– Interconnects
Materials
– Oxides
–SiO2
– Boro-Silicate
Glass
– Nitrides
–BN
– polymers
Importance of Dielectrics to Silicon Chips
Size of devices
– Electron Tunneling dimension
Chip Cooling- Device Density
– Heat Capacity
– Thermal Conductivity
Chip Speed
– Capacitance in RC interconnects
Band theory of Dielectrics
Forbidden Zone–Energy Gap-LARGE
Conduction
Band
Valence
Band
Difference between
Semiconductors and Dielectrics
Material
Eg(eV)
Ge
0.67
Si
1.12
GaAs
1.43
SiO2
8
UO2
5.2
Ga2O3
4.6
Fe2O3
3.1
ZnO
3.2
NiO
4.2
Al2O3
8
kBT =0.0257 eV
at 298˚K
Fermi-Dirac Probability
Distribution for electron energy, E
Probability, F(E)=
(e{[E-Ef]/kBT}+1)-1
–Ef is the
Fermi Energy
Number of Occupied States
Density of States
Fermi-Dirac
T>1000K only
Probability of electrons in
Conduction Band
Lowest Energy in CB
E-Ef Eg/2
Probability in CB
F(E)= (exp{[E-Ef]/kBT} +1)-1 )
= (exp{Eg/2kBT} +1)-1
exp{-Eg/2kBT} for Eg>1 eV @ 298K
exp{-(4eV)/2kBT}= exp{-100} @ 298K
kBT =0.0257 eV
at 298˚K
Intrinsic Conductivity of Dielectric
Charge Carriers
– Electrons
– Holes
– Ions, M+i, O-2
= ne e e + nh e h
# electrons = # holes
– ne e (e+ h)
– ne C exp{-Eg/2kBT}
Non-Stoichiometric Dielectrics
Metal Excess
M1+x O
Metal with Multiple valence
Metal Deficiency
M1-x O
Metal with Multiple valence
Reaction
Equilibrium
Keq (PO2)±x/2
x
TiO2
TiO2 x O2 ( g )
2
+3 .. 1
+4
'
2TiTi OO
2TiTi VO O2 ( g )
2
x
ZnO O2 ( g )
Zn1 xO
2
+2
+3
x
NiO O2 ( g )
Ni1 xO
2
Density Changes with Po2
SrTi1-xO3
Non-Stoichiometric Dielectrics
Excess
M1+x O
Deficient
M1-x O
Non-Stoichiometric Dielectrics
Ki=[h+][e-]
K”F=[O”i][V”O]
Conductivity
=f(Po2 )
Density =f(Po2 )
Dielectric Conduction due to Non-stoichiometry
N-type
P-type
Dielectric Intrinsic Conduction due to Non-stoichiometry
N-type
P-type
+h
+h
Excess
Zn1+xO
Deficient
Cu2-xO
Extrinsic Conductivity
Donor Doping
n-type
Ed = -m*e e4/(8 (o)2 h2)
Ef=Eg-Ed/2
Acceptor Doping
p-type
Ef=Eg+Ea/2
Extrinsic Conductivity of Non-stoichiometry oxides
Acceptor Doping
p-type
x
x
Li2O (1 x) NiO O2
( Lix Ni122 x Nix3 )O
2
4
p= 2(2 m*h kBT/h2)3/2 exp(-Ef/kBT)
Law of Mass Action, Nipi=ndpd or =nndn
@ 10 atom % Li in NiO
conductivity increases by 8 orders of magnitude
@ 10 atom % Cr in NiO no change in conductivity
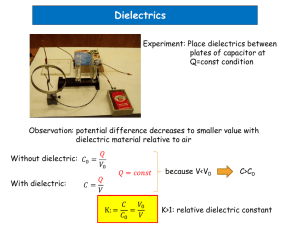
Capacitance
C=oA/d
=C/Co
=1+e
e =
electric
susceptibility
Polarization
P = e E
e = atomic
polarizability
Induced polarization
P=(N/V)q
Polar regions align with E field
P=(N/V) Eloc
i(Ni/V) i=3 o (-1)/(+2)
Local E Field
Local Electric Field
Eloc=E’ + E
E’ = due to
surrounding dipoles
Eloc=(1/3)(+2)E
Ionic Polarization
P=Pe+Pi
Pe = electronic
Pi= ionic
Pi=(N/V)eA
Thermal vibrations prevent
alignment with E field
Polar region follows E field
opt= (Vel/c)2
opt= n2
n=Refractive index
Dielectric Constant
Material
(=0)
opt=n2
Diamond
5.68
5.66
NaCl
5.90
2.34
LiCl
11.95
2.78
TiO2
94
6.8
Quartz(SiO2)
3.85
2.13
Resonant Absorption/dipole relaxation
Dielectric Constant
imaginary number
’ real part
dielectric storage
” imaginary part
dielectric loss
o natural frequency
Dipole Relaxation
d 2x
dx
e i t
2
0 x e
2
dt
dt
m
Resonant frequency,o Relaxation time,
2
2
2
N
e
o
'
opt
2
2 2
2 2
V o mi ( o )
2
2
2
N
e
o
"
V o mi ( o2 2 ) 2 2 2
s opt
opt
1 2 2
( s opt)
"
1 2 2
'
Relaxation Time,
Dielectric Constant vs.
Frequency
Avalanche Breakdown
Avalanche Breakdown
Like nuclear fission