Force-spectroscopy of single proteins Force as a regulator of: -protein folding
advertisement
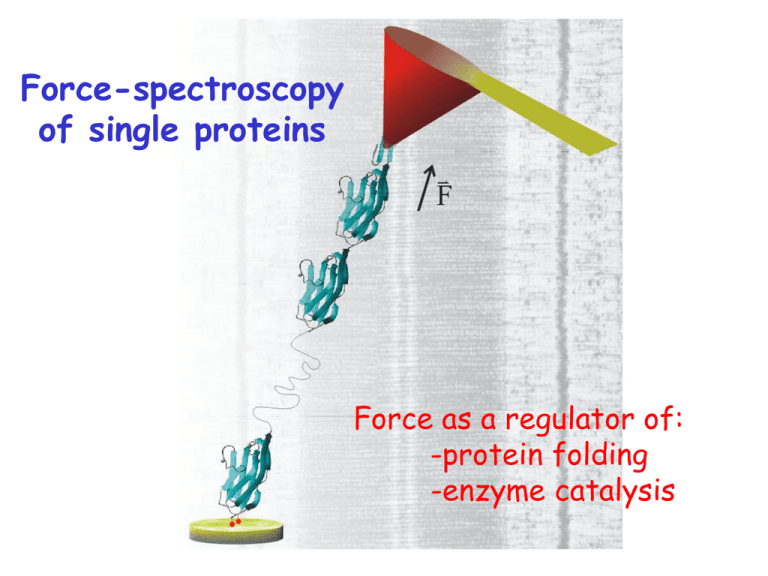
Force-spectroscopy of single proteins Force as a regulator of: -protein folding -enzyme catalysis Proteins are exposed to mechanical forces in-vivo extracellular matrix fibronectin connector complex synapse P0 Schwann cell Cytoskeleton: actin, etc. tenascin cell S-S bonds are common fascilin NCAM cell-matrix Basal Lamina tenascin fibronectin etc. Anchor proteins Cytoskeleton Dystrophin titin F ion channels Cadherin muscle We engineer polyproteins with 8-12 repeats extension (nm) Pulling a polyprotein at constant force produces a staircase of elongation events ubiquitin time (s) Tilting the energy landscape of a reaction k Ae 0 u G0 / kT W W* G0 W ku k e 0 W * / kT u * W Fx * x The probabilistic nature of unfolding is apparent in the dwell times A protein length (20 nm/div) 20 nm B td four different molecules pulled at the same force (110 pN) F= 100 pN Time (s) Each unique folding pathway is still largely hidden from view due to low time resolution length (20 nm/div) At t ~ 1ms we will be able to observe bond-by-bond details force (pN) ? 100 ms too slow Force dependent protein unfolding k k0 e Fx k BT k0= 0.015 s-1 xu= 1.7 Å MD simulations of I27 unfolding A`-G patch of I27 F 6 hydrogen bonds in A`-G patch F Unfolding transition state contains water molecules Use solvent substitution to probe for role of water molecules in TS 0 % glycerol 20 % glycerol 30 % glycerol 50 % glycerol D2O increases G0 but does not change x H2O D2O H2O ΔXU = 0.25 nm D2O ΔXU = 0.27 nm Force-dependent chemical reactions F The (I27G32C-A75C)8 polyprotein F “Trapped” residues “Unsequestered” residues F Mechanical unfolding exposes the buried disulfide to nucleophilic attack nucleophile Identifying single disulfide reduction reactions I27G32C-A75C [DTT] = 0 mM [DTT] = 12.5 mM Unsequestered unfolding up to disulfide; ~10.5 nm Single disulfide reduction & trapped unfolding; ~14.2 nm Force accelerates the rate of disulfide reduction by DTT Force dependency measures the elongation of the S-S bond at the transition state. 54 r= [Trx] r0exp(FΔxr/kBT) xr=0.34 Å S-S bond elongation at TS depends on the reducing agent 2.5 mM TCEP x=0.47±0.03 Å rate (s-1) rate (s-1) 12.5 mM GSH x=0.29±0.06 Å Force (pN) Force (pN) Studying enzyme catalysis is a big challenge its all done by rearranging atoms over very short distances.... below 1Å! distances below 1Å cannot be easily resolved Probing the chemistry of thioredoxin catalysis with force Chemistry: SN2 attack of thiolate anion on disulfide Arne Holmgren ; Eur. J. Biochem, 1968, 6:475-484 Arne Holmgren et al; PNAS, 1975, 72:2305–2309 A single molecule thioredoxin system Identifying disulfide reduction by single Trx enzymes Trx= 0 Trx= 8mM The rate of reduction is both force and [Trx] dependent [Trx]= 8 mM F= 100 pN Trx catalysis has a bimodal force dependency 15:1 k01 = k01(0) [Trx] k12 = k12(0) exp(FΔx/kBT)) k02 = k02(0) [Trx] exp(FΔx/kBT)) • two pathways for Trx reduction (I & II) • Fits reveal a ratio of 15:1 Path I • Inhibited by force • complex shortens by Δx12 = -0.73 Å k01 = k01(0) [Trx] k12 = k12(0) exp(FΔx12/kBT)) Nucleophilic attacks are directional Reorientation of the stretched bond is required to have all three S atoms in