Sea Anemone….To Treat or not to Treat: That is the Question?
advertisement

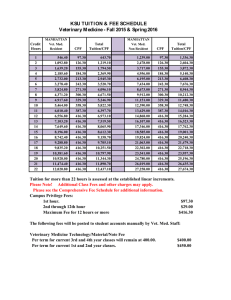
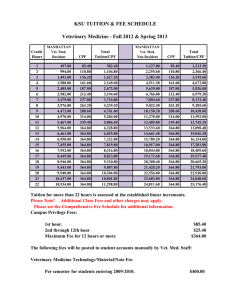
Sea Anemone….To Treat or not to Treat: That is the Question? CT Surgery/Cardiology Conference Shadwan Alsafwah, MD Cardiology Fellow University of Tennessee at Memphis Case 53 YO M with OSA was referred for OP routine TTE for evaluation of pulmonary HTN. PMH: OSA HTN Hyperlipedemia Asthma Colon Polyposis BPH LUE weakness and tremor since 6 months Case…… Meds: Albuterol Lisinopril Simvastatin Terazosin PSH: Hernia repair SH: Smoker 1ppd X 30 y No ETOH, illicit drugs Allergies: Sulfa Metronidazol Codien Case… Physical exam: Vitals: 154/77, 65, 16, 97.7 Neck: No JVD, No Carotid Bruit. Chest: CTAB CVS: RRR, normal S1, S2, no extra sounds Abdomen: Soft, NT, ND, NABS Ext: No E/C/C Neuro: Normal except for Motor 4/5 in LUE 2 D Echo EF: normal estimated 75% Borderline mild pulmonary hypertension (peak PA pressure 35-40 mm Hg. Mild –moderate LVH Fimbria-like structure on the aortic valve, most likely papillary fibromatous tumor. Less likely to be vegitation or Lambl’s Excrescence. TEE recommended TEE Fimbriae-like structure on the right coronary cusp of the aortic valve C/W Papilary fibroelastoma (not likely to be a lambl’s excrescence, or vegetations) Otherwise normal aorta Normal LV function, EF 75% Better Be Prepared for Questions like: What does this “structure” mean? What caused it? What should we do about it? Outline Nomenclature Historical Reference Incidence Natural History Etiologies Anatomy: - Gross - Micro Clinical Manifestations Diagnostic Modalities Differential Diagnosis Treatment Prognosis Summary Nomenclature Fibroma Cardiac papiloma Valvar papiloma Myxofibroma Fibroelastic hamartoma Endocardiac papillary fibroma Giant Lambl’s excrescences Cardiac Papillary Fibroelastoma (CPF) Historical Reference The first cardiac tumor ever described was a left atrial myxoma described in 1845 by King TW : “ On simple vascular growth in the left auricle of the heart” Lancet 1845;2:428-429. Yater in 1931 was the first to describe the valvular tumors Cheitlin et al in 1975 used the term “papillary fibroelastoma” for the first time. Lichtenstein et al in 1979 were the first to report a CPF found incidentally during VSD repair. Flotte et al diagnosed this tumor on Echo 1980 Incidence Historically was the third most common benign primary cardiac tumor after Myxomas, Lipomas More recent series has placed it as the second most common benign primary tumor of the adult heart. The most common primary tumor of the cardiac valves (3/4th) Has an estimated incidence of 0.0017%-0.33% in autopsy series, and an estimated echocardiography incidence of 0.019% Incidence… 90% arise from valvular tissue, most commonly aortic (44%) or mitral valves (35%). They may arise from papilary muscles and chordae tendineae, but rarely from the mural endocardium Most commonly they arise from the mid portion of the valve. They project into the arterial lumen of semilunar valves and the atrial surface of AV valves Reported from neonates to 92 years, but in general rarely seen below age 20, with mean age of 60 years, and 29% were 70 years of age or older. Males = Females Benign Primary Cardiac Tumors Natural History Significant percentage of patients have concomittent valvular disease, suggesting that prior endocardial damage predisposes to papiloma formation Generaly, Small in size: - 99% <20 mm in largest dimension (mean 9 mm) - Range 2-70 mm in size More than 90% are solitary Slow- grwoing tumor Etiologies Remains under discussion, possible etiologies: Truly neoplastic Viral Iatrogenic: 1. Post cardiac surgery 2. Post radiation therapy Other possible etiologies (?) Viral Small study at Hospital Cardiologique, Chulille, France. 