Bivalirudin (Angiomax ) The Gold Standard Anticoagulant during PCI?
advertisement

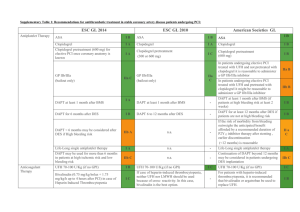
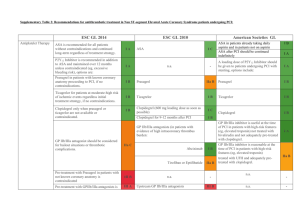
® (Angiomax ) Bivalirudin The Gold Standard Anticoagulant during PCI? Carrie Oliphant, Pharm.D., BCPS Cardiology Clinical Specialist Methodist University Hospital PCI procedure Thrombin Generation Tissue Factor Platelet Activation Adhesion Molecules Vessel Wall Injury Inflammation Ann Pharmacother 2004;38:99-109. Role of Thrombin • Conversion of fibrinogen to fibrin – Fibrin strands trap platelets and RBCs • Factor XIII activation – Stabilizes fibrin clot by cross linking strands • Activates Factor VIII and V – Enhance production of thrombin • Platelet activation – Secretes tissue factors, expression of GP IIb/IIIa receptors Am Heart J 2005;149:S43-53. Heparin Limitations • Indirect inhibition of thrombin – Cannot inhibit fibrin-bound thrombin • Nonlinear pharmacokinetics, dose response variability • Causes platelet activation • Inhibition by PF4 • Heparin:PF4 complex formation Am Heart J 2005;149:S43-53. N Engl J Med 2005;353:1028-40. Bivalirudin vs. other DTIs Drug Bivalirudin Lepirudin (Refludan, Hirudin) Argatroban Binding site Exosite-1; active Exosite-1; active Active Type of binding Reversable Irreversable Reversable PCI studies • • • • BAT, BAT reanalysis CACHE REPLACE-1 REPLACE-2 BAT • Unstable angina or postinfarction angina • n=4098 (excluded 214 enrolled patients) • Protocol (All patients: ASA 300-325 mg) – Bivalirudin 1 mg/kg bolus, 2.5 mg/kg/hr infusion x 4 hours, 0.2 mg/kg/hr infusion x 20 hours – UFH 175 units/kg bolus, 15 units/kg/hr infusion x 18-24 hours • ACT < 350: Additional 60 units/kg bolus N Engl J Med 1995;333:764-9. BAT Major Bleeding 12 9.8 10 8 Bivalirudin UFH 6 4 3.8 2 0 p<0.001 N Engl J Med 1995;333:764-9. BAT reanalysis • Intention to treat analysis – n=4312 • Primary endpoint – Death, revasc., MI at 7, 90 and 180 days Bivalirudin 7 days 135 (6.2%) 90 days 340 (15.7%) 180 days 496 (23%) Heparin 169 (7.9%) 399 (18.5%) 532 (24.7%) p value 0.039 0.012 0.153 Am Heart J 2001;142:952-9. BAT reanalysis • Major bleeding – Bivalirudin 76 (3.5%) – UFH 199 (9.3%) p<0.001 Am Heart J 2001;142:952-9 CACHET • Elective angioplasty or stenting • n=268 •Abciximab 0.25 mg/kg bolus, 0.125 mcg/kg/min infusion x 12 hours. •UFH 70 U/kg bolus, additional boluses PRN for ACT > 200 sec •ASA for all patients. •Clopidogrel x 30 days (load?) Am Heart J 2002;143:847-53. CACHET Am Heart J 2002;143:847-53. REPLACE-1 • Elective or urgent PCI • n=1056 • Protocol (All patients: ASA, Clopidogrel) – Bivalirudin 0.75 mg/kg bolus, 1.75 mg/kg/hr infusion for the duration of the procedure – UFH 60-70 units/kg bolus, adjusted to ACT of 200-300 sec – GP IIb/IIIa inhibitors at recommended doses • Use at the discretion of the MD Am J Cardiol 2004;93:1092-1096. REPLACE-1 • GPI use – UFH 72.5% vs. Bivalirudin 71.1% • 34% Abciximab, 30% Eptifibatide, 6% Tirofiban • Clopidogrel use prior to procedure – UFH 57.4% vs. Bivalirudin 54.7% • 29% 2-48 hours before procedure • Primary endpoint – Composite of death, MI or repeat revasc. by discharge or within 48 hours of randomization Am J Cardiol 2004;93:1092-1096. Am J Cardiol 2004;93:1092-1096. REPLACE-2: Study Design 6002 Urgent or elective PCI patients 2994 aspirin clopidogrel stent 3008 Bivalirudin 