a line Path II k02 = k02(0) [Trx] exp(FΔx02/kBT)) • Accelerated by force • complex elongates by Δx02 = 0.22 Å Transition states for SN2 thiolate attack in water. Pedro Alexandrino Fernandes and Maria Joào Ramos: Chem. Eur. J. 2004, 10, 257 ―266 The P34H mutation reduces ratio I/II 5:1 • reduced k01 unchanged Δx12 and Δx02 Trx mutations can change both a0 and x 1.5 WT 10 µM -1 rate (s ) 2.0 1.0 G74S 10 µM 0.5 P34H/G74S 10 µM 0 200 400 Force (pN) 600 Rate vs Force plots are sensitive to chemical mechanism well into the sub-Å level. No other technique can do this. 6 hTrx 5 DTT -1 r (s ) 4 3 E. Coli Trx 2 1 0 0 100 200 300 400 Force (pN) 500 600 Dwell times to reduction, in log binned plots readily uncovers the number of rate constants Mechanical injury leads to oxidative stress and high mechanical forces swelling, inflammation ROS F Oxidative stress fibronectin promotes formation of disulfide bonds, stiffening tissues Chemical reactions catalyzed by enzymes are affected by mechanical force, then... trauma, swelling, inflamation High forces in vascular damage Stent placement Understanding protein folding is one of the grand challenges of modern biology. Can we say something new with the AFM? So far we have mostly studied proteins using chemical denaturation. urea unfolded state (undefined) folded fluorescence (indirect) reaction coordinate (unknown) ku k e 0 m[ D ] u time The force-quench experiment length (20 nm/div) 3 4 2 1 5 force (pN) ubiquitin time (s) The second pull measures the extent to which mechanical stability is recovered after folding The fraction of stable proteins recovers exponentially The unstable component elongates in fast steps The time to recovery is measured from the moment that the protein has collapsed Folding is slowed by force k0= 100 s-1 xf= 8.2 Å Identical force quench experiments, very different outcomes Folding trajectories Failures (majority) After extension, the protein is mostly a very stiff polymer and those that readily contract typically fold Every extension resets the dihedral space to a new starting point Reduced hydrophobic collapse in a 40 % ethanol solution Saline Saline + 40% ethanol Change in free energy for entropic and hydrophobic collapse Entropy 3Å Hydrophobic collapse Energy landscape of an extended protein relaxed to ~10 pN ±180° at Lcontour Purely entropic “flat” region Hydrophobic forces are strong, steep funnel Folding after a force-quench entropic (chain) collapse hydrophobic (solvent) collapse folded unstable folded stable Carmelu Badilla Kirstin Walther Jasna Brujic Arun Wiita Sergi Garcia Manyes Robert Szoszkiewicz Frauke Grater Lorna Dougan Raul Perez-Jimenez Koti Ainavarapu A few conclusions -Force-spectroscopy tracks the conformations and chemistry of single proteins with sub-Ångstrom and millisecond resolution (will get better). -Force probes novel regions of the folding and unfolding energy landscape of proteins. -Force regulates the rate of chemical reactions. And thanks to my collaborators: -Bruce Berne; Columbia Chemistry -Jose M. Sanchez-Ruiz, Universidad de Granada -Arne Holmgren, Karolinska Institute -Hui Lu; University of Illinois
![Anti-Thioredoxin 2 antibody [71G4] ab16857 Product datasheet 1 Abreviews 1 Image](http://s2.studylib.net/store/data/012095853_1-72f5dbc2ebbf408fbe2cfbbfa171f0c1-300x300.png)



