MLAB 1415-Hematology Keri Brophy-Martinez Hypoproliferative Anemias
advertisement

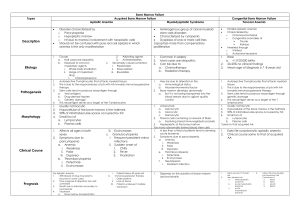
MLAB 1415-Hematology Keri Brophy-Martinez Hypoproliferative Anemias Overview Disorders Anemia Acquired Congenital Normocytic or Macrocytic Normochromic Bone marrow hypocellularity Defect Depletion, damage or inhibition of stem and/or progenitor cells APLASTIC ANEMIA Aplastic anemia Disorder (or group of disorders) characterized by aplasia of bone marrow or its destruction by chemical agents or physical factors. All cell lines are affected. Aplasia Failure of a tissue or organ to develop normally Pathophysiology Aplastic anemia is caused by an immune process, either antibodies directed against the stem cell or a cellular immune mechanism (T-lymphocyte) that suppresses stem cell prolieferation Bone marrow fails due to the immunologically mediated tissue-specific destruction Phases I: Onset After an initiating event (i.e. viral infection) the hematopoietic cells are destroyed by the immune system Small numbers of surviving stem cells can support adequate hematopoiesis for some time, but eventually the circulating cell counts become very low and clinical symptoms appear Phases II: Recovery Either a partial response or a complete response can occur, initially, without increased number of stem cells III: Late Disease Years after recovery, blood counts may fall as pancytopenia occurs or as abnormal clones of stem cells emerge. Often leads to PNH( paroxysmal noctural hemoglobinuria), AML (acute myelogenous leukemia) or MDS (myelodysplasia) Aplastic Anemia Two Groups Acquired Majority of cases Surface after age 60 Congenital 2-5 years old 15-25 years old APLASTIC ANEMIA Acquired Idiopathic or primary with no clear cause Secondary due to Chemical agents such as benzene, insecticides, weed killers Drugs such as chloramphenicol (antibiotic) and phenylbutazone (anti-inflammatory), chemotherapy drugs (busulfan, vincristine), anticonvulsants (Dilantin) Ionizing radiation from nuclear fallout, x-rays or radiation therapy, radium Infections such as hepatitis, Epstein-Barr virus, CMV, HIV Miscellaneous - pregnancy, malnutrition, immunologic dysfunction APLASTIC ANEMIA Congenital/ Inherited Aplastic Anemia Fanconi’s anemia Genetic predisposition to bone marrow failure Chromosomal breaks, gaps, exchanges 25- 30% cases of childhood aplastic anemia Twice as common in males than females Clinical Features Low birth weight Skin hyperpigmentation Short statue Dyspnea Bleeding Infections Skeletal disorders Aplastic anemia : Lab Features Total Bone marrow failure Hypocellular: 70% fat Pancytopenia= all cell lines affected Aplastic Anemia: Lab Features ↓ RBC, hgb (<7), hct (<20), retic (absolute and corrected) ↓ WBC (<1.5) , absolute neutrophil count (<0.5) Relative lymphocytosis due to the neutropenia ↓ platelets (<20,000-60,000) P.B Normocytic/macrocytic,normochromic Moderate anisocytosis, poikilocytosis NRBCs, teardrops* Treatment Treatment of choice is bone marrow transplantation. If this is not an option, immunotherapy is given. Spontaneous recovery may occur if the offending agent is removed. Prognosis is poor, high mortality rate..2 years Complications include Bleeding Iron overload due to transfusions Pure red cell aplasia Rare condition Red cell precursors in the bone marrow are decreased Presence of peripheral blood anemia Pure Red Cell Aplasia Congenital Diamond-Blackfan anemia Defect in erythroid precursors Acquired Hemolytic crises Infection with parvovirus, EBV, viral hepatitis Malnutrition Certain drugs or neoplasms Other Hypoproliferative Anemias Defective hormonal stimulation of erythroid progenitor cells Kidney Disease Endocrine Disorders Lab findings reflect anemia and pathologies of primary disorder References Harmening, D. M. (2009). Clinical Hematology and Fundamentals of Hemostasis. Philadelphia: F.A Davis. http://www.aamdsglossary.co.uk/glossary/b http://blass.com.au/definitions/aplastic%20anemia McKenzie, S. B., & Williams, J. L. (2010). Clinical Laboratory Hematology . Upper Saddle River: Pearson Education, Inc.