0.75 mg/kg bolus, 1.75 mg/kg infusion with provisional GP IIb/IIIa UFH 65 units/kg abciximab or eptifibatide JAMA 2003; 289: 853-863. REPLACE-2 • Low risk PCI patients – Excluded primary PCI patients • Suggested indications for provisional GPI: – Abrupt or side branch closure, obstructive dissection, new or suspected thrombus, impaired or slow coronary flow, distal embolization, persistent residual stenosis, unplanned stent placement, prolonged ischemia, or other clinical instability. JAMA 2003;289:853-863. Endpoint Definitions Death 2º 1º • Any cause • Q wave in 2 leads • CKMB 3 fold ULN if <48 hr PCI 2 fold ULN if no revasc. MI Urgent revasc Major bleeding • Precipitated by ischemia • Re-intervention within 24 hours • • • • Intracranial or retroperitoneal Observed bleed with fall in Hgb >3g/dL No observed bleed with fall in Hgb >4g/dL Transfusion 2 units PRBC or whole blood JAMA 2003; 289: 853-863. Antiplatelet therapy JAMA 2003;289:853-863. Outcomes – 30 days p = 0.32 p = 0.40 p = 0.26 p = 0.23 p = 0.44 p < 0.001 heparin + GP IIb/IIIa bivalirudin 10.0% 9.2% 7.1% 7.6% 7.0% 6.2% 4.1% 2.4% 1.4% 1.2% 0.4% 0.2% Quadruple Triple composite composite Death MI Urgent Protocol revasc major bleed JAMA 2003; 289: 853-863. Hemorrhagic End Points D -43% p < 0.001 D -47% p < 0.001 D -33% p = 0.30 D -55% p < 0.001 D -63% p < 0.001 D -59% p < 0.001 25.7% 25.8% heparin + GP IIb/IIIa Bivalirudin with provisional GP IIb/IIIa 4.1% 13.6% 3.0% 2.5% 2.4% 1.7% 1.3% 0.9% 0.6% Major bleed Minor bleed TIMI TIMI major bleed minor bleed 0.8% Access site bleed 0.7% Platelets < 100,000 JAMA 2003; 289: 853-863. Bleeding vs. other clinical trials 5% 4% TIMI-any TIMI-minor TIMI-major 4.3% 4.3% 3.9% 3% 3.0% 3.0% 2.9% 2% 1.9% 1.5% 1% 1.3% 1.3% 0.9% 0.6% 0% EPISTENT ESPRIT REPLACE-2 UFH + abciximab UFH +eptifibatide UFH +GPI N=794 N=1040 N=3002 REPLACE-2 Bivalirudin N=2994 Lancet 1998;352:87-92.; Lancet 2000;356:2037-2044. 6 month- Ischemic end points JAMA 2004;292:696-703. 1-year Mortality All patients 3.0 heparin+GPI bivalirudin with provisional GPI 2.5 2.5% 2.0 Cumulative Percentage 1.5 of Mortality 1.9% p-value=0.16 1.0 0.5 0.0 0 60 120 180 240 300 360 Days (time from randomization) JAMA 2004;292:696-703. Clopidogrel in REPLACE-2 • Pretreatment N=5893 • No Pretreatment N=841 30.00% * * Bivalirudin-no pretreatment Bivalirudinpretreatment UFH-no pretreatment 20.00% * 10.00% * UFH- pretreatment 0.00% Quad Triple Major Minor endpoint endpoint bleeding bleeding *p=0.007 Bivalirudin pretreatment vs. no pretreatment; *p<0.001 for Bivalirudin vs. UFH J Am Coll Cardiol 2004;44:1194-9. REPLACE-2 Controversies • Study design – Non-inferiority study – Primary endpoint included efficacy and safety • Ineffective drugs with better safety profile may appear better • Increased ACT values w/UFH (median 317 sec) vs. previous GPI trials (EPISTENT, ESPRIT) • Increased non-Q wave MIs, CPK MB >5-10x Meta-analysis p<0.001 11% 10% 9% 8% 7% 6% 5% 4% 3% 2% 1% 0% p=0.049 p=0.02 p<0.001 10.8% 7.8% Bivalirudin 5.8% UFH 3.0% 2.7% 2.0% 0.1% 0.2% Death Revasc. Major bleeding Quad endpoint Death, MI, revasc., major bleeding J Am Coll Cardiol 2005;45 Suppl A:36A. Economic analysis- Drug cost Heparin + GPI Bivalirudin Cost n Total cost n Total cost $10 2896 $28,960 0 $0 $395 0 $0 2994 $1,182,630 $1350 1290 $1,741,500 106 $143,100 $600 1606 $963,600 111 $66,600 $2,734,060 $1,392,330 $909 $465 Drug Heparin Bivalirudin Abciximab Eptifibatide Total Average per patient Patient savings > $400 The Medicines Company- Data on File. Economic Analysis-Outcomes Average reduction in cost: $405 (95% CI $ 37773, p<0.001) J Am Coll Cardiol 2004;44:1792-1800 Drug Properties • Synthetic compound based on the structure of hirudin • Onset of action: 2 minutes • Predictable response • Clearance – Proteolytic cleavage and renal elimination (< 20%) • No antidote for rapid reversal Drugs 2005;65(13): 1869-1891. Half life Renal function (GFR, ml/min) Mild renal impairment-normal renal function (> 60 ml/min) Moderate renal impairment (30-59 ml/min) Severe renal impairment (10-29 ml/min) Dialysis-dependent patients Half life (min) 25 34 57 210 Inhibition of Platelet Aggregation Pharmacotherapy 2002;22:97S-104S. FDA approval • PCI with provisional GPI – Only studied in patients also receiving aspirin • Patients with HIT/HITTS or at risk of HIT/HITTS undergoing PCI The Medicines Company. Angiomax: prescribing information. www.angiomax.com Preparation • 250 mg vial – Add 5 ml sterile water for reconstitution • Add to D5W or 0.9% NS – Final concentration 5 mg/ml • Standard 250 mg/50 ml Conversion from Heparin/LMWH to Bivalirudin • UFH – Wait 30 minutes after discontinuation • LMWH – Wait 8 hours after last LMWH dose Pharmacotherapy 2002;22:105S-111S. Dosing • Bolus 0.75 mg/kg – Five minutes after bolus given, measure ACT • If ACT<225, give additional 0.3 mg/kg bolus • Infusion – Normal to mild renal impairment: 1.75 mg/kg/hr – Severe renal impairment (GFR 10-29 ml/min): 1 mg/kg/hr – Dialysis dependent: 0.25 mg/kg/hr The Medicines Company. Angiomax: prescribing information. www.angiomax.com Indications for Provisional GPI • • • • • • • • • Decreased TIMI flow (0-2) or slow reflow Dissection with decreased flow New or suspected thrombus Persistent residual stenosis Distal embolization Unplanned stent; suboptimal stenting Side branch closure; abrupt closure Clinical instability Prolonged ischemia www.angiomax.com ACT monitoring REPLACE-2 Bivalirudin Median ACT:358 UFH Median ACT:317 Pharmacotherapy 2002;22(8):1007-1018.; JAMA 2003;289:853-863. Sheath Removal Am J Cardiol 2004;94:1417-19. Side Effects- REPLACE-2 Drugs 2005;65:1869-1891. Place in Therapy- Guidelines • ACC/AHA – Class IIa- reasonable alternative to UFH + GPI in low risk patients. – Class I- replace UFH in patients with HIT. • ESC – Class IIa- replace UFH or LMWHs to reduce bleeding complications. – Class I- replace UFH or LMWHs in patients with HIT. www.acc.org; Eur Heart J 2005;26:804-47. Methodist University • ~ 100 PCI procedures/month • July 2005-January 2006 – Bivalirudin used in 532 cases – ~90% of PCI cases – Bivalirudin + GPI: 8.1% Ongoing Clinical Trials • ACUITY (n=13,800)- ACS (NSTEMI) – Bivalirudin + GPI vs. Bivalirudin + provisional GPI vs. UFH or LMWH + GPI – 1° endpoint-death, MI, revasc., major bleeding – GPI subgroup upstream vs. PCI • BIAMI (n=300) – Bivalirudin during primary PCI for STEMI • ISAR-REACT 3 (n=3000) – Bivalirudin + Clopidogrel 600 mg vs. UFH + Clopidogrel 600 mg in PCI www.clinicaltrials.gov Ongoing Clinical Trials • EVOLUTION (n=300) – Bivalirudin vs. UFH + protamine during CABG. • CHOOSE (n=100) – Bivalirudin vs. UFH + protamine during CABG in patients with HIT. www.clinicaltrials.gov