4 patients with valvular CPF: 2 with prior neuro embolic events 2 without prior embolic events CPFs were surgically removed, and all samples were histologically confirmed Specific immunohistochemical (IHC) studies were conducted on all samples Grandmougin D, et al. Heart Valve Dis 2000;9(6):832-41 (?) Viral The first 2 patients: there was good correlation between the neuro events and the presence of thrombus aggregated on the injured superficial endothelial layer. The other 2 patients: no endothelial damage or thrombus were found. IHC studies showed: -A centrifugal mesenchymal cellular migration arising from the central layer to the superficial layer with differentiation steps. -The presence of dendritic cells and remnants of CMV in the intermediate layer. Is CPF a chronic form of viral endocarditis. Grandmougin D, et al. Heart Valve Dis 2000;9(6):832-41 (?) Iatrogenic A study at Mayo clinic and Armed forces Institute of Pathology in washington, DC found 12 iatrogenic CPF cases (6 post CT surgery, 6 post thoracic irradiation) between 19902000: 1. Common: It represented 18% of all surgically excised CPF during that period! 2. Timing: mean interval was 18 years (range 9-31 years) 3. Multiple: about 58% were multiple! 4. Location: found in the chamber closest to the procedure, or within the radiation field 5. Atypical: often involve nonvalvular endocardial surfaces Kurup AN, et al. Hum Pathol 2002;33(12):1165-9 (?) Other Possible Etiologies Mechanical damage to the endothelium Organizing thrombi Hamartomous origin or congenital etiologies in neonates/infants (very rare) Gross Anatomy Resemble a sea anemone: Friable, white to tan multiple branching and nonbranching fingerlike fronds emanating from a stalked central core Microscopically Each frond is avascular and consists of a collagenous core surrounded by elastic fibers and loose mucopolysaccharide matrix with rare smooth muscle cells And covered by a single layer of endocardial endothelial cells Clinical Manifestations More than 60% asymptomatic, found incidentally Do not generally cause valvular dysfunction But, sometimes can cause: 1. Embolic Phenomena leading to TIAs and CVAs: - Can be as high as 25% over 3 years, and 6% in asymptomatic incidental CPF -Results from fragmentation of the papillary spikelets of the tumor or from thrombi formed by platelets and fibrin adhering to the uneven surface of CPF -A/C of ? effect (3 cases with recurrent strokes while on A/C) Other Clinical Manifestations.. - The tumor mobility was the only independent predictor of CPF related death or nonfatal embolization 2. Angina Pectoris, sometimes AMI if it involves the coronary ostium 3. Outflow tract obstruction, presyncope or syncope 4. Sudden death 5. It can get infected! (SBE prophylaxis?) Diagnosis Should be suspected in young patients with no evidence of cerebrovascular disease who present with an embolic cerebral stroke, especially in the presence of NSR Before 1977, they were diagnosed exclusively at postmortem examination Up to 1991 only 132 cases were reported in the literature Now, it is generally an incidental finding by routine TTE echocardiography (sensitivity 62%) Best seen by TEE (sensitivity 77%) Either TTE, TEE sensitivity is up to 90% if size >20 mm Typical Echocardiographic Features Round, oval, irregular in appearance Well-demarcated borders Homogenous texture Nearly half have small mobile stalk TEE with its high resolution, may distinguish the collagen center of the tumor from other cardiac structures, due to its shining echo appearance It can rarely become calcified Cardiac MRI and Ultrafast CT CPF are usually not seen at MRI or CT, due to their size (very small in general) and location (moving valves) Detects only exceptionally large CPF, or atypical CPF (away from valves) MRI is generally preferred to CT as it reflects the chemical microenvironment within the tumor (better soft-tissue characterization), offering clues to the type of tumor Will have more role in near future with new emerging advances in technology? Differential Diagnosis Lambl’s excrescences Myxoma Bacterial vegetations Organizing marantic (thrombotic) endocarditis CPF Vs Lambl’s Excrescences Location: Valve surface Rarely multiple Gross: Small, branching Micro: abundant subendothelial myxoid ground substance Etiology: Multiple theories Very rare At sites of valve closure > 90% multiple Smaller, non branching Less abundant subendothelial myxoid ground substance Endothelial damage, followed by thrombosis and organization. Common: more than 70% of adults Treatment Controversial, due to the absence of randomized controlled data available Long-term oral A/C +/- Antiplatelet therapy could be offered to symptomatic patients who are not surgical candidates, but its efficacy in preventing embolic events is unclear. SBE prophylaxis (?) Sun JP, et al. Circualtion 2001;103:2687 Study Design Retrospective + Prospective 16-year study (1983- 1999) using echo (total 109502 echos) and pathology data base at CCF. 162 patient found to have pathologically confirmed CPFs: - in 141 an Echo (126 TTE, 107 TEE) was performed -of those 93 CPFs identified: - 26 identified pre-surgery (prospectively) - 67 identified post-surgery (retrospectively) An additional 45 patients with presumed CPF identified by echo database were followed for symptoms attributable to CPF. Sun JP, et al. Circulation 2001;103:2687 Sun, JP, et al. Circulation 2001;103:2687 Results 23/26 patients in the Prospective group developed symptoms. 5/45 patients in the presumed group developed symptoms. Stalks with mobility were present in almost all the symptomatic ones Sun JP, et al. Circulation 2001;103:2687 Treatment of Right-sided CPF Right-sided CPF are less risky, surgery is not completely agreed upon, but generally surgery is indicated if: 1. Symptomatic 2. Large mobile tumors 3. Presence of PFO with a sizable right to left shunt Treatment of Left-sided CPF Somewhat less controversial: In general: it should be removed, especially: 1. Symptomatic 2. CPF≥ 1 cm, especially if mobile 3. Young patients with low risk of surgery and high risk for embolization 4. Patients with other cardiovascular disease. Asymptomatic patients with small, left-sided nonmobile CPF can be followed-up closely with periodic clinical evaluations and echo, and receive surgical intervention whenever symptoms develop or the tumor becomes mobile Prognosis Surgical removal is usually curative after complete resection, never reported to recur in the same location CPF can recur in another location More than 90% can be resected using conservative valve- sparing approaches Incidental CPF found on the aortic or mitral valves during other surgery should be removed. Long-term f/u is recommended Back to Our Question: Sea anemone: To treat or not to treat, that is the question? The best advise is: Individualize, look at each case separately Consider in your Decision.. The Patient: -Age : the younger the pt the higher the cumulative risk of embolization -Other co-morbidities… Symptomatic CPF or not If symtomatic: what strength of association of the tumor with symptoms CPF Size (≥ or < 1 cm) CPF Location (L sided or R sided, valvular or nonvalvular…) CPF mobility (i.e. presence of stalk or not) Now Back to Our Patient He is 53 Y.O. No major co morbidities/contraindications for surgery. His CPF is on the Aortic valve < 1 CM nonmobile The major question is whether the LUE weakness represent an ischemic event or not. Summary CPF is increasingly recognized with the widespread use of TTE, TEE, and with new imaging modalities It should be differentiated from other valvular pathologies especially Lambl’s excrescences. It can be symptomatic, mainly manifesting as embolic disease Controverseries still ongoing about the pathogenesis and treatment of incidental CPF More studies are needed to clarify its pathogenesis, and treatment. Thank You